Abstract
Background
Stereotactic body radiotherapy (SBRT) in pancreatic cancer can be limited by its proximity to organs at risk (OAR). In this analysis, we evaluated the toxicity and efficacy of two different treatment approaches in patients with locally recurrent or oligometastatic pancreatic cancer.
Materials and methods
According to the prescription method, patients were divided in two cohorts (C1 and C2). The planning target volume (PTV) was created through a 4 mm expansion of the internal target volume. In C2, a subvolume was additionally created, a simultaneous integrated protection (SIP), which is the overlap of the PTV with the planning risk volume of an OAR to which we prescribed a reduced dose.
Results
In all, 18 patients were treated (7 with local recurrences, 9 for oligometastases, 2 for both). Twelve of 23 lesions were treated without SIP (C1) and 11 with SIP (C2). The median follow-up was 12.8 months. Median overall survival (OS) was 13.2 (95% confidence interval [CI] 9.8–14.6) months. The OS rates at 6 and 12 months were 87 and 58%, respectively. Freedom from local progression for combined cohorts at 6 and 12 months was 93 and 67% (95% CI 15–36), respectively. Local control was not statistically different between the two groups. One patient in C2 experienced grade ≥3 acute toxicities and 1 patient in C1 experienced a grade ≥3 late toxicity.
Conclusion
The SIP approach is a useful prescription method for abdominal SBRT with a favorable toxicity profile which does not compromise local control and overall survival despite dose sacrifices in small subvolumes.
Zusammenfassung
Hintergrund
Die stereotaktische Strahlentherapie (SBRT) ist bei Pankreaskarzinomen durch die enge Lagebeziehung der Risikoorgane (OAR) zum Zielvolumen erschwert. In dieser Analyse evaluierten wir die Toxizität und die Lokalkontrolle von zwei unterschiedlichen Therapiestrategien bei Patienten mit rezidivierendem oder oligometastatischem Pankreaskarzinom.
Material und Methoden
Die Patienten wurden anhand der Verschreibungsmethode in zwei Kohorten geteilt (C1 und C2). Das Planungszielvolumen (PTV) wurde durch eine Expansion des internen Zielvolumens (ITV) um 4 mm erzeugt. In C2 wurde zusätzlich ein Subvolumen (simultan integrierte Protektion, SIP) definiert, welches durch die Überlappung des PTV mit dem Planungsrisikovolumen (PRV) eines OAR generiert wurde, um die Grenzdosen für das jeweilige OAR einhalten zu können.
Ergebnisse
Insgesamt 18 Patienten wurden behandelt (7 Lokalrezidive, 9 Oligometastasen, 2 kombiniert). Zwölf von 23 Läsionen wurden ohne SIP (C1) und 11 mit SIP (C2) therapiert. Bei einem medianen Follow-up von 12,8 Monaten lag das mediane Überleben bei 13,2 Monaten (95 %-Konfidenzintervall [KI] 9,8–14,6). Das Gesamtüberleben nach 6 und 12 Monaten betrug je 87 % und 58 %. Die Lokalkontrolle für das Gesamtkollektiv betrug nach 6 und 12 Monaten jeweils 93 % und 67 % (95 %-KI 15–36); sie war statistisch nicht unterschiedlich zwischen den beiden Gruppen. Ein Patient in C2 entwickelte eine akute Grad-4-Toxizität und 1 Patient in C1 entwickelte eine Grad-4-Spättoxizität.
Schlussfolgerung
Die SIP-Verschreibungsmethode ist eine hilfreiche Strategie bei der SBRT mit einem günstigen Nebenwirkungsprofil. Trotz der Dosisreduktion in kleinen Subvolumina waren die lokale Kontrolle und das Gesamtüberleben identisch.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreatic cancer has a devastating prognosis with 5‑year survival rates of 7% [1]. Surgical excision is the treatment choice with a 5-year survival rate of approximately 20%, but resection is only possible in 15–20% of patients [2, 3]. Published data suggest that stereotactic ablative radiotherapy (SBRT) could be an alternative approach to chemoradiotherapy (CRT) for locally advanced pancreatic cancer (LAPC) with promising local control (48–94% at 12 months) [4–12]. Furthermore, reported local control for the treatment of hepatic metastases with SBRT ranges from 75–95% at 1 year and 60–90% at 2 years [13, 14].
