Abstract
Purpose
Against the background of the increasing dilemma in the scientific community regarding protected versus unprotected carotid artery stent (CAS) placement and the disputed points in interpreting the results of scientific studiesas well as the difficulty in conducting such randomized controlled studies, this article gives a review of experiences with carotid stent placement without the use of protection devices.
Methods
This series comprised 133 consecutive patients with 136 carotid stenoses of which 128 carotid arteries (94%) were either symptomatic (93 out of 136 = 68.4%) or had a greater than 70% stenosis (35 out of 136 = 25.7%) and 8 out of 136 were asymptomatic and had stenoses between 50% and 70%. Patients underwent neurologic and sonographic evaluation before the procedure and during follow-up (mean 18 months).
Results
Primary stent placement was carried out in 110 out of 136 lesions and predilatation was necessary before stent deployment in 26 lesions,. Neurologic periprocedural complications included 3 disabling and 1 non-disabling strokes. During the follow-up period there were 6 deaths all unrelated to the carotid disease and no major strokes. The degree of stenosis decreased from a mean of 81% to a mean of 12.3% immediately after the procedure, 22 patients were defined as restenosis of which 9 were symptomatic.
Conclusions
Carotid stent placement without the use of distal protection devices was found to be a safe and effective procedure with a relatively low incidence of periprocedural complications.
Zusammenfassung
Zielsetzung
Vor dem Hintergrund des wachsenden Dilemmas der „scientific community“ bezüglich der geschützten oder ungeschützten Platzierung eines Karotis-Stent sowie der umstrittenen Aspekte bei der Interpretationen der Ergebnisse wissenschaftlicher Studien und auch der Probleme bei der Ausführung solcher Studien werden Erfahrungen mit Platzierungen von Karotis-Stents ohne Protektionssysteme präsentiert.
Methoden
Die Untersuchungsserie umfasste 133 konsekutive Patienten mit 136 Karotisstenosen, darunter 128 Stenosen (94 %), die entweder symptomatisch (93 von 136 = 68,4 %) waren oder eine Größe über 70 % hatten (35 von 136 = 25,7 %). Acht der 136 Stenosen waren asymptomatisch und lagen zwischen 50 und 70 %. Die Patienten wurden vor der Stent-Platzierung und während der Nachuntersuchung (im Median 18 Monate) neurologisch und sonografisch betreut.
Ergebnisse
Eine primäre Stent-Platzierung wurde bei 110 der 136 Stenosen durchgeführt. Bei den restlichen 26 Läsionen war vor der Stent-Platzierung eine Prädilatation notwendig. Während der Prozedur traten neurologische Komplikationen in Form von 3 schweren und einem leichten Schlaganfall auf. Während der „Follow-up“-Periode verstarben 6 Patienten; die Todesursachen waren unabhängig von der Karotiskrankheit, und es traten keine schweren Schlaganfälle auf. Der Stenosegrad verringerte sich von einem Median von 81 % vor der Platzierung zu 12,3 % unmittelbar nach dem Verfahren. Es wurden 22 Fälle als Restenose klassifiziert, und 9 dieser Patienten waren symptomfrei.
