Abstract
The syndrome heart failure with preserved ejection fraction (HFpEF) represents patients with different comorbidities and specific etiologies, but with a key and common alteration: an elevation in left ventricular (LV) filling pressure or pulmonary capillary wedge pressure (PCWP). Expert consensuses, society guidelines, and diagnostic scores have been stated to diagnose HFpEF syndrome based mainly on the determination of elevated LV filling pressure or PCWP by transthoracic echocardiography (TTE). Echocardiographic parameters such as early (E) and late diastolic mitral inflow velocity (mitral E/A ratio), septal and lateral mitral annular early diastolic velocity (E′), ratio of the early diastolic mitral inflow and annular velocity (E/E′-ratio), maximal left atrial volume index (LAVImax), and tricuspid regurgitation peak velocity (VTR) constitute the pivotal parameters for determining elevated LV filling pressure or PCWP in patients with suspected HFpEF symptoms. Notwithstanding this, taking into consideration the heterogeneity of patients with HFpEF symptoms, the term “HFpEF” should be considered as a syndrome rather than an entity since HFpEF results from different pathological entities that should and can be characterized by echocardiography and multimodality imaging. Comprehensive TTE might help diagnose specific diseases and etiologies by characterization of specific cardiac phenotypes.
Zusammenfassung
Das Syndrom der Herzinsuffizienz mit erhaltener Ejektionsfraktion („heart failure with preserved ejection fraction“, HFpEF) steht für Patienten mit verschiedenen Komorbiditäten und spezifischen Grunderkrankungen, die jedoch eine gemeinsame charakterisierende Veränderung zeigen, nämlich den pathologischen Anstieg der linksventrikulären (LV-)Füllungsdrücke oder des pulmonalkapillären Wedge-Drucks (PCWP). Expert Consensus Statements, nationale und internationale Leitlinien sowie Diagnose-Scores wurden publiziert, um das HFpEF-Syndrom zu diagnostizieren, vornehmlich basierend auf der Bestimmung eines erhöhten LV-Füllungsdrucks oder PCWP mittels transthorakaler Echokardiographie (TTE). Die echokardiographischen Parameter der frühen (E) und späten (A) diastolischen transmitralen Flussgeschwindigkeit sowie deren E/A-Ratio, die septale und laterale mitralringnahe Myokardgeschwindigkeit (E′), das Verhältnis der frühen transmitralen Flussgeschwindigkeit zur mitralringnahen Myokardgeschwindigkeit (E/E′-ratio), der maximale linksatriale Volumenindex (LAVImax) und die maximale transtrikuspidale Regurgitationsgeschwindigkeit (VTR) bilden die grundlegenden Pfeiler, um einen erhöhten LV-Füllungsdruck oder PCWP bei Patienten mit HFpEF-Symptomen festzustellen. Nichtsdestotrotz sollte in Anbetracht der Heterogenität der Patienten mit HFpEF-Symptomen die Bezeichnung „HFpEF“ immer als Syndrom anstelle einer Diagnose betrachtet werden, da HFpEF durch verschiedene pathologische Gegebenheiten begründet sein kann, die durch Echokardiographie und weitere multimodale Bildgebung detektiert werden können und sollten. Eine fachkundige TTE kann über die exakte Charakterisierung von echokardiographischen Phänotypen zur Diagnose spezifischer Erkrankungen und Ätiologien beitragen.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The syndrome of “heart failure with preserved ejection fraction” (HFpEF) represents patients with different comorbidities and specific etiologies, but with a key and common alteration, that is, an elevation of left ventricular (LV) filling pressure or pulmonary capillary wedge pressure (PCWP). Expert consensus, society guidelines, and diagnostic scores have been put forward to diagnose the syndrome of HFpEF based mainly on the determination of elevated LV filling pressure or PCWP by transthoracic echocardiography. Echocardiographic parameters—such as early (E) and late (A) diastolic mitral inflow velocity as well as transmitral E/A ratio, septal and lateral mitral annular early diastolic velocity (E′), ratio of the early diastolic mitral inflow and annular velocity (E/E′ ratio), maximal left atrial volume index (LAVImax), and tricuspid regurgitation peak velocity (VTR)—are pivotal to determine elevated LV filling pressure or PCWP in patients with suspected HFpEF symptoms [1,2,3,4,5].