With regard to toxicity, published data show an excellent acute tolerance but late gastrointestinal toxicity is dose-limiting [4, 15, 16] because of the close proximity to critical organs. Some authors introduced risk adaptive strategies, dependent on the location of the tumor, with reduced dose to the entire planning target volume (PTV) [15], while others prioritized the duodenal dose constraints over all other organs and accepted dose reductions to the total PTV [11]. By reducing the dose to the entire PTV, a severely reduced tumor control probability (TCP) has to be assumed as the price for lower normal tissue complication probability (NTCP). Other groups suggested the reduction of PTV margins [17]. In order to overcome the problem without reducing the dose to the whole PTV, we developed a novel prescription method termed simultaneous integrated protection (SIP) for quantifiable and comparable dose prescription to targets close to dose-limiting structures [18]. The current retrospective analysis was performed to analyze the toxicity and efficacy in patients who underwent SBRT for locally advanced or oligometastatic pancreatic cancer. We compared two cohorts without (C1) and with (C2) simultaneous integrated protection (SIP) for hollow organs in the vicinity of the target volumes.
Materials and methods
Patient selection
This single institutional retrospective analysis was approved by the ethics board of the University Medical Center. Patients eligible for this study had histologically proven pancreatic adenocarcinoma (PDAC), had been deemed to be surgically or medically inoperable, and were discussed in a multidisciplinary board. A complete staging evaluation with physical examination, positron emission computed tomography (PET/CT) or magnetic resonance imaging (MRI) of the abdomen and CT thorax was performed prior to therapy. Treatment was according to our institutional standard operating procedures and in analogy to a planned single center phase I trial developed to test the toxicity profile of the SIP approach [18].
SBRT techniques
Patients were immobilized in supine position in a high precision customized vacuum cushion (BlueBAG BodyFIX, Innovative Technologies Völp, Innsbruck, Austria) using abdominal compression during 4D-CT (Brilliance CT Big Bore, Philips Medical Systems, Cleveland, OH, USA) or 4D-PET-CT (Gemini TF BigBore, Philips Medical Systems, Cleveland, OH, USA). For the four-dimensional (4D) acquisition, breathing motion was monitored with a belt (Mayo Clinic Respiratory feedback system) using a phase-based binning method. The internal target volume (ITV) was created accounting for the extent and the position of the tumor in all motion phases in three dimensions using the 4D image data. The PTV was a uniform 4 mm expansion of the ITV in all dimensions (both cohorts). All hollow visceral organs were expanded isotropically by 4 mm to demarcate volumes of increased risk of high dose exposure (planning risk volume, PRV). For lesions where dose constraints could not be achieved without reducing the dose to the whole PTV, we utilized a simultaneous integrated protection (SIP) dose prescription (C2), an intensity-modulated radiotherapy (IMRT) technique described in detail elsewhere ([18]; Fig. 1). In short, we defined a subvolume for the intersection of the PTV and the PRV of hollow organs termed simultaneous protection volume (PTVSIP). The subvolume of the PTV without overlap with the PRV represented the dominant PTV (PTVdom). The term dominant was chosen to imply that the SIP approach is only valid for small volumes of PTVSIP. Dose was prescribed to PTVdom with a dose reduction made in the small PTVSIP subvolume. The dose in the PTVSIP was required to be as high as possible within the constraints to avoid local relapse. Treatment planning was performed using either Oncentra Masterplan (Nucletron BV [Elekta], Veenendaal, Netherlands) or Eclipse (Varian Medical Systems, Palo Alto, CA) treatment planning systems.
