Abstract
Introduction
In vivo dosimetry is desirable for the verification, recording, and eventual correction of treatment in intraoperative electron radiotherapy (IOERT). Our aim is to share our experience of metal oxide semiconductor field–effect transistors (MOSFETs) and radiochromic films with patients undergoing IOERT using a general-purpose linac.
Materials and methods
We used MOSFETs inserted into sterile bronchus catheters and radiochromic films that were cut, digitized, and sterilized by means of gas plasma. In all, 59 measurements were taken from 27 patients involving 15 primary tumors (seven breast and eight non-breast tumors) and 12 relapses. Data were subjected to an outliers’ analysis and classified according to their compatibility with the relevant doses. Associations were sought regarding the type of detector, breast and non-breast irradiation, and the radiation oncologist’s assessment of the difficulty of detector placement. At the same time, 19 measurements were carried out at the tumor bed with both detectors.
Results
MOSFET measurements (\(\overline{D}\) = 93.5 %, sD = 6.5 %) were not significantly shifted from film measurements (\(\overline{D}\) = 96.0 %, sD = 5.5 %; p = 0.109), and no associations were found (p = 0.526, p = 0.295, and p = 0.501, respectively). As regards measurements performed at the tumor bed with both detectors, MOSFET measurements (\(\overline{D}\) = 95.0 %, sD = 5.4 % were not significantly shifted from film measurements (\(\overline{D}\) = 96.4 %, sD = 5.0 %; p = 0.363).
Conclusion
In vivo dosimetry can produce satisfactory results at every studied location with a general-purpose linac. Detector choice should depend on user factors, not on the detector performance itself. Surgical team collaboration is crucial to success.
Zusammenfassung
Hintergrund und Ziel
Die In-vivo-Dosimetrie ist wünschenswert für die Überprüfung, Registrierung und die eventuelle Korrektur der Behandlungen in der IOERT („Intraoperative Electron Radiation Therapy“). Unser Ziel ist die Veröffentlichung unserer Erfahrungen beim Gebrauch von MOSFETs und Radiochromfilmen bei den Patienten, die sich einer IOERT-Behandlung mit einem Allzweck-Linac unterzogen.
Material und Methoden
Es wurden in sterile Bronchialkatheter eingeführte MOSFETs sowie zerschnittene und digitalisierte Radiochromfilme, die durch Gasplasma sterilisiert wurden, verwendet. Insgesamt wurden 59 Messungen bei 27 Patienten durchgeführt. Dazu zählten 15 Primärtumore (7 Brust- und 8 anderweitige Tumore) und 12 erneut aufgetretene Tumore. Die Daten wurden einer Analyse mit atypischen Werten unterzogen und entsprechend ihrer Kompatibilität mit den relevanten Dosen klassifiziert. Gesucht wurden Assoziationen bezüglich der Art des Detektors, Brust- und Nichtbrustbestrahlung und der Bewertung durch den Radioonkologen hinsichtlich des Schwierigkeitsgrads der Detektorplatzierung. Außerdem wurden 19 Messungen im Tumorbett mit beiden Detektoren durchgeführt.
Ergebnisse
Es ergaben sich keine bedeutenden Unterschiede bei den Messergebnissen mit MOSFET (\(\overline{D}\) = 93,5 %, sD = 6,5 %) und den Messergebnissen mit Radiochromfilmen (\(\overline{D}\) = 96,0 %, sD = 5,5 %; p = 0,109). Assoziationen wurden nicht gefunden (p = 0,526; p = 0,295; p = 0,501). Auch die im Tumorbett durchgeführten Messungen mit den beiden Detektoren ergaben, dass die Messergebnisse mit MOSFET (\(\overline{D}\) = 95,0 %, sD = 5,4 %) nicht wesentlich von den Messergebnissen mit Radiochromfilmen abwichen (\(\overline{D}\) = 96,4 %, sD = 5,0 %; p = 0,363).
Schlussfolgerung
Die In-vivo-Dosimetrie mit einem Allzweck-Linac kann an jeder untersuchten Stelle zu zufriedenstellenden Ergebnissen führen. Ausschlaggebend für die Wahl des Detektors sollten Benutzerfaktoren und nicht das Verhalten des Detektors selbst sein. Die Zusammenarbeit mit dem chirurgischen Team ist entscheidend für den Erfolg.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Intraoperative electron radiotherapy (IOERT) is a highly selective radiotherapy technique aimed at restricted anatomic volumes during surgical oncology treatment. It consists of single-fraction irradiation with a high delivered absorbed dose after direct visual examination of the tumor bed by means of an electron beam [1]. Currently, it is being revisited and reported on intensively [2–4].