However, there is an ongoing discussion about whether HFpEF should be regarded as a single diagnosis or as a symptom complex or a syndrome [3, 4]. Patients with HFpEF symptoms obviously form a cohort of different diseases with the same clinical phenotype. As a consequence, the underlying varying individual diagnoses combined with further comorbidities need different specific treatment. Depending on the specific diagnosis, individual outcomes can significantly vary [6]. Consequently, the single diagnosis of HFpEF should be discarded because the clinical phenotype characterized by HFpEF symptoms is due to heterogeneous syndromes with different etiologies [7]. Thus, diagnostic procedures for patients with HFpEF symptoms should focus on the detection of specific underlying diseases. Between 1998 and 2021, recommendations regarding heart failure (HF) were published to characterize patients with HFpEF symptoms [2,3,4,5, 8,9,10,11,12] centering on the question of “how to diagnose HFpEF” [1,2,3,4,5, 8,9,10,11,12]. The initial terminology or definition of diastolic HF as “an increased resistance to filling one or both ventricles, leading to symptoms of congestion due to an inappropriate upward shift of the diastolic pressure–volume relation” [13] was later replaced by the term “heart failure with normal ejection fraction” (HFnEF), and finally by the term “HFpEF” [9] presupposing cardiac causes of the HFpEF symptoms.
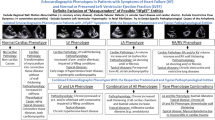
Patients with diastolic HF or with HFpEF symptoms were originally characterized by the presence of signs or symptoms of congestive HF with a normal or mildly reduced LV systolic function and by the evidence of abnormal LV relaxation, filling, diastolic distensibility, and diastolic stiffness. In the past, functional parameters—such as LV relaxation and stiffness—were measured via invasive investigations [8, 9]. However, over time, noninvasive techniques—especially Doppler echocardiography—have been increasingly used to characterize LV diastolic function and elevated LV filling pressure (Fig. 1; [1, 14]).
Schemes illustrating the diagnostic workflow to estimate left ventricular (LV) filling pressures in patients with HFpEF symptoms according to the diagnostic EACVI criteria (a) and the HFA-PEFF approach (b). E early diastolic mitral inflow velocity, A late diastolic mitral inflow velocity, E/A transmitral E/A ratio, E′ septal and lateral mitral annular early diastolic velocity, E/E′ ratio of early diastolic mitral inflow and annular velocity, LA left atrial, LAVImax left atrial volume index, VTR tricuspid regurgitation peak velocity
With respect to the HFpEF definition and the diagnostic procedures in patients with HFpEF symptoms, two important aspects must be mentioned:
First, the 2021 recommendation describes only three criteria for evaluating HFpEF syndrome: symptoms, LVEF ≥ 50%, and LV diastolic dysfunction (DD; [4]). The importance of normal LV size as a prerequisite for diastolic HF was highlighted in the recommendations until 2012 [8,9,10,11] and has not been emphasized since 2016 [1, 8, 9] introducing obliviscence of basic pathophysiological principles that normal LV relaxation depends on normal end-systolic LV load and LV volume [15]. Thus, eccentric LV hypertrophy is an exclusion criterion for diastolic HF. Consequently, the proper assessment of LV diameters and LV volumes by transthoracic echocardiography (TTE) as a prerequisite to characterize a pathophysiological state is an essential focus of attention [16]. Therefore, comprehensive TTE must satisfy methodological requirements to assess proper LV volumes. The accuracy of LV volume assessment by TTE is highly debated in clinical routine [17].