Example of a treatment using the simultaneous integrated protection (SIP) method. PTV: Total planning target volume (104 cm3), PRV planning risk volume, PTV SIP : subvolume of the PTV with overlap of the PRV that was prescribed a reduced dose (21 cm3), PTV dom : subvolume of the PTV without overlap with the PRV
Patients were treated every other day with 3–12 fractions, depending on the proximity to OARs. Dose was prescribed either to a specific isodose (60%, 80%) or according to ICRU 83. Dose constraints were in concordance with the SIP protocol and published literature [18, 19]. Three fraction regimens were preferred in patients with lesions away from critical structures, 12 fraction regimens were preferred in patients with intimate contact to bowel structures, and 5 fraction regimens were intermediate in terms of closeness to the bowel. Daily on-line correction using cone beam CTs (CBCT) was applied with oral contrast in order to visualize the stomach and duodenum.
The prescribed doses were converted to biological effective doses (BED) and equieffective doses for 2 Gy fractions (EQD2), assuming that tumor and late reacting bowel tissue α/βratios were 10 Gy and 3 Gy, respectively.
Toxicity and follow-up
Patients were examined clinically at least once per week during treatment by radiation oncologists. During follow-up, complete history, physical examination, and imaging (CTs, MRIs, or PET-CTs) were acquired every 3 months. Toxicity was scored using the National Cancer Institute Common Terminology Criteria for Adverse Events v. 4.03. Toxicities reported within 3 months after treatment completion were considered acute; any toxicity thereafter was considered to be late.
Statistical analysis
End points were toxicity assessment and freedom from local progression (FFLP) at 1 year; the latter was defined as the absence of progressive disease within the PTV, as well as overall survival. Local failure was defined as per Response Evaluation Criteria in Solid Tumors (RECIST) v1.1. Lesions that developed or progressed outside the PTV, in the same organ, were scored as local out of field progression and those developed in other organs as distant progression. Survival and control times were calculated from the start of SBRT. Time to progression and survival were evaluated with the Kaplan–Meier method and multivariate analysis using the Cox proportional hazards model and logistic regressions at a statistical significance level of p ≤ 0.05 (two sided). Analyses were performed using SPSS (v. 21, SPSS Inc., Chicago, IL, USA).
Results
Patients and treatment characteristics
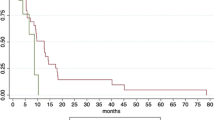
Between 2009 and 2014, a total of 18 patients with 23 lesions were treated (Suppl. Table 1, Table 1). Nine patients were treated for local progression or recurrence after resected PDAC (3 and 6 patients, respectively), two of whom had an additional solitary metastasis (liver). Nine patients were treated for oligometastases (liver and/or lymph nodes). Of these lesions, 12 were treated without SIP (C1) and 11 were treated with SIP (C2, Fig. 2). Twelve patients were diagnosed with oligometastatic disease prior to treatment and 16 received systemic therapy prior to SBRT.
Patients in the C1 group received a median total physical dose to the PTV of 35 Gy (30–40 Gy), with a median BED10 of 60 Gy (range 45–84 Gy) and a median EQD210of 50 Gy (range 38–70 Gy). The median PTV volume was 21.3 cm3 (range 6.6–106 cm3).
In C2, the median PTV volume was 65 cm3 (range 10.6–210 cm3) and the median PTVSIP volume 17 cm3 (range 0.4–66 cm3). The median ratio of PTVSIP/PTV was 12% (range 0.4–44%). The median total prescription dose was 48 Gy (range 32–50 Gy); the median PTVSIP prescription was 42 Gy (range 28–42 Gy). The median biological effective dose (BED10) and equieffective dose in 2 Gy fractions (EQD210) prescribed to the whole PTV were 67 Gy (range 46–100 Gy) and 56 Gy (range 38–83 Gy), respectively.
