Abstract
Background
Radiotherapy has been reported to promote the invasion of glioblastoma cells; however, the underlying mechanisms remain unclear. Here, we investigated the role of the Wnt/β-catenin pathway in radiation-induced invasion of glioblastoma cells.
Methods
U87 cells were irradiated with 3 Gy or sham irradiated in the presence or absence of the Wnt/β-catenin pathway inhibitor XAV 939. Cell invasion was determined by an xCELLigence real-time cell analyser and matrigel invasion assays. The intracellular distribution of β-catenin in U87 cells with or without irradiation was examined by immunofluorescence and Western blotting of nuclear fractions. We next investigated the effect of irradiation on Wnt/β-catenin pathway activity using TOP/FOP flash luciferase assays and quantitative polymerase chain reaction analysis of β-catenin target genes. The expression levels and activities of two target genes, matrix metalloproteinase (MMP)-2 and MMP-9, were examined further by Western blotting and zymography.
Results
U87 cell invasiveness was increased significantly by ionizing radiation. Interestingly, ionizing radiation induced nuclear translocation and accumulation of β-catenin. Moreover, we found increased β-catenin/TCF transcriptional activities, followed by up-regulation of downstream genes in the Wnt/β-catenin pathway in irradiated U87 cells. Importantly, inhibition of the Wnt/β-catenin pathway by XAV 939, which promotes degradation of β-catenin, significantly abrogated the pro-invasion effects of irradiation. Mechanistically, XAV 939 suppressed ionizing radiation-triggered up-regulation of MMP-2 and MMP-9, and inhibited the activities of these gelatinases.
Conclusion
Our data demonstrate a pivotal role of the Wnt/β-catenin pathway in ionizing radiation-induced invasion of glioblastoma cells, and suggest that targeting β-catenin is a promising therapeutic approach to overcoming glioma radioresistance.
Zusammenfassung
Hintergrund
Studien haben gezeigt, dass eine Strahlentherapie die Invasivität von Glioblastomzellen erhöht. Zwar wurden mehrere Signalwege mit diesem strahleninduzierten Eindringen in Zusammenhang gebracht, doch die genauen Mechanismen sind bisher unklar. In der vorliegenden Studie wurden die proinvasive Wirkung einer Bestrahlung auf U87-Zellen überprüft und die Beteiligung des Wnt/ß-Catenin-Signalwegs als möglicher zugrundeliegender Mechanismus diskutiert.
Methoden
U87-Zellen wurden einer Strahlung von 3 Gy oder einer Scheinbestrahlung ausgesetzt, einige Zellen waren zuvor mit dem Wnt/ß-Catenin-Inhibitor XAV 939 behandelt worden. Der Einfluss dieser verschiedenen Konditionen auf die Invasivität wurde anschließend mit dem xCELLigence Zellanalysesystems und einem Matrigel-Invasion-Assay überprüft. Die intrazelluläre ß-Catenin-Verteilung mit bzw. ohne Bestrahlung wurde anhand von Western-Blot-Analysen unterschiedlicher Kernfraktionen und Immunfluoreszenzanalysen untersucht, die Wirkung der Strahlung auf die Aktivität des Wnt/ß-Catenin-Signalwegs durch einen Luciferase-Assay. Die Expressionslevel und Aktivität von Zielgenen, wie MMP-2 und MMP-9, wurden durch eine Western-Blot-Analyse und einen Zymographie-Assay determiniert.
Ergebnisse
Die Invasivität von U87-Zellen wurde durch ionisierende Strahlung signifikant erhöht. Immunfluoreszenzanalysen zeigten eine strahleninduzierte nukleäre Translokation und eine ß-Catenin-Akkumulation. Nach Bestrahlung zeigten sich im Luciferase-Assay zudem eine erhöhte ß-Catenin/TCF-Transkriptionsaktivität, gefolgt von einer Hochregulation der Downstream-Zielgene in den Wnt/ß-Catenin-Signalwegen in bestrahlten U87-Zellen. XV 939 unterdrückte die strahleninduzierte Hochregulierung von MMP-2 und -9 und inhibierte die Aktivität dieser Gelatinasen.