Second, the switch from invasive to noninvasive diagnostics to define HFpEF syndrome is mandatory because pathological LV filling patterns and elevated LV end-diastolic pressure (LVEDP) are hallmarks in these patients and measurable by TTE. Hence, both aspects have implications on diagnostic algorithms to characterize cardiac diagnoses in patients with HFpEF symptoms (Fig. 1). In addition, pathological LV filling patterns are not necessarily linked to increased E/E′ ratio [18, 19] and to increased N‑terminal pro-brain natriuretic peptide (pro-NT-BNP) levels [20].
Diagnostic scores for patients with HFpEF symptoms
In addition to the conventional diagnostic assessment of patients with HFpEF symptoms [9,10,11,12], scores have been introduced to improve the diagnostic accuracy: the H2FPEF score [2, 21] and the HFA-PEFF score [3]. The main principle of these scores is the attempt to increase the probability of detecting cardiac causes of HFpEF symptoms due to multifactorial considerations. The H2FPEF score is based on weight, age, LV filing pressure measured with the E/E′ ratio, and the history of hypertensive heart disease, atrial fibrillation, and pulmonary hypertension. The H2FPEF score might overestimate atrial fibrillation in patients with HFpEF symptoms in comparison with the HFA-PEFF score. The HFA-PEFF score (Fig. 1; Table 1) is based on a stepwise approach with an initial workup characterizing HF symptoms, comorbidities, and risk factors by ECG, TTE, and natriuretic peptides; a special diagnostic workup by comprehensive TTE; an advanced workup by functional testing using diastolic stress echocardiography or invasive measurements; and finally by an etiological workup to clarify the final etiology using cardiac magnetic resonance imaging (MRI), cardiac or noncardiac biopsies, scintigraphy, computed tomography (CT), and positron emission tomography (PET), genetic testing, and/or specific laboratory tests. The HFA-PEFF score eliminated the E/A ratio as a criterion of DD.
The problems of these scores are obviously that the factors contributing to the points of the H2FPEF score are interrelated (e.g., obesity is linked to LV hypertrophy, increased E/E′-ratio, and pulmonary hypertension), and that the most important target—the specific underlying diagnosis responsible for the HF symptoms—is addressed in the last step of the HFA-PEFF score. Thus, both scores largely disagree in classifying patients with HFpEF symptoms due to diastolic HF [22, 23].
Consequently, there are still ongoing diagnostic challenges in managing patients with HFpEF symptoms. Firstly, how to improve the detection of noncardiac causes of symptoms. And how to distinguish between early subclinical stages of diseases due to risk factors and comorbidities.
Secondly, how to characterize the “masqueraders” [3] such as coronary artery disease (CAD), valvular heart disease (VHD), arrhythmias, and pericardial constriction. And how to handle specific pathological entities that are recently not integrated into the masqueraders described here. Systemic inflammatory diseases and coronary microvascular endothelial inflammation play an important role as the cause of myocardial structural and functional alterations that can be commonly observed in obesity, diabetes, consuming neoplasms, and rheumatological diseases at subclinical stages [24, 25].
Thirdly, how to deal with atrial fibrillation (AF) in patients with HFpEF symptoms. If arrhythmias are described as masqueraders, why is AF not integrated as a masquerader to exclude patients with HFpEF symptoms in the scores? Normally, AF is a symptom of pathophysiological circumstances or an underlying cardiac diagnosis.
Fourthly, the simplification of echocardiographic analysis of diastolic dysfunction by solely determining the E/E′ ratio raises the fundamental question of why comprehensive TTE should not focus directly on specific diagnoses instead of describing a multifactorial HFpEF hodgepodge.
In summary, the so-called HFpEF diagnosis according to the scores merges a cohort of patients with HFpEF symptoms, in which patients with chronic hypertensive heart disease and storage diseases are mixed with patients of unknown, unclear, or undetected specific cardiac or noncardiac diagnoses. Thus, the diagnostic HFpEF mishmash of similar, but still heterogeneous, clinical phenogroups with extremely varying disease outcome is presumably the reason of treatment failure in several HFpEF trials [26,27,28].