Response
After treatment, patients were routinely examined physically and received a CT or MRI every 3 months. In cases where the response could not be otherwise assessed, a PET CT was performed for further evaluation. Of the 23 lesions treated, 18 did not progress and 5 progressed in-field at a median interval of 7.7 months (range 6.6–13.7 months) after therapy. The FFLP at 6 months was 93% and at 12 months 67% (95% CI 15–35 months, Fig. 3). Neither use of the SIP technique (p = 0.757, log-rank), nor prior metastases, PTV volume, physical dose, EQD210, D95%, D90% D02, Dmean, or corresponding EQD210 for PTV, PTVsip, PTVdom were significant for local control (Table 2). Of the 5 in-field uncontrolled lesions, three were treated with SIP and the remaining two without SIP. Sites of in-field progression were liver (n = 2), lymph nodes (n = 1), and pancreas (n = 2). Regarding the patterns of failure, no patient developed an in-field only progression; two patients developed an in-field in combination with an out of field but local (same organ, both in liver) progression and two in combination with a distant progression (Fig. 3). The median BED10 for the lesions that progressed was 67 Gy (range 53–86 Gy) and the median BED10 for the SIP subvolumes was 57 Gy (range 54–57 Gy) and the median BED10 for the lesions that did not progress was 69.5 (range 46–119) and the median BED10 for the SIP subvolumes was 57 (range 43–60; p = 0.458). CA19-9 concentrations at start of SBRT were available in all but two patients and did not predict local failure (p = n. s.).
Time to chemotherapy after SBRT was 3.6 months (median) from the last day of radiotherapy. A total of 14 patients had chemotherapy after SBRT and 3 patients had no further chemotherapy, data were missing in 1 patient.
Survival
At the time of the analysis 7 of the 18 patients had died, 5 from progressive disease and 2 from other causes. The median overall survival (OS) after treatment was 13.2 months (95% CI 16.3–34.5). The OS rates at 6 and 12 months were 87 and 58%, respectively. Survival from primary diagnosis was 34.9 months (range 9.2–88 months) postresection and 32.4 months (range 15.5–90.1 months) in patients with primary chemotherapy and oligometastases. Only the PTV volume was statistically significant for survival on multivariate analysis but not on univariate analysis (Table 2).
Toxicity
Toxicities are listed in Table 3. Most patients tolerated the treatment without or with mild symptoms (CTC grade I) mostly nausea (26%) and diarrhea (16%). One patient suffered from abdominal pain CTC grade 1 after the first fraction (1 × 9 Gy) and subsequently fractionation was changed to 10 × 4 Gy every other day. One patient (C2) suffered from an occlusive ileus (CTC grade 3) during radiotherapy due to tumor compression requiring a stent which resulted in termination of treatment at 31.5 Gy (7 × 4.5 Gy, EQD210 38 Gy, and BED10 46 Gy). The Dmean of the duodenum at the time of treatment interruption was 5.1 Gy; the Dmax for 0.5cc was 20.5 Gy, for 5cc 18.3 Gy and for 10cc 16.5 Gy. A few days later the same patient suffered from an erosive bleeding from a Forrest III ulcer which was treated with proton pump inhibitors. The Dmean of the stomach at the time of treatment interruption was 5.6 Gy; the Dmax for 0.5cc was 19.8 Gy, for 5cc 17.8 Gy and for 10cc 16.5 Gy.
There was only one late toxicity; one patient (C1) experienced a CTC grade 4 bleeding of the common hepatic artery, 9 months after SBRT in a region where the tumor was primarily infiltrating the vessel, which was successfully treated by radiological coiling of the vessel. At the time of bleeding the tumor showed a partial response but the patient simultaneously developed a peritoneal dissemination. The prescription dose to the tumor was 40 Gy (8 × 5 Gy at the 60% isodose, EQD210 60 Gy, BED10 72 Gy). The Dmean at the common hepatic artery was 66 Gy (EQD210 106 Gy, EQD23 164 Gy) and the Dmax at 0.5cc was 66.7 Gy. No radiation-induced liver disease occurred.
Discussion
During the past years, several prospective and retrospective trials have tested the efficacy of SBRT in the treatment of LAPC with 1–15 fractions (4–25 Gy single doses) corresponding to an EQD210 of 31.25–204 Gy and a BED10 of 37.5–244.8 Gy [5]. Most acute toxicities were mild but several studies raised concerns due to a significant rate of gastrointestinal toxicities (ulcerations, bleedings, and perforations) [20]. The frequency of high-grade gastrointestinal toxicities (grade ≥ 3) rises over time [6] and ranges from 0–23 (median 7%).