Schlussfolgerungen
Die Platzierung eines Karotis-Stent ohne Einsatz eines distalen Protektionssystems ist ein sicheres und effektives Verfahren mit einer relativ niedrigen Rate an periprozeduralen Komplikationen.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Carotid artery stent (CAS) placement has emerged as an alternative revascularization technique for extracranial carotid stenotic disease [1–3]. Nevertheless, one of the limitations of CAS is the potential for embolic stroke caused, for example, by plaque dislodgement of atheromatous material [4, 5]. To prevent embolic events a variety of cerebral protection devices (CPDs) has been developed in recent years andpreliminary results have shown that these devices can significantly reduce thromboembolic complications during CAS [4, 5]. However, concerns have been raised regarding these protection devices because they add further manipulation, costs and risks to the procedure [5–7]. It has been suggested that reducing the number of endovascular maneuvers in the supra-aortic vasculature can decrease the risk of plaque material dislodgment [6, 7]. Based on this information the role of CPDs in protecting the distal circulation could be questionable if the number of devices involved at the site of stenosis could be reduced. With the increasing trend to using CPDs as part of CAS and with the increasing probability for these devices to become routinely applied without achieving a high level of evidence and even after publication of the EVA-3S study some interventionalists do not see any medicolegal need to use protection devices or even express some doubts concerning the recommendation to stop unprotected CAS [8]. On the other hand it is difficult to imagine that a randomized controlled study of protected versus unprotected CAS will ever be conducted because in such a study too many patients will be needed to show a difference between the groups considering the reported low complication rates of the procedures. Therefore the need for building a cumulative data bank regarding unprotected CAS will remain the only and the honest source to compete with the flood of data coming from the routine use of protection devices which have been incorporated in most current clinical trials in spite of the fact that the benefit has not been proven in a prospective randomized trial. Therefore experiences with 136 consecutive carotid stent placement procedures without the use of CPDs are reported here.
Materials and Methods
Patient Population
Between June 2005 and June 2007, 133 consecutive patients with 136 carotid stenoses underwent stent placement and included 103 men and 30 women with a mean age of 70.7 years (range 42–88 years). The indications for treatment and exclusion criteria are summarized in Table 1. Of these patients 88 (66.1%) had comorbidities that placed them in the North American Symptomatic Carotid Endarterectomy Trial (NASCET) high-risk cohort of patients for endarterectomy ([9]; Table 2), 63 stenoses were located in the right internal carotid artery, 57 in the left internal carotid artery and there were 6 patients with bilateral stenoses. In another 3 patients the stenosis was in the right common carotid artery (CCA), while in another 3 the stenosis was in the left CCA.
Of the stenoses 93 (68.4%) were symptomatic with a median National Institutes of Health Stroke Scale (NIHSS) score of 12 (range 8–21). Clinically, non-disabling stroke was diagnosed in 54 patients, disabling stroke in 6 patients and transient ischemic attack in 33 patients. The remaining 43 stenoses (31.6%) were asymptomatic and the degree of stenosis was more than 70% in 35 (25.7%) and between 50% and 70% in 8 (5.8%) according to NASCET [9]).
Carotid Stenting Protocol
Preprocedural carotid ultrasonography was technically feasible in all subjects, so retrospectively and based on recent literature the plaque morphology was classified into 4 grades [10–12]. Cases with grades 1 and 2 were considered as high-risk plaque morphology, grade 3 was medium risk and grade 4 was low risk for stroke. All patients with a high-risk plaque as identified by reviewing the preprocedural carotid ultrasonography were treated according to the NASCET criteria. Biplane angiography (Advantix, GE Medical Systems, Milwaukee WI) was performed immediately before the endovascular intervention. The degree of stenosis before stent placement was quantified using the NASCET [9] criteria and ranged from 50% to 99%, with a mean of 81%. Before treatment a baseline cerebral computed tomography (CT) or magnetic resonance (MR) imaging was performed for all patients. All patients underwent neurological examination by a neurologist before and 24 h after the procedure with a preprocedural mean NIHSS score of 5.4 and 4.4 immediately after the procedure, with a significant improvement of the score related to the procedure (p = 0.002). Patients received oral aspirin (100 mg) and clopidogrel (75 mg) for at least 5 days before the procedure or alternatively a loading dose of 300 mg clopidogrel at least 4 h before carotid stent placement. Stent placement was performed under local anesthesia in all of the patients. At the beginning of the procedure patients received an intravenous (IV) bolus injection of heparin (5000–7500 IU) which was controlled by measuring the activating clotting time (ACT). A femoral approach was used in all but one of the cases (patient with bilateral lesions) which had an extremely tortuous aortic arch, and the carotid artery was sounded by a 5 F vertebral configuration catheter via a direct carotid approach.