Comprehensive echocardiography for specific diagnosis in HFpEF
One of the cornerstones of the diagnostic HFpEF scores is to perform TTE [1,2,3,4,5,6,7,8,9,10,11,12]. Pathological TTE findings in patients with HFpEF symptoms are characterized by the parameters LV ejection fraction (LVEF), LV end-diastolic volume (LVEDV), E′, E/E′ ratio, E/A ratio, transmitral deceleration time (DT), the time duration of ArD − AD (ArD, = duration of reverse pulmonary vein atrial systole flow; AD = duration of mitral valve atrial wave flow), the maximum left atrial (LA) volume index (LAVImax), and the LV mass index (LVMI; [8, 9]). Recently, the maximum tricuspid regurgitant velocity (VTR), calculated systolic pulmonary artery pressure (sPAP), and LV global longitudinal strain (GLS) have been added [3, 4]. However, the accuracy of these pivotal parameters to characterize diastolic HF is limited due to a sensitivity between 26 and 70% and a specificity between 56 and 86% [2]. Thus, the diagnostic approach of comprehensive TTE must be improved to detect specific cardiac or noncardiac diagnoses through specific echocardiographic findings in patients with HFpEF symptoms. Generally, TTE imaging can be completed by additional imaging modalities [5, 29,30,31,32].
An advanced diagnostic TTE workflow includes a systematic morphological and functional classification with additional conventional as well as modern echocardiographic parameters [1, 16, 33,34,35]. It is obvious that the masqueraders VHD, CAD, and pericardial constriction as well as eccentric LV hypertrophy must be detected as specific diagnoses [3]. Hereafter, the echocardiographic characterization might focus on the proper detection of echocardiographic cardiac phenotypes, which are indicative of specific diagnoses (Fig. 2). Echocardiographic phenotypes are determined by isolated morphological and functional abnormalities of the left ventricle, the left atrium, and the right cardiac chambers as well as by their combination (Fig. 2). This conventional echocardiographic phenotyping using a “human intelligence approach” corresponds to current methodological developments of machine learning-derived echocardiographic phenotyping [36].
Alterations of LV wall size and consecutive LV geometry can be easily detected by conventional TTE assessing the LV wall, LV mass, LV volume, and LV geometry. The parameters that are necessary for characterizing this echocardiographic LV phenotype are LV wall thickness, relative wall thickness (RWT), LVMI, LV end-diastolic and end-systolic diameter (LVEDD, LVESD) as well as volume (LVEDV, LVESV), LVEF, LVLV remodeling index (LVRI), and LV sphericity ratio and index [16]. In addition, mitral annular plane systolic excursion (MAPSE) and LV strain assessment are also established in the clinical scenario.
Alterations in LA size and function are commonly detectable by LA planimetry and Doppler echocardiography. Parameters to characterize this echocardiographic LA phenotype are LAVI, E/A ratio, DC, and ArD-AD [1]. However, parameters such as minimum LA volume index (LAVImin), LA emptying fraction, LA strain including the compounds of reservoir, conduit, and contraction LA strain as well as E′, E/E′ ratio, isovolumetric relaxation time (IVRT), VTR, and sPAP to characterize DD should be added [34, 35].
Alterations in right chamber morphology and function can be primarily detected by right atrial and right ventricular (RA, RV) volume assessment. Due to the bipyramidal RV shape, three-dimensional RV volume assessment is necessary with respect to objectification and accuracy. In general, the volumes of the cardiac chambers can be assessed more precisely by three-dimensional or triplane datasets [33]. In addition, diastolic RV wall thickness, radial RV thickening, RV eccentricity index, tricuspid annular plane systolic excursion (TAPSE), and RV as well as RA strain can be added to assess the RA/RV phenotype.