These are the first published data reporting toxicity and outcomes with the novel SIP prescription technique described by Brunner et al. [18]. The treatment was very well tolerated without compromising the local control, despite of the prescription of a reduced dose to small subvolumes (SIP). The two observed grade 3 acute toxicities (occlusive ileus and gastrointestinal bleeding) occurred during therapy in one and the same patient at a very low cumulative dose. Thus, we concluded that the mechanical ileus was due to tumor infiltration and the gastrointestinal bleeding probably through the manipulation due to prior stenting of the duodenum a few days before. There were no other grade ≥ 3 acute or late toxicities in patients treated with this method. One late toxicity occurred (CTC grade 4) in the C1 group, namely bleeding from the common hepatic artery. This vessel was previously infiltrated by the tumor prior to treatment; bleeding was completely resolved following coiling of the vessel. The observed late toxicities grade ≥ 3 of 5% after SBRT of the upper abdomen are in concordance with published literature (Table 4).
Murphy et al. [4] demonstrated a dose-dependent relationship of duodenal toxicity after single fraction therapy concluding that the multiple dose–volume histogram endpoints and a Lyman NTCP model are strongly predictive of duodenal toxicity. This dose-dependent relationship was also confirmed in other series [5, 20–23]. Some authors [5] reported that late toxicity grade ≥ 2 and grade ≥ 3 correlated highly with prescribed EQD2/BED after linear (R2 = 0.85 and 0.77, respectively) and Lyman–Kutcher–Burman modelling. Linear regression lines indicated grade ≥ 2 and grade ≥ 3 toxicity frequencies of 5% at 65 Gy and 80 Gy EQD23, respectively.
Several attempts were made to reduce the incidence of late toxicities. The Stanford group found significantly fewer occurrences of gastrointestinal toxicities with fractionated SBRT than with single fraction [10], while Schellenberg et al. [11] prioritized the duodenal dose constraints and accepted dose reductions in order to keep at least 50% of the duodenum near the PTV under the 50% dose and only 5% of the duodenum was allowed to receive 95% of the dose. Mahadevan et al. [15, 16] determined the fraction size for a total of three fractions by the distance between the tumor and bowel. The group reported a median OS of 20 months, although this was calculated from the time of diagnosis and late grade 3 toxicity was 9% (3/39 with gastrointestinal bleeding).
In our series the FFLP at 6 and 12 months was 93 and 67%, respectively, at a median EQD210 of 56 Gy and a BED10 of 67 Gy which correspond to the published data. The reported local control rates at 12 months ranged from 48–94% (Table 4). Furthermore, we could show an encouraging overall survival (OS) of 13.8 months in a patient collective with an unfavorable prognosis due to tumor recurrence and/or the presence of metastases at the time of treatment. The median OS in the SBRT series without metastases ranges from 5.7–20 months (median 14.4 months) from diagnosis and 6.4–14.3 months (median 11 months) from radiotherapy (Table 4). Over the past decades, the concept of oligometastases has emerged as an intermediate stage between locoregional tumor spread and disseminated metastases. Preventing or delaying local recurrence with SBRT not only decreases tumor burden but also offers palliative benefit. SBRT can be well tolerated without significant toxicity and may play a role in the prolongation of survival in highly selected patients. This group of patients with prolonged survival have a higher risk of developing late morbidities so that a calculated treatment approach is important in order to reduce the appearance of late toxicities but also to intensify treatment.
Conclusion
The SIP treatment approach is a useful adaptive prescription method which tailors the dose to each tumor, with a favorable toxicity profile, while respecting normal tissue constraints. Local control and overall survival were very good despite of dose sacrifices in small subvolumes in half of the patients. Based on these data, we are now conducting a phase 1 prospective study in patients with OARs adjacent to the PTV using the SIP concept.