A long exchange 0.035 inch stiff guidewire (Terumo, Somerest, NJ) was advanced into the external carotid artery and the 5 F selective catheter was then exchanged for a long 6 F or 8 F sheath (80 cm). Using road-mapping the stenotic lesion was crossed using a 0.014 inch microguidewire followed by advancement and deployment of an Acculink (Guidant/Abbot, Giessen, Germany) in 39 stenoses, a carotid wall stent (Boston Scientific, Natick MA) in 68 stenoses or a sinus-carotid conical-RX stent, (OptiMed, Ettlingen, Germany) in 30 stenoses of appropriate dimensions across the stenosis. In 26 patients (19.1%) the stenosis (tight stenosis) or severe calcifications (Fig. 1) could not be passed with the stent delivery system and predilatation with a 2 mm or 2.5 mm angioplasty balloon was necessary. After stent deployment a cerebral angiogram was obtained where postdilatation was indicated when homogenous stent expansion failed due to the presence of hard calcifications or there was an unsatisfactory degree of stent expansion due to residual stenosis of more than 25% or failure of reconstitution of an acceptable intacranial perfusion. Postdilation was performed in 130 lesions in this series. Finally, a cervical and cerebral angiogram was obtained with the same projections to compare changes of stenosis grade and changes in hemodynamics of cerebral perfusion. Hemostasis of the puncture site was achieved using a percutaneous closure device (Angio-Seal, St Jude Medical, Eschborn, Germany).
Clinical and Imaging Protocol
Neurological reassessments and NIHSS scoring and carotid Doppler sonography were performed in the first 0–48 h postoperation with a mean delay of 18 h. Postoperative medications included daily doses of aspirin (100 mg), clopidogrel (75 mg) and events were classified using the modified Rankin scale [13]. Follow-up assessment was carried out with CT or MR imaging examination 48 h after stent placement. After discharge the patients were followed-up clinically and Doppler sonography was carried out at 1, 3, 6, and 12 months and then annually thereafter. The 133 patients with 136 lesions had been examined by ultrasonography before and after the procedure which was repeated for all patients after the first month with a mean interval between the 2 examinations of 62 days. A further examination was carried out on 120 patients after 3 months with a mean period of 146 days.
Statistical Analysis
The data were analyzed using Student’s t-test for paired samples and Fisher’s exact test for small samples when required.
Results
Procedural Outcomes and Restenosis
Primary stent placement was successful in 110 out of 136 lesions in 107 patients. In 26 lesions in 26 patients, predilatation was necessary before stent deployment and in most patients angiography showed a significant improvement in the degree of stenosis which decreased from a mean of 81% before the procedure to a mean of 12.3% immediately after stent placement. In some patients the stents expanded almost completely after the use of postdilatation (Fig. 2). On the Doppler ultrasonography obtained during the follow-up the average degree of stenosis continued to decrease (the severity of residual stenosis impinging on the stent was measured using the NASCET [9] formula) from 12.3% immediately after stent placement to 11.1% within the first 30 days, 11% from day 30 to 6 months (follow-up period) and 10.9% after 6 months and beyond (Fig. 3). A follow-up CT or MR imaging was performed in 129 patients and showed no changes compared to the prestenting CT or MR images. During follow-up, intrastent restenosis occurred in 22 patients according to the modified Zwiebel classification [14]. Only 9 patients showed symptomatic restenosis with recurrences of symptoms localized to the stented territory of which 8 were detected by Doppler sonography on average 216 days after stenting and1 was detected 11 months after stenting (Fig. 2). Intrastent angioplasty using exclusively dilatation balloon (Sterlings balloon, Boston Scientific; submarine balloon) was performed in all patients with neither CPD nor restenting. At the latest sonography in follow-up the lumen of these stents has remained patent with no signs of restenosis.