In vivo dosimetry is desirable for the verification, recording, and eventual correction of treatment and is advised to be performed [5]. Detectors based on metal oxide semiconductor field–effect transistors (MOSFETs) [6–9] and radiochromic films [10, 11] have been investigated for these purposes. MOSFETs can be used as dosimeters when appropriately operated [12–14]. Radiochromic films are made of materials that obtain color upon irradiation and thus it is possible to use pieces of film as a detector when properly calibrated [15, 16].
The literature has focused on IOERT performed with mobile linacs. With respect to MOSFET studies, Consorti et al. [6] used a NOVAC7 mobile linac and measured the absorbed doses delivered to the tumor bed of 19 patients undergoing definitive treatment or boost treatment of the breast and radiotherapy for the pancreas. The differences between intended and measured absorbed doses ranged from − 6.9 to 11.6 %.
Ciocca et al. [7] performed entrance absorbed dose measurements on 45 breast cancer patients irradiated with NOVAC7 and LIAC mobile linacs. The differences between intended and measured absorbed doses ranged from − 7.6 to 10 %.
Soriani et al. [8] also used a NOVAC7 mobile linac. The difference in the delivered absorbed dose to the target of 12 prostate cancer patients ranged between − 10.7 % and 10.5 % with respect to the intended maximum absorbed dose.
Agostinelli et al. [9] reported measurements performed on 91 patients suffering from breast cancer and treated with a mobile linac LIAC. Their substantially spread data show the difficulty of matching the measured and prescribed absorbed dose, which made the authors develop an optimization tool to better calculate the theoretical absorbed dose delivered to the MOSFET.
With regard to film experiences, Ciocca et al. [10] included 35 patients affected by breast carcinoma and treated with a NOVAC7 mobile linac. Deviation between the measured and expected absorbed dose ranged from − 9.9 to 9.9 %.
Krengli et al. [11] presented a study of 38 patients with locally advanced prostate cancer irradiated with a Mobetron mobile linac. Film dosimetry focused on measuring absorbed dose to the rectum.
Here, our aim was to share our experience in in vivo dosimetry with both MOSFETs and radiochromic films with patients undergoing IOERT using a general-purpose linac. We report the intrinsic precision of both kinds of dosimeters, their suitability for regular treatment, and the robustness of measurements concerning a breast treatment or other sites and detector positioning difficulties assessed during the treatment.
Patients and methods
Inclusion and exclusion criteria
In principle, every case and all locations were included. Despite the difficulties caused by bleeding surgical beds, deep sites, difficult access, or inclined surfaces, no measurement was excluded until it was subjected to outlier statistical analysis.
Dosimeters
Reinforced TN-502RDM-H mobile MOSFETs (Best Medical Canada Ltd., Ontario, Canada) and Gafchromic MD-55−2 film (International Specialty Products, N.J.) were used. The absorbed doses utilized in the calibration procedure were accurately measured by means of routine dosimetric equipment following the IAEA TRS-398 protocol [17] immediately before irradiation. An Elekta Precise linac (Elekta AB, Stockholm, Sweden) was used both for calibrations and treatments.
Uncertainty assessments
Dosimetric uncertainties were considered, assessed, and added as indicated by metrology reports [18]. Three main sources of uncertainty were identified.
Uncertainty associated with absorbed dose measurements in water were estimated using the IAEA TRS-398 protocol [17].
Nonlinearity of the response of linac monitor chambers was also considered. We plotted the readout of the monitor chambers against the calibrated reference chamber.
Finally, a major source of uncertainty is that associated with detector calibration and dosimeter response. Both kinds of detectors are affected by the use of plastic water instead of water in the calibration procedure, and the associated uncertainty was considered. In the case of MOSFETs, we included uncertainty of calibration factors, their resolution, and the reproducibility of measurements. In the case of films, the uncertainty associated with the fitting of the calibration polynomial and reproducibility was added.
Surgical procedure and dosimeter handling
MOSFETs were inserted into sterile bronchus catheters. Catheters were attached to the surgical bed (or tumor in nonremovable cases) to avoid their movement. After the readout they were cleaned and stored to be reused until their exhaustion.