References
Siegel RL, Miller KD, Jemal A (2015) Cancer statistics, 2015. CA Cancer J Clin 65(1):5–29. doi:10.3322/caac.21254
Herman JM, Wild AT, Wang H, Tran PT, Chang KJ, Taylor GE, Donehower RC, Pawlik TM, Ziegler MA, Cai H, Savage DT, Canto MI, Klapman J, Reid T, Shah RJ, Hoffe SE, Rosemurgy A, Wolfgang CL, Laheru DA (2013) Randomized phase III multi-institutional study of TNFerade biologic with fluorouracil and radiotherapy for locally advanced pancreatic cancer: final results. J Clin Oncol 31(7):886–894. doi:10.1200/JCO.2012.44.7516
Ducreux M, Cuhna AS, Caramella C, Hollebecque A, Burtin P, Goéré D, Seufferlein T, Haustermans K, Van Laethem JL, Conroy T, Arnold D (2015) Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 26(suppl 5):v56–v68. doi:10.1093/annonc/mdv295
Murphy JD, Christman-Skieller C, Kim J, Dieterich S, Chang DT, Koong AC (2010) A dosimetric model of duodenal toxicity after stereotactic body radiotherapy for pancreatic cancer. Int J Radiat Oncol Biol Phys 78(5):1420–1426. doi:10.1016/j.ijrobp.2009.09.075
Brunner TB, Nestle U, Grosu AL, Partridge M (2015) SBRT in pancreatic cancer: what is the therapeutic window? Radiother Oncol 114(1):109–116. doi:10.1016/j.radonc.2014.10.015
Chang DT, Schellenberg D, Shen J, Kim J, Goodman KA, Fisher GA, Ford JM, Desser T, Quon A, Koong AC (2009) Stereotactic radiotherapy for unresectable adenocarcinoma of the pancreas. Cancer 115(3):665–672. doi:10.1002/cncr.24059
Herman JM, Chang DT, Goodman KA, Dholakia AS, Raman SP, Hacker-Prietz A, Iacobuzio-Donahue CA, Griffith ME, Pawlik TM, Pai JS, O’Reilly E, Fisher GA, Wild AT, Rosati LM, Zheng L, Wolfgang CL, Laheru DA, Columbo LA, Sugar EA, Koong AC (2015) Phase 2 multi-institutional trial evaluating gemcitabine and stereotactic body radiotherapy for patients with locally advanced unresectable pancreatic adenocarcinoma. Cancer 121(7):1128–1137. doi:10.1002/cncr.29161
Koong AC, Christofferson E, Le QT, Goodman KA, Ho A, Kuo T, Ford JM, Fisher GA, Greco R, Norton J, Yang GP (2005) Phase II study to assess the efficacy of conventionally fractionated radiotherapy followed by a stereotactic radiosurgery boost in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys 63(2):320–323. doi:10.1016/j.ijrobp.2005.07.002
Koong AC, Le QT, Ho A, Fong B, Fisher G, Cho C, Ford J, Poen J, Gibbs IC, Mehta VK, Kee S, Trueblood W, Yang G, Bastidas JA (2004) Phase I study of stereotactic radiosurgery in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys 58(4):1017–1021. doi:10.1016/j.ijrobp.2003.11.004
Pollom EL, Alagappan M, von Eyben R, Kunz PL, Fisher GA, Ford JA, Poultsides GA, Visser BC, Norton JA, Kamaya A, Cox VL, Columbo LA, Koong AC, Chang DT (2014) Single- versus multifraction stereotactic body radiation therapy for pancreatic adenocarcinoma: outcomes and toxicity. Int J Radiat Oncol Biol Phys 90(4):918–925. doi:10.1016/j.ijrobp.2014.06.066
Schellenberg D, Kim J, Christman-Skieller C, Chun CL, Columbo LA, Ford JM, Fisher GA, Kunz PL, Van Dam J, Quon A, Desser TS, Norton J, Hsu A, Maxim PG, Xing L, Goodman KA, Chang DT, Koong AC (2011) Single-fraction stereotactic body radiation therapy and sequential gemcitabine for the treatment of locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys 81(1):181–188. doi:10.1016/j.ijrobp.2010.05.006
Trakul N, Koong AC, Chang DT (2014) Stereotactic body radiotherapy in the treatment of pancreatic cancer. Semin Radiat Oncol 24(2):140–147. doi:10.1016/j.semradonc.2013.11.008