Follow-up and Complications
The neurological complications are listed in Table 3 and motor dysphasia (n = 1), brachiofacial hemiparesis (n = 3) and circumstances of the neurological complications are described in Table 4. During the initial 30-day follow-up, neurological complications persisted in 4 patients (3%), 3 (3.2%) were encountered in patients with initially symptomatic lesions while 1 complication (2.3%) was reported in a patient with an initially asymptomatic lesion according to NASCET (Table 3). Partial recovery within 72 h was observed in the patient with motor aphasia and 1 patient with hemiparesis. Partial disability was present in only 3 patients with hemiparesis at the 9-month follow-up. During the follow-up period (mean 18 months, range 10–26 months) there were 6 non-procedure-related deaths (postcardiac surgery) and the causes of death included myocardial infarction (n = 2), ventricular arrhythmia (n = 1), massive pulmonary embolization (n = 1), multiorgan failure (n = 1) and fatal stroke (n = 1) which occurred 62 days after the procedure and was not procedure-related: 2 patients developed hyperperfusion syndrome and 1 patient showed haemorrhagic transformation
Plaque Morphology and Risk Stratification
High-risk morphology plaques were found in 42 patients, 12 of whom were asymptomatic and 30 symptomatic. Periprocedural complications were observed in 10.5% (n = 4) of a total of 42 patients with high-risk plaque morphology and in 0% (n = 0) of the low-risk plaque morphology group. Not only was the neurological complications rate higher in the high-risk group but the severity was also significantly higher and 4 complications in this group consisted of 3 major strokes and 1 minor stroke.
Discussion
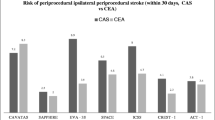
In this study CAS has proved to be as effective as carotid endarterectomy (CEA) in alleviating carotid stenosis in high-risk patients [11], however, the potential for cerebral embolism during the procedure generates great concern regarding the safety of CAS. Furthermore, some studies contributed more to the confusion by showing that filter-protected CAS is associated with an increase in new lesions on diffusion-weighted imaging (DWI) and significantly higher rates of total and particulate microembolization on transcranial pulsed Doppler (TCD) ultrasound than unprotected CAS [15]. The American Heart Association Consensus Conference Recommendations have set the maximum acceptable complication rate of stroke after carotid intervention as 6% for patients with symptomatic lesions and 3% for asymptomatic patients [16–18] and any alternative technique with a complication rate exceeding these recommendations is considered unacceptable. Clinical experience with CAS has shown relatively high 30-day stroke death rates ranging from 3.98% to 10% [3, 19–21] and because of this relatively high incidence of adverse events, efforts have been made to reduce the incidence by using CPDs during CAS [2, 4, 20, 22]. An extensive literature review indicated that the use of CPD can decrease the incidence of the 30-day combined stroke and death rate after CAS from 5.5% without the use of CPDs to 1.8% with CPDs [23]. In the global carotid artery stent registry a reduction in stroke and procedure-related deaths was reported as 5.29% in patients without protection to 2.23% when using protection. The recently published SAPPHIRE-study (Stenting and Angioplasty in Patients at High Risk for Endarterectomy) demonstrated that stroke rates are similar between CAS with cerebral protection and CEA in high-risk patient populations [11]. In the Stent-Supported Percutaneous Angioplasty of the Carotid Artery versus Endarterectomy trial (SPACE) study there were no statistically significant differences between stenting procedures with and without protection devices [36]. Although these reports are encouraging, there is evidence that neuroprotected CAS is associated with predominantly silent cerebral ischemia in approximately 25% of the patients [22]. Other reports have pointed out that the use of CPDs does not offer a full guarantee of completely eliminating the risk of embolic complications during CAS [9, 23–28]. Even the efficacy margin of CPD is problematic for some, where the issue of failure to capture all emboli with filter protection with preservation of flow through the pores of CPDs, may mean preserving the possibility for the emboli to travel to the brain. A number of groups have evaluated the efficacy of a filter in ex vivo models and, although the filter was able to capture the vast majority of particles, especially the large ones, it did not capture 100% [29]. Particles are released during the initial passage phase and particles smaller than the filter pores pass through or around the filter.