Pieces of 1.5 × 1.5-cm film were digitized, packed between two 2 × 2-cm pieces of transparent polyester film, and sterilized by means of gas plasma. After the irradiation, enveloping materials were removed and the film was sterilized again to ensure safe processing. Film reading led to a red channel net mean pixel value calculated inside an approximately 1 × 1-cm centered region of interest (ROI). Pixel readout variation within this region was controlled to not be greater than the total film uncertainty.
Clinical measurements
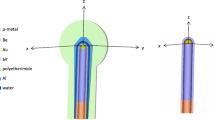
In all, 59 measurements were taken on 27 patients. Of these, 30 measurements were performed with MOSFETs and 29 with films. We carried out 19 measurements at the tumor bed with both detectors, one next to the other, to be representative of the absorbed dose delivered by the central region of the electron field to the same bed plane. We assumed that, even with a small applicator, low energy, and maximum bevel angle, lateral differences in absorbed dose can be neglected in comparison with the high gradient caused by depth. In Fig. 1 we present isodoses in water with the minimum applicator (6 cm) combined with the maximum bevel angle (45°) and the minimum energy (9 MeV) used in this study. Our intention was to verify the prescribed absorbed dose by detecting the upward region of the beam depth dose. Full contact of the applicator edge to the tumor bed was always achieved, and thus only tumor bed relief could alter a uniform irradiation, except in two cases in which lead protection was used (discussed in the next section).
Isodoses in water corresponding to the electron beam irradiated with minimum applicator size (6 cm) combined with larger bevel angle (45°) and minimum energy (9 MeV) used in our study. The represented plane is defined by depth and the lateral dimension along which the bevel acts. The legend on the right contains represented isodoses expressed in percentage. A rather flat central area could be seen to allocate dosimeters
Treatment locations involved 15 primary tumors: four rectal tumors, seven breast tumors, two soft tissue sarcomas (one abdominal and one scapular), one lower limb skin tumor, one nerve tumor, and 12 relapses (four presacral, four nodal, one pancreatic, one intestinal, one inguinal, and one retroperitoneal). Table 1 summarizes the irradiation parameters utilized.
Relevant doses—that is, the prescribed dose, the expected maximum dose, and the expected surface dose—were gathered for comparison with the measured dose.
Statistical analysis
First, the absorbed doses measured by the dosimeters were normalized considering the corresponding expected maximum absorbed doses to be 100 %. The expected absorbed doses had been determined by means of routine scanning of beams with an Electron Field Diode (IBA Dosimetry GmbH, Schwarzenbruck, Germany) when commissioning the linac for IOERT. This diode of 2-mm active width has been reported to have little energy dependence and almost no field dependence [19]. Once these D i values were obtained, we calculated their z-scores [20], defined as:
\({{z}_{i}}=\frac{{{D}_{i}}-\overline{D}}{{{s}_{D}}}\) (1)
where \(\overline{D}\) and s D were the mean and the standard deviation of the sample, in order to detect the presence of outliers and remove them. We used the Wilcoxon rank sum test to test whether the absorbed dose distribution of data derived from the MOSFETs was shifted with respect to the distribution from films, without assuming any in particular.
Second, a threshold of compatibility for the included measurements was fixed. If a measurement fell into the band formed by the expected surface dose minus the uncertainty of this measurement considering k = 1 (with k the coverage factor as stated by the cited reports [18]) and the expected maximum dose plus this uncertainty, it was considered as compatible. A measurement was considered as incompatible if it could not reach this band. Compatible and incompatible measurements were represented in three contingency tables against the following variables: type of detector, breast and non-breast irradiation, and radiation oncologist (RO) assessment of the difficulty of detector placement. Fisher’s exact tests were performed to reveal associations.
Lastly, we calculated the descriptive statistics of the samples taken at the same plane and assessed whether the data distribution from the MOSFETs was shifted with respect to the distribution originating from films.
Results
Assessment of uncertainties
The uncertainty associated with absorbed dose measurements in water measured by means of a chamber calibrated with Co-60 like ours is 2.1 % (k = 2). Regarding the non-linearity of linac monitor chambers, we first had to consider that the maximum deliverable fraction of a treatment under clinical operation of the treatment unit was 1,000 MU, with an estimated uncertainty of 0.034 Gy (k = 2). Therefore, treatments of 2,000 MU, 3,000 MU, and 4,000 MU are n sums of the maximum delivered fraction with uncertainties of this kind of \(\sqrt{n}\ 0.034\), that is, 0.048 Gy, 0.059 Gy, and 0.068 Gy (k = 2), respectively. When adding uncertainty associated with detector calibration and dosimeter response, the total relative uncertainty of the MOSFETs was about 2.2 % (k = 1) and the total relative uncertainty of the film was about 2.8 % (k = 1) during the treatments. In one film, pixel variation was greater than this quantity. Since this measurement was not able to predict the delivered absorbed dose after the next analysis, it was not considered as correct for further tests.