Aitken KL, Hawkins MA (2015) Stereotactic body radiotherapy for liver metastases. Clin Oncol 27(5):307–315. doi:10.1016/j.clon.2015.01.032
Sterzing F, Brunner TB, Ernst I, Baus WW, Greve B, Herfarth K, Guckenberger M (2014) Stereotactic body radiotherapy for liver tumors: principles and practical guidelines of the DEGRO Working Group on Stereotactic Radiotherapy. Strahlenther Onkol 190(10):872–881. doi:10.1007/s00066-014-0714-1
Mahadevan A, Jain S, Goldstein M, Miksad R, Pleskow D, Sawhney M, Brennan D, Callery M, Vollmer C (2010) Stereotactic body radiotherapy and gemcitabine for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys 78(3):735–742. doi:10.1016/j.ijrobp.2009.08.046
Mahadevan A, Miksad R, Goldstein M, Sullivan R, Bullock A, Buchbinder E, Pleskow D, Sawhney M, Kent T, Vollmer C, Callery M (2011) Induction gemcitabine and stereotactic body radiotherapy for locally advanced nonmetastatic pancreas cancer. Int J Radiat Oncol Biol Phys 81(4):e615–622. doi:10.1016/j.ijrobp.2011.04.045
Kumar R, Wild AT, Ziegler MA, Hooker TK, Dah SD, Tran PT, Kang J, Smith K, Zeng J, Pawlik TM, Tryggestad E, Ford E, Herman JM (2013) Stereotactic body radiation therapy planning with duodenal sparing using volumetric-modulated arc therapy vs intensity-modulated radiation therapy in locally advanced pancreatic cancer: A dosimetric analysis. Med Dosim 38(3):243–250. doi:10.1016/j.meddos.2013.02.003
Brunner TB, Nestle U, Adebahr S, Gkika E, Wiehle R, Baltas D, Grosu AL (2016) Simultaneous integrated protection: a new concept for high-precision radiation therapy. Strahlenther Onkol. doi:10.1007/s00066-016-1057-x
Timmerman RD (2008) An overview of hypofractionation and introduction to this issue of seminars in radiation oncology. Semin Radiat Oncol 18(4):215–222. doi:10.1016/j.semradonc.2008.04.001
Hoyer M, Roed H, Sengelov L, Traberg A, Ohlhuis L, Pedersen J, Nellemann H, Kiil Berthelsen A, Eberholst F, Engelholm SA, von der Maase H (2005) Phase-II study on stereotactic radiotherapy of locally advanced pancreatic carcinoma. Radiother Oncol 76(1):48–53. doi:10.1016/j.radonc.2004.12.022
Kavanagh BD, Pan CC, Dawson LA, Das SK, Li XA, Ten Haken RK, Miften M (2010) Radiation dose-volume effects in the stomach and small bowel. Int J Radiat Oncol Biol Phys 76(3 Suppl):S101–S107. doi:10.1016/j.ijrobp.2009.05.071
Cattaneo GM, Passoni P, Longobardi B, Slim N, Reni M, Cereda S, di Muzio N, Calandrino R (2013) Dosimetric and clinical predictors of toxicity following combined chemotherapy and moderately hypofractionated rotational radiotherapy of locally advanced pancreatic adenocarcinoma. Radiother Oncol 108(1):66–71. doi:10.1016/j.radonc.2013.05.011
Elhammali A, Patel M, Weinberg B, Verma V, Liu J, Olsen JR, Gay HA (2015) Late gastrointestinal tissue effects after hypofractionated radiation therapy of the pancreas. Radiat Oncol 10:186. doi:10.1186/s13014-015-0489-2
Gurka MK, Kim C, He AR, Charabaty A, Haddad N, Turocy J, Johnson L, Jackson P, Weiner LM, Marshall JL, Collins SP, Pishvaian MJ, Unger K (2014) Stereotactic Body Radiation Therapy (SBRT) Combined With Chemotherapy for Unresected Pancreatic Adenocarcinoma. Am J Clin Oncol. doi:10.1097/COC.0000000000000118