Furthermore, CPD itself can cause severe spasms and dissection with subsequent minor stroke [30, 31]. Other potential drawbacks of CPDs include the passage of a CPD through the stenotic lesion which entails a potential risk of dislodgement of unstable plaques and intolerance to balloon occlusion devices in many patients who have contralateral carotid disease or absence of intracranial communicating arteries. Other risks are due to failed deployment of the CPD or difficulty in retrieval because of complicated anatomy and formation of emboli which can potentially be released during removal of the CPD. These disadvantages have led some authors to be sceptical about the value of protection devices [22, 25].
Al-Mubarak et al. [37] demonstrated by TCD that the embolic events are related to the extent of endovascular manipulation. At the same time TCD has proved that the procedure-related embolization during CAS is higher than that during CEA although the clinical significance of microemboli is not clear [32]. The incidence of perioperative stroke in this series was 3% (n = 4) but no deaths were related to the procedure. In the series described here 2.25% (n = 3) of the patients suffered from disabling stroke which is slightly higher than the rate of 2% of permanently disabling stroke and death reported in NASCET [9]. On the other hand, it compares favorably with the overall 30-day stroke and death rate of 7.4% reported by Roubin et al. [20], the stroke rate with the CAS of 6.2% reported in the SAPPHIRE trial [11] and with the findings of Kastrup et al. [2] who found a 5.5% stroke rate for patients treated without CPD and 1.8% for those treated with CPD. Predilatation was necessary in 26 patients because initially the stent could not pass the stenosis but the stents passed without difficulty in the remaining 110 lesions. This could be related to the fact that new generations of self-expanding stents have become smoother with lower profiles allowing a less traumatic passage through stenotic lesions. Several reports have stressed the fact that high-risk morphology plaques have a high propensity to embolize and cause stroke [10, 33, 34]. Although there is no strong evidence at present that supports the selection of patients for treatment based on sonography examination, it has been suggested that measurement of echolucency together with the degree of stenosis may improve the selection of patients for surgical treatment [12]. This is the rationale behind the focus on asymptomatic patients presenting with degrees of stenosis between 50% and 70% associated with high-risk morphology plaques in this series. The higher periprocedural risk of stroke (p < 0.05) found in this study in patients with a high-risk plaque morphology is in agreement with the findings of previous reports [9, 10, 31, 35]. However, there are sporadic descriptions of case series with carotid stent placement without the use of either balloon dilatation or CPD. Little information on efficacy and safety is available from clinical investigations with a sizable patient sample except for the report by Lownie et al. ([7]; 21 patients) and Maynar et al. ([35]; 89 patients) who treated by stenting without balloon dilatation. Taking these data into consideration the higher rate of periprocedural complications reported in this series compared to those reported by Kastrup et al. [2] could be explained by the higher number of patients subjected to dilatation either pre-stenting or post-stenting and by high-risk plaque morphology. All patients with predilatation also needed postdilatation. Predilatation in this case series was inevitable in 26 patients (19.1%), most of whom had tight stenosis or severe calcifications which hindered primary placement of the stent (Fig. 1) and postdilatation was used in 130 lesions while 6 lesions had neither postdilatation nor predilatation. This large number of cases of postdilatation may be mostly due to the fact that the radial expanding force of a self-expanding stent might fail to overcome the resistance presented by a rigid calcified arterial wall without the aid of poststenting balloon dilatation specially with the larger number of wall stent implantations in this cohort when compared to other implanted nitinol stents. This number of cases with neither postdilatation nor predilatation is too low to be evaluated statistically. However, the overuse of postdilation in our cohort may also shed some light on the possibility of reducing the complication rate in this cohort if this step were more cautiously tailored.The 3% of 30-day major neurological events compares favorably with the rates reported with CEA and conventional CAS. The 4.5% overall death rate (6 out of 133) reflects the high-risk patient population involved in this study, none