Fazit
Die vorgestellten Daten zeigen die zentrale Rolle des Wnt/ß-Catenin-Signalwegs für die strahleninduzierte Erhöhung der Invasivität von Glioblastomzellen. In diesem Zusammenhang könnte die Inhibierung von ß-Catenin einen viel versprechenden Ansatz in der Behandlung strahlenresistenter Gliome darstellen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Glioblastoma multiforme (GBM) is the most aggressive and devastating disease in the central nervous system [1]. Despite continuous advances in new clinical therapies, the prognosis and survival of GBM patients remain dismal with a median survival of 9–12 months. The diffusely infiltrative nature of GBM is the main obstacle for the development of effective treatments [2].
The standard therapy for glioblastoma is complete macroscopic resection and adjuvant irradiation of the tumour bed, which can be combined with adjuvant chemotherapy [3]. In the clinic, radiation treatment schemes are often fractionated (54–60 Gy at a daily fraction of 1.8–3 Gy) in which a small fraction of the radiation is delivered at regular intervals over a large time frame. However, sublethal doses of radiation can increase the aggressiveness of the cancer because each exposure may increase the migratory and invasive capacities of the tumour cells [4].
Radiotherapy has been found to promote the invasion of various kinds of cancer cells including GBM [5–9]. Ionizing radiation (IR) activates multiple signalling pathways in tumour cells, which modulate several cellular functions and induce the secretion of growth factors and chemokines, resulting in increased migration and invasiveness of cancer cells [10–12]. A better understanding of the molecular mechanisms that underlie IR-induced invasion and radiation resistance of GBM may provide new possibilities in terms of targeted therapeutic strategies. In this study, we examine the pro-invasive effects of irradiation on U87 cells and the involvement of the Wnt/β-catenin pathway, and discuss the possible underlying mechanism.
Methods
Cell culture and treatment
The GBM cell line U-87MG was obtained from the American Type Culture Collection (Manassas, VA) and cultured according to the supplier’s recommendations. At 70 % confluency, the cells were irradiated with 3 Gy in a Gammacell 40 exactor (Best Theratronics, Ontario, Canada). Control cells received a sham irradiation. At 30 min before irradiation or sham irradiation, XAV 939 (3,5,7,8-tetrahydro-2-[4-(trifluoromethyl)phenyl]-4H-thiopyrano[4,3-d]pyrimidin-4-one) (S1180; Selleckchem, Houston, TX) was added to the culture medium as a Wnt/β-catenin pathway inhibitor at a final concentration of 5 μM. For the control cells, dimethylsulfoxide was added to the medium.
Real-time cell analysis of invasion
Real-time cell analysis (RTCA) of invasion was performed using an xCELLigence DP device (Roche Diagnostics, Mannheim, Germany) according to the manufacturer’s instructions [13]. Briefly, the bottoms of the upper wells of a CIM-plate 16 were coated with 400 µg/ml collagen I (Sigma, Deisenhofen, Germany) and 5 % (v/v) growth factor-reduced matrigel (diluted at 1:20 in basal medium; BD Biosciences, Heidelberg, Germany). After irradiation (or sham irradiation), the cells were harvested and added in quadruplet to the upper chamber at 2.5 × 104 cells/well in serum-free culture medium, whereas the lower chambers were filled with complete medium containing FBS. Cells attach to the bottom, followed by initial invasion through a solid matrix, which leads to an increase in electrical impedance of the integrated gold microelectrodes. The dimensionless cell index, calculated as the relative change in measured electrical impedance caused by migrated cells, reflects the invasive behaviour of the cells.
Matrigel invasion assay
Invasion of GBM cells in vitro was assessed using Boyden chambers (BD Biosciences) in which the upper and lower wells are separated by a porous membrane (8-µm pore size) coated with matrigel. Following pre-treatment as indicated, subconfluent GBM cells were harvested in enzyme-free cell dissociation buffer (Gibco Life Technologies, Darmstadt, Germany) and added in triplicate at 4 × 104 cells in culture medium to each upper chamber. NIH 3T3 cell‐conditioned medium (500 μl) was used as a chemoattractant in the bottom wells. Cell invasion was evaluated by counting the number of cells that had migrated across the membrane within 20 h. Cells on the lower side of the membrane were fixed with ice-cold methanol for 10 min and stained with 4′6-diamidino-2-phenylindole [10 mg/ml in phosphate-buffered saline (PBS), 1:500 dilution] for 10 min in the dark. Images were obtained by scanning the chamber bottoms with a Cell Observer (Zeiss, Oberkochen, Germany), and cell numbers were counted using the ImageJ software (http://rsb.info.nih.gov/ij/). The relative invasion was plotted as the mean ± standard error of the mean against the non-irradiated and un-treated control cells.