Data analysis
The z-scores of the measured absorbed doses guided us in the understanding of anomalous measurements. With MOSFETs we obtained a z-score equal to − 3.9, corresponding to an underdosage caused because the detector unintentionally moved next to the edge of the applicator; − 1.8 corresponding to a detector placed right on the lung protection in a breast cancer treatment, probably irradiated by suboptimal energy; and 1.0 and 1.4 indicating overdosages caused by backscatter near lead protection in two rectal cancer treatments. Another measurement with z equal to − 2.2 was investigated in depth but no plausible explanation was found. With respect to film, one measurement had z equal to −2.1 corresponding to a piece that lay partially in air because of tumor bed relief; and − 1.7 for a piece not perpendicular to the applicator. Data with z equal to − 2.7 could also not be explained. Thus, these data were removed because they do not represent the correct manner of tumor bed irradiation. Finally, the data analyzed further were from 51 measurements in 36 sites involving all of the 27 patients, of which we could assess 15 pairs of measurements performed at the same plane. Descriptive statistics are summarized in Table 2 and histograms are depicted in Fig. 2.
MOSFET measurements were not significantly shifted from film measurements when subjected to the Wilcoxon rank sum test (p = 0.109; two-sided).
Compatibility and incompatibility of measurements
The compatibility and incompatibility of measurement results are summarized in Table 3. We investigated associations between compatibility or incompatibility and the type of detector, the situation in which a breast was treated versus a different localization, and the RO impression of the difficulty of detector placement. The associations were not statistically significant as shown by the p values obtained from Fisher’s exact tests (0.526, 0.295, and 0.501, respectively; one-sided).
Tumor bed absorbed doses
Table 4 presents descriptive statistics regarding the measurements performed at the tumor bed with both detectors. The two distributions (Fig. 3) did not appear as significantly shifted when subjected to the Wilcoxon signed ranks test (p = 0.363; two-sided).
Discussion
Due to the features of a single electron irradiation, as depicted in Fig. 1, verification of correct irradiation is possible by measuring the absorbed dose between the surface absorbed dose and the maximum, whereas an accurate measurement could be awkward because of uncertainties and current technological limitations. Although a wide set of localizations were managed, thus complicating the overall results, very close central values were obtained with both detectors for the whole set of data. These values lie between the band formed by the prescribed dose and the intended maximum dose, and therefore this should be interpreted as the successful delivery of the overall treatments. The maximum range of detected deviations (28.6 % for MOSFETs and 21.1 % for films) is greater than the ranges reported by other studies in breast cancer treatment [6,7,10]. Here, the increased difficulty of irradiating a broader set of localizations involving bleeding tumor beds and zones less accessible to the detectors must be considered.
Nevertheless, with the presented data, it was not possible to show a significantly different performance of in vivo dosimetry versus the variables studied. Thus, we could expect the same degree of treatment success treatment for any localization.
In the case of the films, the process of sterilization does not seem to impede the use of this kind of detector in in vivo dosimetry. A variation of 1.5 % in the response has been reported in films irradiated with 20 Gy and sterilized twice, but we did not consider it because it was not statistically significant [21].
With respect to the uncertainties of the dosimeters, they are comparable with those that can be found in the literature [5, 6, 9] and not greater. The standard deviation of the measured absorbed doses is about two or three times the detector uncertainty, but this is not a pitfall from the point of view of the authors. To the contrary, it indicates the need for planning or imaging tools [9, 22, 23] to determine the in vivo tumor bed anatomy and localize the detectors more accurately.
The ability of MOSFETs to record the absorbed dose right after treatment delivery makes it more attractive to the user. Its associated uncertainty is less than that of film. However, in our opinion, MOSFETs are presumably more difficult to handle by the surgical team at the beginning as they have to be sheathed with sterile components and attached onto the bed while avoiding twisting of the wire and detachment when the applicator comes into contact with the ROI. Special care has to be taken with eventual rotations of the detector as deduced from angular response studies [6, 7, 24]. The commitment of the surgical team is very important for management of these issues.
Further research would lead to setting a rationale to control radiation delivery on-line, check positioning and alignment of the mobile elements of the treatment, and use an action level to detect discrepancies and compensate for eventual misirradiations.