of the cases in this study occurred in the 30 days after the procedure nor were they neurologically related. The maximum stent expansion occurred during the procedure with a decrease in the degree of stenosis from 81% to 12.3% (p = 0.002) but there was no such further significant expansion during the later period of follow-up. Despite the initially acceptable range of stent expansion, 9 patients developed symptomatic restenosis caused by intrastent myointimal hyperplasia during the mean 18-month follow-up. All of these patients were symptomatic and all were successfully treated with intrastent angioplasty. Hyperperfusion occurred in 2 patients (1.5%), 1 of which had only a TCD pattern of mild hyperdynamic cerebral blood flow during the first 24–48 h after stent placement, while the other, besides confirmatory TCD pattern, suffered from hemorrhagic transformation. However, many authors believe that gradual expansion of a self-expanding stent could offer the benefit of potentially reducing the risk of reperfusion-hyperperfusion syndrome after restoration of carotid flow. In this series, the reported reperfusion-hyperperfusion syndrome was lower than that reported by other authors who did not use postdilatation [35] and those results inferred from initial series treated with balloon expandable stents. In the light of previous data the rationale for overcorrecting the initial stenosis in this series could be justified. A separate complication described in the literature on CAS is vagal reflex because of balloon dilatation at the level of the carotid bulb. Some authors foster the concept of eliminating this complication by using neither prestenting nor poststenting balloon dilatation but this concept has several limitations including the cases of patients with extremely tight stenosis in which a stent delivery system may fail to cross without predilatation and occurred in 26 patients in this series. The same is true for calcified stenoses in which the radial expanding force of a self-expanding stent might fail to overcome the resistance presented by a rigid calcified arterial wall without the aid of poststenting balloon dilatation. This was the case in most of the patients in this series.
Conclusions
In this series CAS without the use of distal protection devices was found to be safe and effective with a low incidence of periprocedural complications. The results from this series provide grounds for further clinical investigations, especially for a prospective, randomized study comparing CAS with and without the use of CPDs.
References
Wholey MH, et al. Current global status of carotid artery stent placement. Cathet Cardiovasc Diagn. 1998;44(1):1–6.
Kastrup A, et al. Early outcome of carotid angioplasty and stenting with and without cerebral protection devices: a systematic review of the literature. Stroke. 2003;34(3):813–9.
CAVATAS. Endovascular versus surgical treatment in patients with carotid stenosis in the carotid and vertebral artery transluminal angioplasty study (CAVATAS): a randomised trial. Lancet. 2001;357(9270):1729–37.
Castriota F, et al. Impact of cerebral protection devices on early outcome of carotid stenting. J Endovasc Ther. 2002;9(6):786–92.
Cremonesi A, et al. Protected carotid stenting: clinical advantages and complications of embolic protection devices in 442 consecutive patients. Stroke. 2003;34(8):1936–41.
Eckert B, Zeumer H. Editorial comment—Carotid artery stenting with or without protection devices? Strong opinions, poor evidence! Stroke. 2003;34(8):1941–3.
Lownie SP, et al. Efficacy of treatment of severe carotid bifurcation stenosis by using self-expanding stents without deliberate use of angioplasty balloons. AJNR Am J Neuroradiol. 2005;26(5):1241–8.
Forsting M. Editorial comment—with or without protection? The second important question in carotid artery stenting. Stroke. 2004;35(1):e20–1.
North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325(7):445–53.
Mathiesen EB, Bonaa KH, Joakimsen O. Low levels of high-density lipoprotein cholesterol are associated with echolucent carotid artery plaques: the tromso study. Stroke. 2001;32(9):1960–5.
Yadav JS, et al. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med. 2004;351(15):1493–501.
Gronholdt ML, et al. Ultrasonic echolucent carotid plaques predict future strokes. Circulation. 2001;104(1):68–73.
Wilson JT, et al. Improving the assessment of outcomes in stroke: use of a structured interview to assign grades on the modified Rankin scale. Stroke. 2002;33(9):2243–6.
Zwiebel W, editor. Doppler evaluation of carotid stenosis. Introduction to vascular ultrasonography. 3rd ed. Philadelphia: Saunders; 1992.
Macdonald S, et al. Filter-protected versus unprotected carotid artery stenting: a randomised trial. Cerebrovasc Dis. 2010;29(3):282–9.