Immunofluorescence
For immunofluorescence of β-catenin, cells were cultured on glass coverslips for 24 h before irradiation and then fixed at 12 h post-IR with 2 % paraformaldehyde in PBS. The cells were permeabilized with 0.3 % Triton-X 100 in PBS for 5 min and then incubated for 4 h at room temperature with rabbit anti-human β-catenin IgG (ab6302; Abcam, USA) diluted at 1:100 in 5 mg/ml bovine serum albumin (BSA)/PBS. Subsequently, the cells were washed with PBS plus Tween-20 (PBST), incubated with Alexa Fluor 488 goat anti-rabbit IgG (H+L; 1:1000; Life Technologies, Darmstadt, Germany) in 1 % BSA/PBST for 1 h at room temperature in the dark, and then washed three times with PBST in the dark. Finally, the cells were counterstained with 500 nM propidium iodide (Life Technologies) for 5 min at room temperature and mounted with mounting medium. Images were acquired with the Cell Observer and analysed by Image-Pro® Plus software (version 6.0.0.260).
Transfection and reporter assays
A total of 1.5 × 104U87 cells/well were seeded in 96-well plates. After overnight culture, the cells were transfected with 100 ng TOP-FLASH plasmid (Millipore), which contains six TCF-binding motifs, or 100 ng FOP-FLASH control plasmid (Millipore) containing six mutated TCF-binding motifs and 1 ng pRLSV40 vector (Promega, Madison, WI) in Opti-MEM using 0.25 μl Lipofectamine 2000 per well according to the manufacturer’s protocol (Invitrogen). After 6 h, the transfection solution was changed to serum-containing medium. Eighteen hours later, the cells were treated as indicated above. At 24 h after treatment, the cells were lysed and the luciferase activity was determined using a Dual Luciferase kit (Promega). The relative firefly luciferase activity was normalized to Renilla luciferase activity.
Quantitative reverse-transcription PCR
Total RNA was extracted using an RNeasy RNA isolation kit (Qiagen, Hilden, Germany). cDNA was prepared from the RNA using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Darmstadt, Germany). Gene expression analysis was performed in triplicate in an ABI Prism 7000 Sequence Detection System (Applied Biosystems) using SYBR Green Master Mix (Eurogentec, Cologne, Germany). Standard curves were generated for each transcript demonstrating 90–100 % amplification efficiency, and relative quantification of gene expression was determined by comparing the threshold values. Primers were purchased from Sigma-Aldrich (Taufkirchen, Germany). Primer sequences are provided in Table 1.
Zymography
Cells were treated as indicated in the previous section and cultured in serum-free medium for 24 h. The supernatant was harvested and the soluble proteins were concentrated. Then, 20-µg samples of the proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) in 10 % gels containing 0.1 % gelatine without denaturing agents. To allow digestion of the gelatine structure by gelatinases, the gels were washed twice for 30 min in 50 mM Tris-HCl (pH 7.5) and 2.5 % Triton X-100, and then incubated overnight at 37 °C in 50 mM Tris-HCl (pH 7.6), 10 mM CaCl2, 150 mM NaCl, and 0.05 % NaN3. The gels were stained with Coomassie Brilliant Blue R-250 and then destained with 90 % methanol/H2O (1:1) and 10 % glacial acetic acid. Because of the gelatinolytic activity, bright bands were visible at M r 92,000 for matrix metalloproteinase (MMP)-9 and M r 72,000 for MMP-2.