Conclusions
It is feasible to perform in vivo dosimetry with a general-purpose linac, yielding satisfactory results in every localization studied despite variability. Concordance between the relevant doses and data distributions from MOSFETs and radiochromic films demonstrates that detector choice should depend on user factors such as available budget, detector set-up training, and need for immediate reporting, not on the detector performance itself. The collaboration of the surgical team is crucial for successful program completion.
References
Gunderson LL, Willett CG, Harrison LB, Petersen IA, Haddock MG (1997) Intraoperative irradiation: current and future status. Semin Oncol 24(6):715–731
Calvo FA, Sole CV, Martinez-Monge R, Azinovic I, Aristu J, Zudaire J, Garcia-Sabrido JL, Berian JM (2013) Intraoperative EBRT and resection for renal cell carcinoma: twenty-year outcomes. Strahlenther Onkol 189(2):129–136. doi: 10.1007/s00066-012-0272-3. Epub 2012 Dec 9
Klaver YL, Lemmens VE, Nienhuijs SW, Nieuwenhuijzen GA, Rutten HJ, de Hingh IH (2013) Intraoperative radiotherapy and cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Five consecutive case reports of locally advanced rectal cancer with synchronous peritoneal carcinomatosis. Strahlenther Onkol 189(3):256–260. doi:10.1007/s00066-012-0282-1. Epub 2013 Jan 19
Krengli M, Calvo FA, Sedlmayer F, Sole CV, Fastner G, Alessandro M, Maluta S, Corvò R, Sperk E, Litoborski M, Pisani C, Fillini C, Fusconi F, Osti MF, Tomio L, Marsiglia H, Ciabattoni A, Polkowski W, Di Grazia A, Gava A, Kuten A, Iotti C, Gonzalez C, Sallabanda M, Dubois JB, Catalano G, Valentini V (2013) Clinical and technical characteristics of intraoperative radiotherapy. Analysis of the ISIORT-Europe database. Strahlenther Onkol 189(9):729–737. doi:10.1007/s00066-013-0395-1. Epub 2013 Jul 12
Rosi A, Viti V (ed) (2003) Guidelines for quality assurance in intra-operative radiation therapy. Report ISTISAN 03/1 EN.0. Istituto Superiore di Sanità, Rome. ISSN 1123-3117
Consorti R, Petrucci A, Fortunato F, Soriani A, Marzi S, Iaccarino G, Landoni V, Benassi M (2005) In vivo dosimetry with MOSFETs: dosimetric characterization and first clinical results in intraoperative radiotherapy. Int J Radiat Oncol Biol Phys 63(3):952–960
Ciocca M, Piazzi V, Lazzari R, Vavassori A, Luini A, Veronesi P, Galimberti V, Intra M, Guido A, Tosi G, Veronesi U, Orecchia R (2006) Real-time in vivo dosimetry using micro-MOSFET detectors during intraoperative electron beam radiation therapy in early-stage breast cancer. Radiother Oncol 78(2):213–216. Epub 2005 Dec 15
Soriani A, Landoni V, Marzi S, Iaccarino G, Saracino B, Arcangeli G, Benassi M (2007) Setup verification and in vivo dosimetry during intraoperative radiation therapy (IORT) for prostate cancer. Med Phys 34(8):3205–3210
Agostinelli S, Gusinu M, Cavagnetto F, Garelli S, Zeverino M, Guenzi M, Corvò R, Taccini G (2012) On-line optimization of intraoperative electron beam radiotherapy of the breast. Radiother Oncol 103(2):188–92. doi:10.1016/j.radonc.2012.01.009. Epub 2012 Feb 17
Ciocca M, Orecchia R, Garibaldi C, Rondi E, Luini A, Gatti G, Intra M, Veronesi P, Lazzari R, Tosi G, Veronesi U (2003) In vivo dosimetry using radiochromic films during intraoperative electron beam radiation therapy in early-stage breast cancer. Radiother Oncol 69(3):285–289
Krengli M, Terrone C, Ballarè A, Loi G, Tarabuzzi R, Marchioro G, Beldì D, Mones E, Bolchini C, Volpe A, Frea B (2010) Intraoperative radiotherapy during radical prostatectomy for locally advanced prostate cancer: technical and dosimetric aspects. Int J Radiat Oncol Biol Phys 76(4):1073–1077. doi:10.1016/j.ijrobp.2009.03.037. Epub 2009 Jul 20
Ramani R, Russell S, O’Brien P (1997) Clinical dosimetry using MOSFETs. Int J Radiat Oncol Biol Phys 37(4):959–964