The main problem with TTE is the detection of subclinical or early disease stages in patients with a normal echocardiographic phenotype if abnormal LV relaxation, LV filling, LV diastolic distensibility, and LV diastolic stiffness only are present. Additional parameters such as end-systolic elastance (Ees) and arterial elastance (Ea) might be added to the proposed echocardiographic parameters E′, E/E′ ratio, IVRT, E/A ratio, DC, and ArD-AR. The estimation of increased LV chamber stiffness through the invasive analysis of the LV pressure–volume relationship is the gold standard for characterizing LV distensibility [18, 19]. Ees is determined by the slope of the end-systolic pressure volume relationship (ESPVR), while Ea is reflected by the negative slope between ESPVR and the LVEDV at zero LV pressure and estimates global myocardial afterload [37,38,39]. Compared to LVEF or GLS, Ees, Ea, and global myocardial work are load-independent parameters of LV systolic function [40,41,42]. The single-beat noninvasive echocardiographic approach for calculating Ees and Ea requires the measurement of noninvasive blood pressure recordings as well as the systolic time interval, defined by pre-ejection period and systolic ejection time derived from pulsed-waved Doppler recordings in the LV outflow tract with simultaneous ECG recordings. Noninvasive assessment of myocardial work requires LV strain analysis and noninvasive blood pressure recordings. The complex equations for calculating Ees, Ea, and myocardial work must be facilitated by automated software to establish these measurements in the clinical scenario.
Thus, the detailed characterization of echocardiographic phenotypes and their combinations by an advanced TTE approach might improve the direct detection of specific underlying diagnoses in patients with HFpEF symptoms (Figs. 2 and 3).
Comprehensive echocardiography in patients with HFpEF symptoms. a–d Strain imaging in a patient with hypertensive heart disease (combined LA and LV phenotype). a Regional longitudinal LV strain graphs and the LV strain pattern; b tracking area of the biplane atrial strain assessment; c atrial strain curves adjusted to the r‑wave, and d adjusted to the p‑wave. e–h Corresponding strain imaging in a patient with amyloidosis (combined LA, LV, and RA/RV phenotype). The reduced LV as well as LA deformation in storage disease in comparison with hypertensive heart disease is objectively shown. LA left atrium, LV left ventricle, RA right atrium, RV right ventricle
Diastolic stress echocardiography to unmask diastolic dysfunction in HFpEF
If advanced echocardiographic assessment at rest in patients with HFpEF symptoms is limited in determining cardiac causes due to DD, machine learning-derived echocardiographic phenotypes might help identify subclinical asymptomatic HF patients at rest [36] or diastolic stress echocardiography might help unmask DD by inducing pathological changes of diastolic filling properties due to structural cardiac findings at stress [43]. However, physical diastolic stress echocardiography [3, 43] is a methodological challenge. Standardization of image acquisition at the respective stress levels is a prerequisite to gain verifiable results. In particular, the sample volume position of the pulsed wave Doppler spectra of the transmitral flow velocities and of the basal myocardial tissue velocities must be comparable between rest and stress. In addition, the fusion of E and A wave with increasing heart rate is often observed at low stress levels, limiting the value of stress testing. Therefore, DD detection should be confirmed by invasive hemodynamic exercise testing if the diagnosis with diastolic stress echocardiography is uncertain [44, 45]. Despite high methodological standards, diastolic stress echocardiography has the potential to distinguish between cardiac and noncardiac causes of HFpEF symptoms and to increase the sensitivity to detect DD.
Conclusion
Patients with symptoms of heart failure with preserved ejection fraction (HFpEF) form a heterogeneous cohort with different underlying diseases. The diagnostic approach for characterizing HF patients with normal or preserved LVEF is challenging because HFpEF symptoms can be induced by cardiac as well as noncardiac underlying diagnoses. Algorithms for HFpEF describe a cohort of patients with hypertensive heart disease, storage diseases, and unknown, unclear, or undetected specific cardiac or noncardiac pathologies. Thus, a more detailed echocardiographic approach including stress echocardiography might be helpful for detecting specific diagnoses, because the outcome of the underlying diseases in patients with HFpEF symptoms can vary extremely. Thus, the term “HFpEF” as a diagnosis may be misleading; HFpEF symptoms form a syndrome resulting from different pathological entities. The detection of the specific underlying diagnosis is possible by advanced echocardiography and multimodality imaging.