Taylor S, Alcocer F, Jordan WD Jr. Controversies in carotid stenting. Vasc Endovascular Surg. 2003;37(2):79–87.
Moore WS, et al. Guidelines for carotid endarterectomy. A multidisciplinary consensus statement from the ad hoc Committee, American Heart Association. Stroke. 1995;26(1):188–201.
Ferguson GG, et al. The North American symptomatic carotid endarterectomy trial: surgical results in 1415 patients. Stroke. 1999;30(9):1751–8.
Phatouros CC, et al. Endovascular treatment of noncarotid extracranial cerebrovascular disease. Neurosurg Clin N Am. 2000;11(2):331–50.
Roubin GS, et al. Immediate and late clinical outcomes of carotid artery stenting in patients with symptomatic and asymptomatic carotid artery stenosis: a 5-year prospective analysis. Circulation. 2001;103(4):532–7.
Sadato A, et al. Use of a large angioplasty balloon for predilation is a risk factor for embolic complications in protected carotid stenting. Neurol Med Chir (Tokyo). 2004;44(7):337–42; discussion 343.
Schluter M, et al. Focal ischemia of the brain after neuroprotected carotid artery stenting. J Am Coll Cardiol. 2003;42(6):1007–13.
Wholey MH, Al-Mubarek N. Updated review of the global carotid artery stent registry. Catheter Cardiovasc Interv. 2003;60(2):259–66.
Ouriel K, Yadav JS. The role of stents in patients with carotid disease. Rev Cardiovasc Med. 2003;4(2):61–7.
Pinero P, et al. Silent ischemia after neuroprotected percutaneous carotid stenting: a diffusion-weighted MRI study. AJNR Am J Neuroradiol. 2006;27(6):1338–45.
Hayashi K, et al. Case of internal carotid artery stenosis complicated with shower embolism during filter-protected carotid artery stenting. Brain Nerve. 2009;61(1):83–7.
Takayama K, et al. Initial experience of using the filter protection device during carotid artery stenting in Japan. Radiat Med. 2008;26(6):348–54.
Roffi M, et al. Flow impairment during protected carotid artery stenting: impact of filter device design. J Endovasc Ther. 2008;15(1):103–9.
Muller-Hulsbeck S, et al. Comparison of various cerebral protection devices used for carotid artery stent placement: an in vitro experiment. J Vasc Interv Radiol. 2003;14(5):613–20.
Cardaioli P, et al. Complication with an embolic protection device during carotid angioplasty. Catheter Cardiovasc Interv. 2004;62(2):234–6.
Higashida RT, et al. Reporting standards for carotid artery angioplasty and stent placement. J Vasc Interv Radiol. 2004;15(5):421–2.
Coggia M, et al. Embolic risk of the different stages of carotid bifurcation balloon angioplasty: an experimental study. J Vasc Surg. 2000;31(3):550–7.
Biasi GM, et al. Carotid plaque echolucency increases the risk of stroke in carotid stenting: the imaging in carotid angioplasty and risk of stroke (ICAROS) study. Circulation. 2004;110(6):756–62.
Bicknell CD, Cheshire NJ. The relationship between carotid atherosclerotic plaque morphology and the embolic risk during endovascular therapy. Eur J Vasc Endovasc Surg. 2003;26(1):17–21.
Maynar M, et al. Carotid stenting without use of balloon angioplasty and distal protection devices: preliminary experience in 100 cases. AJNR Am J Neuroradiol. 2007;28(7):1378–83.
Ringleb PA, Hacke W. Stent and surgery for symptomatic carotid stenosis. SPACE study results. Nervenarzt. 2007;78(10):1130–37.
Al-Mubarak N, et al. Effect of the distal-balloon protection system on microembolization during carotid stenting. Circulation. 2001;104(17):1999–2002.
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mansour, O., Weber, J., Niesen, W. et al. Carotid Angioplasty and Stenting Without Protection Devices. Clin Neuroradiol 21, 65–73 (2011). https://doi.org/10.1007/s00062-011-0057-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00062-011-0057-6