Scalchi P, Francescon P (1998) Calibration of a mosfet detection system for 6-MV in vivo dosimetry. Int J Radiat Oncol Biol Phys 40(4):987–993
Cygler JE, Scalchi P (2009) MOSFET dosimetry in Radiotherapy. In: Rogers DWO, Cygler JE (ed) Clinical dosimetry measurements in radiotherapy. Medical Physics, Madison, p 960. ISBN 978-1-888340-84-6
Niroomand-Rad A, Blackwell CR, Coursey BM, Gall KP, Galvin JM, McLaughlin WL, Meigooni AS, Nath R, Rodgers JE, Soares CG (1998) Radiochromic film dosimetry: recommendations of AAPM Radiation Therapy Committee Task Group 55. American Association of Physicists. Medicine Med Phys 25(11):2093–2115
Butson MJ, Yu PKN, Cheung T, Metcalfe P (2003) Radiochromic film for medical radiation dosimetry. Mater Sci Eng: R 41(3–5):61–120
Andreo P, Burns DT, Hohlfeld K, Huq MS, Kanai T, Laitano F, Smyth VG, Vynckier S, IAEA TRS-398 (2000) Absorbed dose determination in external beam radiotherapy: an international code of practice for dosimetry based on standards of absorbed dose to water. IAEA, Vienna. ISSN 1011-4289
BIPM. Evaluation of measurement data—guide to the expression of uncertainty in measurement. JCGM 100:2008
Wang LL, Rogers DW (2007) Monte Carlo study of Si diode response in electron beams. Med Phys 34(5):1734–1742
Mendenhall W, Sincich T (2005) Statistics for engineering and the sciences, 5th ed. Pearson Prentice Hall, New Jersey. ISBN 0-13-187706-2.
Conde Moreno A, Ruiz RJC, Bouché Babiloni A, Suárez DR, Hueso Bernad N, López Tarjuelo J, Ferrer Albiach C, Calvo FA (2009) Sterilization effect on MD-55—2 radiochromic film for in vivo dosimetry in electron intraoperative radiotherapy. Int J Radiat Oncol Biol Phys 75(3 Supplement):S621. doi:10.1016/j.ijrobp. 2009.07.1419
Pascau J, Santos Miranda JA, Calvo FA, Bouché A, Morillo V, González-San Segundo C, Ferrer C, López Tarjuelo J, Desco M (2012) An innovative tool for intraoperative electron beam radiotherapy simulation and planning: description and initial evaluation by radiation oncologists. Int J Radiat Oncol Biol Phys 83(2):e287–e295. doi:10.1016/j.ijrobp.2011.12.063. Epub 2012 Mar 6
Calvo FA, Sole CV, González ME, Tangco ED, López-Tarjuelo J, Koubychine I, Santos JA, Pascau J, Herranz R, Ferrer C (2013) Research opportunities in intraoperative radiation therapy: the next decade 2013–2023. Clin Transl Oncol 15:683–690
Bloemen-van Gurp EJ, Minken AW, Mijnheer BJ, Dehing-Oberye CJ, Lambin P (2006) Clinical implementation of MOSFET detectors for dosimetry in electron beams. Radiother Oncol 80(3):288–295. Epub 2006 Aug 17
Acknowledgments
Authors would like to thank Ms. Kavita Gandhi and Mrs. Sonja Behler for the linguistic assessment of this document in English and German, respectively, Mr. Juan Carlos Ruiz for the choice and the acceptance of the dosimetric equipment, Dr. Irene Torres for her documentation tasks and Prof. Dr. Felipe Calvo for his useful comments.
Compliance with ethical guidelines
All studies on humans described in the present manuscript were carried out with the approval of the responsible ethics committee and in accordance with national law and the Helsinki Declaration of 1975 (in its current, revised form). Informed consent was obtained from all patients included in studies.
Funding
This work has been supported by grant IPT-300000-2010-3. Spanish Government and ERDF funds.
Conflict of interest
J. López-Tarjuelo, A. Bouché-Babiloni, V. Morillo-Macías, N. de Marco-Blancas, A. Santos-Serra, J.D. Quirós-Higueras, and C. Ferrer-Albiach state that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
López-Tarjuelo, J., Bouché-Babiloni, A., Morillo-Macías, V. et al. In vivo dosimetry in intraoperative electron radiotherapy. Strahlenther Onkol 190, 1060–1065 (2014). https://doi.org/10.1007/s00066-014-0689-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-014-0689-y