References
Nagueh SF, Smiseth OA, Appleton CP et al (2016) Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American society of echocardiography and the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging 17(12):1321–1360. https://doi.org/10.1093/ehjci/jew082
Reddy YNV, Carter RE, Obokata M et al (2018) A simple, evidence-based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation 138(9):861–870. https://doi.org/10.1161/CIRCULATIONAHA.118.034646
Pieske B, Tschöpe C, de Boer RA et al (2019) How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the heart failure association (HFA) of the European society of cardiology (ESC). Eur Heart J 40(40):3297–3317. https://doi.org/10.1093/eurheartj/ehz641
McDonagh TA, Metra M, Adamo M et al (2021) 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 42(36):3599–3726. https://doi.org/10.1093/eurheartj/ehab368
Smiseth OA, Morris DA, Cardim N et al (2022) Multimodality imaging in patients with heart failure and preserved ejection fraction: an expert consensus document of the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging. https://doi.org/10.1093/ehjci/jeab154
Braunwald E (2021) Heart failure with preserved ejection fraction: a stepchild no more! Eur Heart J 42(38):3900–3901. https://doi.org/10.1093/eurheartj/ehab601
Fayol A, Wack M, Livrozet M et al (2021) Aetiological classification and prognosis in patients with heart failure with preserved ejection fraction. ESC Heart Fail. https://doi.org/10.1002/ehf2.13717
European Study Group on Diastolic Heart Failure (1998) How to diagnose diastolic heart failure. Eur Heart J 19(7):990–1003. https://doi.org/10.1053/euhj.1998.1057
Paulus WJ, Tschöpe C, Sanderson JE et al (2007) How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the heart failure and echocardiography associations of the European society of cardiology. Eur Heart J 28(20):2539–2550. https://doi.org/10.1093/eurheartj/ehm037
Dickstein K, Cohen-Solal A, Filippatos G et al (2008of) ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the task force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European society of cardiology. Developed in collaboration with the heart failure association of the ESC (HFA) and endorsed by the European society of intensive care medicine (ESICM). Eur J Heart Fail 10(10):933–989. https://doi.org/10.1016/j.ejheart.2008.08.005
McMurray JJ, Adamopoulos S, Anker SD et al (2012of) ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European society of cardiology. Developed in collaboration with the heart failure association (HFA) of the ESC. Eur Heart J 33(14):1787–1847. https://doi.org/10.1093/eurheartj/ehs104
Ponikowski P, Voors AA, Anker SD et al (2016) 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European society of cardiology (ESC) developed with the special contribution of the heart failure association (HFA) of the ESC. Eur Heart J 37(27):2129–2200. https://doi.org/10.1093/eurheartj/ehw128
Brutsaert DL, Sys SU, Gillebert TC (1993) Diastolic failure: pathophysiology and therapeutic implications. J Am Coll Cardiol 22(1):318–325. https://doi.org/10.1016/0735-1097(93)90850-z
Nagueh SF (2020) Diastology: 2020—a practical guide. Echocardiography 37(11):1919–1925. https://doi.org/10.1111/echo.14742
Brutsaert DL, Sys SU (1989) Relaxation and diastole of the heart. Physiol Rev 69(4):1228–1315. https://doi.org/10.1152/physrev.1989.69.4.1228
Lang RM, Badano LP, Mor-Avi V et al (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging 16:233–270. https://doi.org/10.1093/ehjci/jev014
Rigolli M, Anandabaskaran S, Christiansen JP et al (2016) Bias associated with left ventricular quantification by multimodality imaging: a systematic review and meta-analysis. Open Heart 3:388. https://doi.org/10.1136/openhrt-2015-000388