Chuong MD, Springett GM, Freilich JM, Park CK, Weber JM, Mellon EA, Hodul PJ, Malafa MP, Meredith KL, Hoffe SE, Shridhar R (2013) Stereotactic Body Radiation Therapy for Locally Advanced and Borderline Resectable Pancreatic Cancer Is Effective and Well Tolerated. Int J Radiat Oncol 86(3):516–522
Polistina F, Costantin G, Casamassima F, Francescon P, Guglielmi R, Panizzoni G, Febbraro A, Ambrosino G (2010) Unresectable Locally Advanced Pancreatic Cancer: A Multimodal Treatment Using Neoadjuvant Chemoradiotherapy (Gemcitabine Plus Stereotactic Radiosurgery) and Subsequent Surgical Exploration. Ann Surg Oncol 17(8):2092–2101
Rwigema JC, Parikh SD, Heron DE, Howell M, Zeh H, Moser AJ, Bahary N, Quinn A, Burton SA (2011) Stereotactic body radiotherapy in the treatment of advanced adenocarcinoma of the pancreas. Am J Clin Oncol 34(1):63–69. doi:10.1097/COC.0b013e3181d270b4
Tozzi A, Comito T, Alongi F, Navarria P, Iftode C, Mancosu P, Reggiori G, Clerici E, Rimassa L, Zerbi A, Fogliata A, Cozzi L, Tomatis S, Scorsetti M (2013) SBRT in unresectable advanced pancreatic cancer: preliminary results of a mono-institutional experience. Radiat Oncol 8(1):148
Didolkar MS, Coleman CW, Brenner MJ, Chu KU, Olexa N, Stanwyck E, Yu A, Neerchal N, Rabinowitz S (2010) Image-Guided Stereotactic Radiosurgery for Locally Advanced Pancreatic Adenocarcinoma Results of First 85 Patients. J Gastrointest Surg 14(10):1547–1559
Goyal K, Einstein D, Ibarra RA, Yao M, Kunos C, Ellis R, Brindle J, Singh D, Hardacre J, Zhang Y, Fabians J, Funkhouser G, Machtay M, Sanabria JR (2012) Stereotactic Body Radiation Therapy for Nonresectable Tumors of the Pancreas. J Surg Res 174(2):319–325
Moningi S, Dholakia AS, Raman SP, Blackford A, Cameron JL, Le DT, De Jesus-Acosta AMC, Hacker-Prietz A, Rosati LM, Assadi RK, Dipasquale S, Pawlik TM, Zheng L, Weiss MJ, Laheru DA, Wolfgang CL, Herman JM (2015) The Role of Stereotactic Body Radiation Therapy for Pancreatic Cancer: A Single-Institution Experience. Ann Surg Oncol 22(7):2352–2358
Mellon EA, Hoffe SE, Springett GM, Frakes JM, Strom TJ, Hodul PJ, Malafa MP, Chuong MD, Shridhar R (2015) Long-term outcomes of induction chemotherapy and neoadjuvant stereotactic body radiotherapy for borderline resectable and locally advanced pancreatic adenocarcinoma. Acta Oncol 54(7):979–985
Comito T, Cozzi L, Clerici E, Franzese C, Tozzi A, Iftode C, Navarria P, D’Agostino G, Rimassa L, Carnaghi C, Personeni N, Tronconi MC, De Rose F, Franceschini D, Ascolese AM, Fogliata A, Tomatis S, Santoro A, Zerbi A, Scorsetti M (2016) Can Stereotactic Body Radiation Therapy Be a Viable and Efficient Therapeutic Option for Unresectable Locally Advanced Pancreatic Adenocarcinoma? Results of a Phase 2 Study. Technol Cancer Res T. doi:10.1177/1533034616650778
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
E. Gkika, S. Adebahr, S. Kirste, T. Schimek-Jasch, R. Wiehle, R. Claus, U. Wittel, U. Nestle, D. Baltas, A.L. Grosu, and T.B. Brunner declare that they have no competing interests.
Ethical standards
This article does not contain any studies with human participants or animals performed by any of the authors.
Caption Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Gkika, E., Adebahr, S., Kirste, S. et al. Stereotactic body radiotherapy (SBRT) in recurrent or oligometastatic pancreatic cancer. Strahlenther Onkol 193, 433–443 (2017). https://doi.org/10.1007/s00066-017-1099-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-017-1099-8