Western blot analysis
To determine the levels of protein expression in whole cells, cell pellets were harvested and mechanically homogenized in lysis buffer [50 mM Tris-HCl (pH 7.4), 50 mM NaCl, 1 % Triton X-100, 1 mM ethylene diamine tetra-acetic acid, 20 mM NaF, 2 mM Na3VO4, 1 mM phenylmethanesulfonyl fluoride, 1 μg/ml aprotinin, 1 μg/ml leupeptin, and 1 μg/ml pepstatin]. Homogenates were clarified by centrifugation at 14,000 g for 15 min at 4 °C, and total protein extracts were obtained from the supernatants. Nuclear proteins from U87 cells were extracted using NE-PER Nuclear and Cytoplasmic Extraction Reagents (Thermo Scientific, Rockford, USA) according to the manufacturer’s instructions. Protein concentrations were measured by the bicinchoninic acid method. Proteins were fractionated by 10 % SDS-PAGE (100 μg total protein per lane). After electrophoresis, the proteins were electrotransferred to nitrocellulose membranes. The membranes were then blocked in 5 % fat-free dry milk with 0.05 % Tween 20 for 1 h at room temperature with constant agitation, followed by overnight incubation with the primary antibody at 4 °C. Peroxidase-conjugated secondary antibodies were used to detect the labelled proteins, and visualization was performed by enhanced chemiluminescence and exposure to Hyperfilm ECL film (both from Amersham Biosciences, USA). Primary antibodies were purchased from Abcam as follows: anti-β-catenin (ab6302), anti-MMP2 (ab37150), and anti-MMP9 (ab38898) antibodies.
Statistical analysis
Statistical analysis was performed using the Student’s t test. A p value of less than 0.05 was considered significant.
Results
IR induces invasion of U87 cells
First, we evaluated the effect of IR on the invasion of GBM cells by RTCA of invasion and matrigel invasion assays. As shown in Fig. 1, irradiated cells were significantly more invasive than the non-irradiated control, indicating a pro-invasive effect of irradiation. Surprisingly, pre-treatment with XAV 939, an inhibitor of Wnt/β-catenin signalling, reversed the pro-invasive effect of irradiation. By contrast, inhibition of non-irradiated U87 cell invasion by XAV 939 was not significant (Fig. 1).
IR-induced invasion of U87 cells is reversed by XAV 939. a RTCA of invasion. Invasion was monitored in real time by the RTCA system for 24 h, and growth curves were plotted and analysed. The normalized cell index reflects the invasive capacity of tumour cells. For example, invasion by highly aggressive cells leads to large changes in cell impedance. Data represent the mean cell index and standard error of the mean; n = 4, *p < 0.05, t test, compared with non-irradiated cells; # p < 0.05, t test, presence vs. absence of XAV 939, b Matrigel invasion assay. Irradiated and non-irradiated U87 cells treated with/without XAV 939 were analysed for invasiveness in a matrigel invasion assay. Invaded cells were counted in five independent fields. Fifteen wells per condition were counted in three independent experiments. Data are expressed as the mean percentage of invasion relative to non-irradiated U87 cells without XAV 939 treatment and the standard error of mean; n = 3, *p < 0.05, t test, compared with non-irradiated cells; # p < 0.05, t test, presence vs. absence of XAV 939. RTCA real-time cell analysis
IR alters the distribution pattern of β-catenin
β-Catenin is a key component of the Wnt/β-catenin signalling pathway. Therefore, we next examined the activity of β-catenin in irradiated GBM cells. U87 cells were irradiated or sham irradiated for 12 h. Then, immunofluorescence was used to detect the content and distribution of cellular β-catenin. As shown in Fig. 2, β-catenin was mainly located on the cell membrane of non-irradiated cells, especially at adherens junctions. After irradiation, we observed elevations in the fluorescence signals of β-catenin. Moreover, colocalization analysis showed that more β-catenin was localized to the nucleus in irradiated U87 cells compared with the non-irradiated control, indicating that the distribution of β-catenin was mainly in the cytoplasm and nucleus of irradiated U87 cells. Consistent with these data, whole cell and nuclear β-catenin protein levels were increased significantly after irradiation, indicating accumulation of β-catenin in the nucleus after irradiation. In addition, XAV 939, which is known to promote β-catenin degradation, abrogated the up-regulation and nuclear accumulation of β-catenin in irradiated GBM cells (Fig. 3).