Ommen SR, Nishimura RA, Appleton CP et al (2000) Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: a comparative simultaneous Doppler-catheterization study. Circulation 102(15):1788–1794. https://doi.org/10.1161/01.cir.102.15.1788
Kasner M, Westermann D, Steendijk P et al (2007) Utility of Doppler echocardiography and tissue Doppler imaging in the estimation of diastolic function in heart failure with normal ejection fraction: a comparative Doppler-conductance catheterization study. Circulation 116(6):637–647. https://doi.org/10.1161/CIRCULATIONAHA.106.661983
Werhahn SM, Becker C, Mende M et al (2022) NT-proBNP as a marker for atrial fibrillation and heart failure in four observational outpatient trials. ESC Heart Fail 9(1):100–109. https://doi.org/10.1002/ehf2.13703
Paulus WJ (2018) H2FPEF score: at last, a properly validated diagnostic algorithm for heart failure with preserved ejection fraction. Circulation 138(9):871–873. https://doi.org/10.1161/CIRCULATIONAHA.118.035711
Barandiarán Aizpurua A, Sanders-van Wijk S, Brunner-La Rocca HP et al (2020) Validation of the HFA-PEFF score for the diagnosis of heart failure with preserved ejection fraction. Eur J Heart Fail 22(3):413–421. https://doi.org/10.1002/ejhf.1614
Sanders-van Wijk S, Barandiarán Aizpurua A, Brunner-La Rocca HP et al (2021) The HFA-PEFF and H2 FPEF scores largely disagree in classifying patients with suspected heart failure with preserved ejection fraction. Eur J Heart Fail 23(5):838–840. https://doi.org/10.1002/ejhf.2019
Paulus WJ, Tschöpe C (2013) A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 62(4):263–271. https://doi.org/10.1016/j.jacc.2013.02.092
Paulus WJ, Zile MR (2021) From systemic inflammation to myocardial fibrosis: the heart failure with preserved ejection fraction paradigm revisited. Circ Res 128(10):1451–1467. https://doi.org/10.1161/CIRCRESAHA.121.318159
Massie BM, Carson PE, McMurray JJ et al (2008) Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med 359(23):2456–2467. https://doi.org/10.1056/NEJMoa0805450
Pitt B, Pfeffer MA, Assmann SF et al (2014) Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 370(15):1383–1392. https://doi.org/10.1056/NEJMoa1313731
Solomon SD, McMurray JJV, Anand IS et al (2019) Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med 381(17):1609–1620. https://doi.org/10.1056/NEJMoa1908655
Omar AM, Bansal M, Sengupta PP (2016) Advances in echocardiographic imaging in heart failure with reduced and preserved ejection fraction. Circ Res 119(2):357–374. https://doi.org/10.1161/CIRCRESAHA.116.309128
Čelutkienė J, Plymen CM, Flachskampf FA et al (2018) Innovative imaging methods in heart failure: a shifting paradigm in cardiac assessment. Position statement on behalf of the heart failure association of the European society of cardiology. Eur J Heart Fail 20(12):1615–1633. https://doi.org/10.1002/ejhf.1330
Obokata M, Reddy YNV, Borlaug BA (2019) The role of echocardiography in heart failure with preserved ejection fraction: what do we want from imaging? Heart Fail Clin 15(2):241–256. https://doi.org/10.1016/j.hfc.2018.12.004
Lewis GA, Pearce K, Williams SG et al (2021) The utility of cardiovascular imaging in heart failure with preserved ejection fraction: diagnosis, biological classification and risk stratification. Heart Fail Rev 26(3):661–678. https://doi.org/10.1007/s10741-020-10047-9
Lang RM, Badano LP, Tsang W et al (2012) EAE/ASE recommendations for image acquisition and display using three dimensional echocardiography. Eur Heart J Cardiovasc Imaging 13:1–46. https://doi.org/10.1093/ehjci/jer316
Amzulescu MS, De Craene M, Langet H et al (2019) Myocardial strain imaging: review of general principles, validation, and sources of discrepancies. Eur Heart J Cardiovasc Imaging 20(6):605–619. https://doi.org/10.1093/ehjci/jez041
