Abstract
Background
Mesenchymal stem cell (MSC) treatment has emerged as an important adjunct therapy for heart failure. However, the use of MSC to treat heart failure has not been well established. We conducted a systematic review and meta-analysis to evaluate the efficacy of MSC treatment for heart failure.
Methods
PubMed, Embase, and the Cochrane Central Register of Controlled Trials were searched. Randomized controlled trials (RCTs) assessing the influence of MSC treatment on cardiac function in heart failure were included in this analysis. Two investigators independently searched the articles, extracted data, and assessed the quality of the included studies. Meta-analysis was performed using the fixed-effect model or random-effect model when appropriate.
Results
Six RCTs involving 625 patients were included in the meta-analysis. Compared with control interventions in heart failure patients, MSC treatment had no significant influence on cardiovascular death (RR = 0.76; 95% CI = 0.38–1.52; p = 0.43); however, it was associated with significantly increased left ventricular ejection fraction (LVEF; mean = 9.64; 95% CI = 7.56–11.71; p < 0.00001) and reduced rehospitalization rate (RR = 0.41; 95% CI = 0.23–0.73; p = 0.003). In addition, no significant difference between the two groups was observed for the incidence of myocardial infarction (RR = 0.72; 95% CI = 0.10–5.02; p = 0.74), the recurrence of heart failure (RR = 0.88; 95% CI = 0.28–2.81; p = 0.83), and total death (RR = 0.68; 95% CI = 0.37–1.25; p = 0.21).
Conclusion
Although MSC treatment can significantly improve LVEF and reduce rehospitalization rates, it does not have a significant influence on cardiovascular death, myocardial infarction, heart failure, and total death.
Zusammenfassung
Hintergrund
Die Therapie mit mesenchymalen Stammzellen (MSC) hat sich als wichtige ergänzende Therapie bei Herzinsuffizienz herausgestellt. Allerdings ist der Einsatz von MSC zur Behandlung der Herzinsuffizienz noch nicht verbreitet. Die Autoren erstellten eine systematische Übersicht und Metaanalyse zur Bewertung der Wirksamkeit der MSC-Therapie bei Herzinsuffizienz.
Methoden
Die Datenbanken PubMed, Embase und das Cochrane Central Register of Controlled Trials wurden durchsucht. In die Auswertung einbezogen wurden randomisierte kontrollierte Studien (RCT) zum Einfluss der MSC-Therapie auf die Herzfunktion bei Herzinsuffizienz. Zwei unabhängige Untersucher durchsuchten die Artikel, extrahierten Daten und beurteilten die Qualität der ausgewählten Studien. Eine Metaanalyse wurde je nach Bedarf unter Verwendung des Fixed-Effects-Modells oder des Random-Effects-Modells erstellt.
Ergebnisse
Es wurden 6 RCT mit 625 Patienten in die Metaanalyse einbezogen. Im Vergleich zu den als Kontrolle dienenden Interventionen bei Herzinsuffizienzpatienten wies die MSC-Therapie keinen signifikanten Einfluss auf den kardiovaskulär bedingten Tod auf (RR = 0,76; 95%-KI = 0,38–1,52; p = 0,43); allerdings war sie mit einer signifikant erhöhten linksventrikulären Ejektionsfraktion verbunden (LVEF; Durchschnitt = 9,64; 95%-KI = 7,56–11,71; p < 0,00001) und einer verminderten Rehospitalisierungsrate (RR = 0,41; 95%-KI = 0,23–0,73; p = 0,003). Außerdem wurde weder in Hinblick auf die Inzidenz eines Herzinfarkts ein signifikanter Unterschied zwischen den beiden Gruppen festgestellt (RR = 0,72; 95%-KI = 0,10–5,02; p = 0,74) noch bezüglich eines Wiederauftretens der Herzinsuffizienz (RR = 0,88; 95%-KI = 0,28–2,81; p = 0,83) oder der Gesamtmortalität (RR = 0,68; 95%-KI = 0,37–1,25; p = 0,21).