Ionizing radiation alters the cytoplasmic/nuclear distribution of β catenin. Immunofluorescence demonstrating translocation of β catenin to nuclei. Five fields of non-irradiated and irradiated U87 cells were chosen randomly. All images were acquired using the same settings. Neither autofluorescence nor saturation was detected. Red represents nuclei, while green represents β-catenin. The colocalization area was analysed and indicated as white. The upper images are non-irradiated U87 cells. β-Catenin was mainly located in the cell membrane, while very little β-catenin was in the nuclei (indicated by white dots). The lower images are irradiated U87 cells. The cytoplasmic/nuclear distribution of β-catenin changed in irradiated U87 cells with much more β-catenin accumulating in the nuclei. The colocalization Mender’s coefficients for β-catenin overlapping with the nuclei of irradaited-U87 cells (0.0858 ± 0.0313) were significantly higher compared with non-irradiated U87 cells (0.00298 ± 0.00181. Data represent the mean Mender’s coefficient and standard error of the mean; n = 5, p < 0.05, t test
IR increases the mRNA levels of β-catenin target genes
Up-regulation and nuclear translocation of β-catenin are known to indicate activation of the Wnt/β-catenin signalling pathway. To further evaluate the effects of irradiation on Wnt/β-catenin signalling in U87 cells, we employed TOP/FOP Flash dual reporter assays. Significantly higher relative TOP/FOP luciferase activities were observed in irradiated U87 cells compared with control U87 cells, indicating enhancement of β-catenin/TCF transcriptional activity by radiation, which could be suppressed by XVA 939 (Fig. 4a). Next, we screened the expression of several β-catenin target genes in non-irradiated U87 cells at 12 and 24 h post-irradiation. Quantitative polymerase chain reaction (PCR) analyses showed that the mRNA levels of β-catenin target genes were significantly up-regulated after 3-Gy irradiation, and the up-regulation could be suppressed by XAV 939 (Fig. 4b). These results demonstrate that the Wnt/β-catenin pathway is activated in U87 cells by irradiation.
Irradiation promotes β-catenin/TCF transcriptional activities and up-regulates β-catenin target genes in U87 cells. a Luciferase reporter assays in U87 cells. Prior to irradiation, U87 cells were treated with or without the Wnt/β-catenin inhibitor XAV 939 as indicated. Data represent the relative TopFlash luciferase activity (means ± standard error) of three independent experiments, indicating the transcriptional activity of the β-catenin/TCF complex. *p < 0.05, t test, compared with non-irradiated cells; # p < 0.05, t test, presence vs. absence of XAV 939. b Quantitative PCR analysis of β-catenin target genes. Non-irradiated U87 cells and cells at 12 and 24 h post-irradiation (with or without XAV 939 treatment) were harvested in triplicate for quantitative PCR analysis. Data are expressed as the mean fold change of the mRNA level relative to non-irradiated U87 cells without XAV 939 treatment and the standard error of the mean; n = 3, *p < 0.05, t test, compared with non-irradiated cells; # p < 0.05, t test, presence vs. absence of XAV 939
IR alters MMP expression and activity
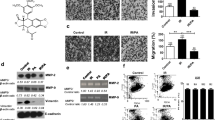
Among β-catenin target genes, MMP-2 and MMP-9 have been reported to be major contributors to GBM invasion. Consistent with the mRNA levels, the results of Western blotting showed that the protein levels of MMP-2 and MMP-9 were increased significantly after 3Gy irradiation (Fig. 5a). To determine whether the activities of MMP-2 and MMP-9 were also elevated by irradiation, we employed gelatine zymography and densitometric analysis. As demonstrated in Fig. 5b, samples from irradiated cells had brighter bands than the control cells at both 72 kDa and 92 kDa, and densitometric analysis showed statistical significance. Importantly, XAV 939 treatment suppressed the up-regulation of MMP-2 and MMP-9 induced by IR and inhibited the activities of these gelatinases.