Voigt JU, Cvijic M (2019) 2‑ and 3‑dimensional myocardial strain in cardiac health and disease. JACC Cardiovasc Imaging 12(9):1849–1863. https://doi.org/10.1016/j.jcmg.2019.01.044
Kobayashi M, Huttin O, Magnusson M et al (2022) Machine learning-derived echocardiographic phenotypes predict heart failure incidence in asymptomatic individuals. JACC Cardiovasc Imaging 15(2):193–208. https://doi.org/10.1016/j.jcmg.2021.07.004
Borlaug BA, Lam CSP, Roger VL et al (2009) Contractility and ventricular systolic stiffening in hypertensive heart disease. Insights into the pathogenesis of heart failure with preserved ejection fraction. J Am Coll Cardiol 54(5):410–418. https://doi.org/10.1016/j.jacc.2009.05.013
Ky B, French B, Khan AM et al (2013) Ventricular-arterial coupling, remodeling, and prognosis in chronic heart failure. J Am Coll Cardiol 62(13):1165–1172. https://doi.org/10.1016/j.jacc.2013.03.085
Schwarzl M, Ojeda F, Zeller T et al (2016) Risk factors for heart failure are associated with alterations of the LV end-diastolic pressure-volume relationship in non-heart failure individuals: data from a large-scale, population-based cohort. Eur Heart J 37(23):1807–1814. https://doi.org/10.1093/eurheartj/ehw120
Shishido T, Hayashi K, Shigemi K et al (2000) Single-beat estimation of end-systolic elastance using bilinearly approximated time-varying elastance curve. Circulation 102(16):1983–1989. https://doi.org/10.1161/01.cir.102.16.1983
Chen CH, Fetics B, Nevo E et al (2001) Noninvasive single-beat determination of left ventricular end-systolic elastance in humans. J Am Coll Cardiol 38(7):2028–2034. https://doi.org/10.1016/s0735-1097(01)01651-5
Russell K, Eriksen M, Aaberge L et al (2012) A novel clinical method for quantification of regional left ventricular pressure-strain loop area: a non-invasive index of myocardial work. Eur Heart J 33(6):724–733. https://doi.org/10.1093/eurheartj/ehs016
Belyavskiy E, Morris DA, Url-Michitsch M et al (2019) Diastolic stress test echocardiography in patients with suspected heart failure with preserved ejection fraction: a pilot study. ESC Heart Fail 6(1):146–153. https://doi.org/10.1002/ehf2.12375
Maeder MT, Thompson BR, Brunner-La Rocca HP et al (2010) Hemodynamic basis of exercise limitation in patients with heart failure and normal ejection fraction. J Am Coll Cardiol 56(11):855–863. https://doi.org/10.1016/j.jacc.2010.04.040
Nauta JF, Hummel YM, van der Meer P et al (2018) Correlation with invasive left ventricular filling pressures and prognostic relevance of the echocardiographic diastolic parameters used in the 2016 ESC heart failure guidelines and in the 2016 ASE/EACVI recommendations: a systematic review in patients with heart failure with preserved ejection fraction. Eur J Heart Fail 20(9):1303–1311. https://doi.org/10.1002/ejhf.1220
Funding
Research of Carsten Tschöpe was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)—SFB 1470, project: B02. Research of Rudolf A. de Boer was funded by the Netherlands Heart Foundation (CVON grants 2017-21; 2017-11; 2018-30; 2020B005) and the European Research Council (ERC CoG 818715). For all other authors this research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
A. Hagendorff, S. Stöbe, J. Kandels, R. de Boer and C. Tschöpe declare that they have no competing interests.
For this article no studies with human participants or animals were performed by any of the authors. All studies mentioned were in accordance with the ethical standards indicated in each case.
Rights and permissions
About this article
Cite this article
Hagendorff, A., Stöbe, S., Kandels, J. et al. Diagnostic role of echocardiography for patients with heart failure symptoms and preserved left ventricular ejection fraction. Herz 47, 293–300 (2022). https://doi.org/10.1007/s00059-022-05118-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00059-022-05118-6