Ionizing radiation increases MMP-2 and MMP-9 expression and activity. a Immunoblot analysis of MMP-2 and MMP-9. α-Tubulin served as a loading control. b. Zymography of gelatinases. Unstained bright bands indicate gelatine digestion by MMPs at their respective molecular weights. Data are expressed as the mean and standard error of the mean; n = 3, *p < 0.05, t test, compared with non-irradiated cells; # p < 0.05, t test, presence vs. absence of XAV 939
Discussion
Wnt/β-catenin signalling plays important roles in maintaining the stemness of cancer stem cells in various types of cancer [14–17] and is considered to be an important regulator that promotes cellular invasiveness through regulation of epithelial–mesenchymal transition (EMT) in many neoplasms [18]. More importantly, accumulated evidence suggests that Wnt/β-catenin signalling contributes to the acquisition of radioresistance and the development of an invasive phenotype [19–21]. In the context of radiation-induced EMT, there is crosstalk between the Wnt/β-catenin pathway and several other pathways, which has been demonstrated to be involved in IR-induced cell invasion [11, 22–24]. For example, epidermal growth factor (EGF)/EGF receptor signalling activates extracellular signal-regulated kinases 1/2 and casein kinase-2 in glioma cells, resulting in phosphorylation of α-catenin at serine 641, which correlates with glioma malignancy. Interestingly, α-catenin phosphorylation promotes β-catenin transactivation and glioma cell invasion [25]. Furthermore, the phosphoinositide 3-kinase/Akt signalling pathway stabilizes β-catenin by blocking the activation of glycogen synthase kinase 3 (GSK3) [26].
To examine the activation of the Wnt/β-catenin pathway by irradiation, we evaluated intracellular translocation of β-catenin, which is regarded as a characteristic event of Wnt/β-catenin pathway activation. β-Catenin has dual functions by performing a crucial role in cell–cell adhesion and participating in the Wnt/Wingless signalling pathway [27], corresponding to its localization in the cell membrane and nucleus, respectively. Immunofluorescence showed that the majority of β-catenin was present in the cell–cell junctions of non-irradiated cells with very little β-catenin in cytoplasmic or nuclear fractions because of the rapid turnover of β-catenin promoted by the APC/GSK3β/axin complex. However, GSK3β activity is inactivated in Wnt canonical signalling, leading to translocation of β-catenin from the cytoplasm to the nucleus and accumulation of nuclear β-catenin, which was observed in irradiated U87 cells.
Next, we employed a dual luciferase (TOF/HOP) reporter system and quantitative PCR to evaluate whether IR-induced nuclear accumulation of β-catenin would result in activation of typical β-catenin target genes. In the absence of Wnt signals, TCF/LEF acts as a repressor of β-catenin target genes in a complex with Groucho. β-Catenin converts TCF/LEF into a transcriptional activator of the same genes that are repressed by TCF/LEF alone. Our results demonstrated that IR significantly increased β-catenin/TCF transcriptional activity in U87 cells, followed by up-regulation of downstream genes [19, 28–30] such as AXIN2, FZD 7, TCF 1, CCND1, CD44, VEGF, MMP-2, and MMP-9. MMPs, especially MMP-2 and MMP-9, are greatly involved in cell invasion and metastasis. Increased enzymatic degradation of extracellular matrix components by MMPs induces cell invasion and tumour spreading [31, 32]. As demonstrated in our study, IR increased the expression of gelatinases (MMP-2 and MMP-9) through activation of the Wnt/β-catenin pathway, resulting in enhancement of the invasive potential of U87 cells.
Considering the strong pro-invasive effects of canonical Wnt signalling upon IR, β-catenin might be a potential target to abrogate the unwanted pro-invasive effects of irradiation. Therefore, we investigated the Wnt/β-catenin pathway using a specific pathway inhibitor. XAV 939 is a potent inhibitor of tankyrases. Tankyrases 1 and 2 stimulate proteasomal degradation of axin, thereby inhibiting the degradation of β-catenin. Thus, pharmacological inhibition of both tankyrases by XAV 939 stabilizes the β-catenin destruction complex, decreases the levels of β-catenin, and selectively and efficiently inhibits canonical Wnt signalling [33]. In our study, application of XAV 939 to irradiated U87 cells promoted the degradation of β-catenin, inhibited activation of the Wnt/β-catenin pathway induced by IR, decreased the expression levels and activities of MMP-2 and MMP-9, and resulted in abrogation of radiation-induced cell invasion. These in vitro results suggest the feasibility of XAV 939 for use in GBM radiotherapy. Although some in vivo studies have demonstrated certain effective administration approaches, such as targeted injection in the spinal cord [34] and nanoparticle constructs to deliver XAV 939 [35], there are still a few technical challenges for the in vivo application of XAV 939. Therefore, further study of systemic administration is needed to determine any possible toxicity and the efficacy of XAV 939 in the treatment of neurological conditions.
Conclusion
Our results have demonstrated a pivotal role of the Wnt/β-catenin signalling pathway in IR-induced invasion of U87 GBM cells, indicating that β-catenin is a potential therapeutic target for overcoming evasive radioresistance. Because targeted inhibition of canonical Wnt signalling has long been complicated by the lack of pathway components that are amenable to pharmacological inhibition [36], the availability of XAV 939 as a potent and selective inhibitor may assist drug discovery and provide a radiosensitizer for more effective radiotherapy of GBM. Thus, further in vivo experiments and pre-clinical trials are warranted.
References
Gralow J, Ozols RF, Bajorin DF et al (2008) Clinical cancer advances 2007: major research advances in cancer treatment, prevention, and screening–a report from the American society of clinical oncology. J Clin Oncol 26:313–325
Stupp R, Mason WP, van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996
Gerstein J, Franz K, Steinbach JP et al (2010) Postoperative radiotherapy and concomitant temozolomide for elderly patients with glioblastoma. Radiother Oncol 97:382–386
Gladstone M, Su TT (2012) Radiation responses and resistance. Int Rev Cell Mol Biol 299:235–253
Cheng JC, Chou CH, Kuo ML et al (2006) Radiation-enhanced hepatocellular carcinoma cell invasion with MMP-9 expression through PI3K/Akt/NF-kappaB signal transduction pathway. Oncogene 25:7009–7018
De Bacco F, Luraghi P, Medico E et al (2011) Induction of MET by ionizing radiation and its role in radioresistance and invasive growth of cancer. J Natl Cancer Inst 103:645–661
Madani I, De Neve W, Mareel M (2008) Does ionizing radiation stimulate cancer invasion and metastasis? Bull Cancer 95:292–300
Fujita M, Otsuka Y, Yamada S et al (2011) X-ray irradiation and Rho-kinase inhibitor additively induce invasiveness of the cells of the pancreatic cancer line, MIAPaCa-2, which exhibits mesenchymal and amoeboid motility. Cancer Sci 102:792–798
Wild-Bode C, Weller M, Rimner A et al (2001) Sublethal irradiation promotes migration and invasiveness of glioma cells: implications for radiotherapy of human glioblastoma. Cancer Res 61:2744–2750
Gliemroth J, Feyerabend T, Gerlach C et al (2003) Proliferation, migration, and invasion of human glioma cells exposed to fractionated radiotherapy in vitro. Neurosurg Rev 26:198–205
Zhai GG, Malhotra R, Delaney M et al (2006) Radiation enhances the invasive potential of primary glioblastoma cells via activation of the Rho signaling pathway. J Neurooncol 76:227–237
Moncharmont C, Levy A, Guy JB et al (2014) Radiation-enhanced cell migration/invasion process: a review. Crit Rev Oncol Hematol 92:133–142
Scrace S, O’Neill E, Hammond EM et al (2013) Use of the xCELLigence system for real-time analysis of changes in cellular motility and adhesion in physiological conditions. Methods Mol Biol 1046:295–306
Teng Y, Wang X, Wang Y et al (2010) Wnt/beta-catenin signaling regulates cancer stem cells in lung cancer A549 cells. Biochem Biophys Res Commun 392:373–379
Vermeulen L, De Sousa EMF, van der Heijden M et al (2010) Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol 12:468–476
King TD, Suto MJ, Li Y (2012) The Wnt/beta-catenin signaling pathway: a potential therapeutic target in the treatment of triple negative breast cancer. J Cell Biochem 113:13–18
Yamashita T, Ji J, Budhu A et al (2009) EpCAM-positive hepatocellular carcinoma cells are tumor-initiating cells with stem/progenitor cell features. Gastroenterology 136:1012–1024
Pala A, Karpel-Massler G, Kast RE et al (2012) Epidermal to mesenchymal transition and failure of EGFR-targeted therapy in glioblastoma. Cancers (Basel) 4:523–530
Kim Y, Kim KH, Lee J et al (2012) Wnt activation is implicated in glioblastoma radioresistance. Lab Invest 92:466–473
Jin X, Jeon HY, Joo KM et al (2011) Frizzled 4 regulates stemness and invasiveness of migrating glioma cells established by serial intracranial transplantation. Cancer Res 71:3066–3075
Zheng H, Ying H, Wiedemeyer R et al (2010) PLAGL2 regulates Wnt signaling to impede differentiation in neural stem cells and gliomas. Cancer Cell 17:497–509
Kil WJ, Tofilon PJ, Camphausen K (2012) Post-radiation increase in VEGF enhances glioma cell motility in vitro. Radiat Oncol 7:25
Timke C, Zieher H, Roth A et al (2008) Combination of vascular endothelial growth factor receptor/platelet-derived growth factor receptor inhibition markedly improves radiation tumor therapy. Clin Cancer Res 14:2210–2219
Park CM, Park MJ, Kwak HJ et al (2006) Ionizing radiation enhances matrix metalloproteinase-2 secretion and invasion of glioma cells through Src/epidermal growth factor receptor-mediated p38/Akt and phosphatidylinositol 3-kinase/Akt signaling pathways. Cancer Res 66:8511–8519
Ji H, Wang J, Nika H et al (2009) EGF-induced ERK activation promotes CK2-mediated disassociation of alpha-Catenin from beta-Catenin and transactivation of beta-Catenin. Mol Cell 36:547–559
Zhou BP, Deng J, Xia W et al (2004) Dual regulation of Snail by GSK-3beta-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat Cell Biol 6:931–940
Luu H, Zhang R, Haydon R et al (2004) Wnt/β-catenin signaling pathway as novel cancer drug targets. Current Cancer Drug Targets 4:653–671
Wu B, Crampton SP, Hughes CC (2007) Wnt signaling induces matrix metalloproteinase expression and regulates T cell transmigration. Immunity 26:227–239
King TD, Zhang W, Suto MJ et al (2012) Frizzled7 as an emerging target for cancer therapy. Cell Signal 24:846–851
MacDonald BT, Tamai K, He X (2009) Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell 17:9–26
Wick W, Platten M, Weller M (2001) Glioma cell invasion: regulation of metalloproteinase activity by TGF-beta. J Neurooncol 53:177–185
Platten M, Wick W, Weller M (2001) Malignant glioma biology: role for TGF-beta in growth, motility, angiogenesis, and immune escape. Microsc Res Tech 52:401–410
Huang SM, Mishina YM, Liu S, et al (2009) Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature 461:614–620
Fancy SP, Harrington EP, Yuen TJ et al (2011) Axin2 as regulatory and therapeutic target in newborn brain injury and remyelination. Nat Neurosci 14:1009–1016
Zhao JW, Dyson SC, Kriegel C, et al (2014) Modelling of a targeted nanotherapeutic ‘stromaʼ to deliver the cytokine LIF, or XAV939, a potent inhibitor of Wnt-beta-catenin signalling, for use in human fetal dopaminergic grafts in Parkinsonʼs disease. Dis Model Mech 7:1193–1203
Barker N, Clevers H (2006) Mining the Wnt pathway for cancer therapeutics. Nat Rev Drug Discov 5:997–1014
Acknowledgements
This research was in part supported by a grant from the National Natural Sciences Foundation of China (No. 81272780). We gratefully thank Dr. Fengjuan Fan and Dr. Anna-Lena Scherr, Centrum für Tumorerkrankungen, Heidelberg, Germany, for help with the manuscript. Authorsʼ contributions: Z.D. cultured the U87 cells and conducted RTCA and matrigel invasion assay, participated in real-time quantitative PCR and drafted the manuscript. L.Z. carried out the immunofluorescence staining and colocalization analysis. N.H. participated in the zymography and immunoblotting. M.Z. participated in the design of the study and performed the statistical analysis. X.L. conceived of the study, and participated in its design and coordination and prepared the final manuscript. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Z. Dong, L. Zhou, N. Han, M. Zhang, and X. Lyu state that there are no conflicts of interest. The accompanying manuscript does not include studies on humans or animals.
Rights and permissions
About this article
Cite this article
Dong, Z., Zhou, L., Han, N. et al. Wnt/β-catenin pathway involvement in ionizing radiation-induced invasion of U87 glioblastoma cells. Strahlenther Onkol 191, 672–680 (2015). https://doi.org/10.1007/s00066-015-0858-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-015-0858-7