Schlussfolgerung
Zwar kann sich durch eine MSC-Therapie die LVEF signifikant verbessern und die Rehospitalisierungsrate vermindern, aber sie hat keinen wesentlichen Einfluss auf das Auftreten eines kardiovaskulär bedingten Todes, eines Herzinfarkts, einer Herzinsuffizienz oder auf die Gesamtmortalität.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
It is well known that heart failure results in a significantly reduced quality of life, is a major societal burden, and has become a leading cause of mortality and morbidity [1,2,3]. Ischemic heart diseases account for two thirds of all cases of systolic heart failure [4]. Poor outcomes are determined by extensive myocardial remodeling and chamber enlargement, and currently there is lack of effective treatment [5,6,7]. Cardiac transplantation and mechanical circulatory support as destination therapy are high-risk therapeutic options that are limited by donor availability, patient eligibility, and costs [8].
Cell-based therapies have become the important paradigm-shifting alternatives [9,10,11]. However, clinical studies report the inconsistent efficacy of cell-based therapies, possibly ascribed to the unpredictable potency of cell products and their limited retention. Methods of cell therapy optimization include myocardial priming to improve cell homing, exploiting resident cell populations, and leveraging combined cell regimens [12,13,14,15]. Mesenchymal stem cells (MSCs) are reported to enhance cardioreparative functionality and induce a restorative response in failing hearts [16]. The Congestive Heart Failure Cardiopoietic Regenerative Therapy trial confirmed the efficacy and safety of cardiopoietic cells delivered via a retention-enhanced catheter for advanced symptomatic heart failure of ischemic etiology [17, 18].
However, the use of MSC treatment for heart failure has not been well established. Recently, several studies on the topic have been published, and the results have been conflicting [19,20,21,22]. Considering these inconsistent effects, we therefore conducted a systematic review and meta-analysis of randomized controlled trials (RCTs) to evaluate the efficacy of MSC treatment in patients with heart failure.
Methods
Ethical approval and patient consent were not required since this was a systematic review and meta-analysis of previously published studies. The systematic review and meta-analysis were conducted and reported in adherence to PRISMA guidelines (Preferred Reporting Items for Systematic Reviews and Meta-Analyses; [23]).
Search strategy and study selection
Two investigators independently searched the following databases (inception to May 2018): PubMed, Embase, and the Cochrane Register of Controlled Trials. The electronic search was performed using the following keywords: mesenchymal stem cell or MSC, and heart failure. We also checked the reference lists of the screened full-text studies to identify other potentially eligible trials.
The following selection criteria were applied: (a) population: patients with heart failure; (b) intervention: MSC treatment; (d) comparison: placebo treatment; and (d) study design: RCT. The exclusion criteria were acute coronary syndrome, valvular heart disease, and malignant tumor.
Data extraction and outcome measures
We used a piloted data-extraction sheet, which covered the following information: first author, number of patients, age, male, body mass index (BMI), New York Heart Association (NYHA) class, details of methods used in two groups. Data were extracted independently by two investigators, and discrepancies were resolved by consensus. We contacted the corresponding author to obtain data when necessary. No simplifications and assumptions were made.
The primary outcome was cardiovascular death. Secondary outcomes included left ventricular ejection fraction (LVEF), rehospitalization, myocardial infarction, the recurrence of heart failure, and total death.
Quality assessment in individual studies
The Jadad Scale was used to evaluate the methodological quality of each RCT included in this meta-analysis [24]. This scale consists of three evaluation elements: randomization (0–2 points), blinding (0–2 points), dropouts and withdrawals (0–1 points). One point is allocated to each element if they are mentioned in the article, and another one point is given if the methods of randomization and/or blinding are appropriately described. If the methods of randomization and/or blinding are inappropriate, or dropouts and withdrawals are not recorded, then one point is deducted. The Jadad Scale score varies from 0 to 5 points. An article with a Jadad score of ≤ 2 is considered to be of low quality. If the Jadad score is ≥ 3, the study is thought to be of high quality [25].
Statistical analysis
We estimated mean differences (MDs) with 95% confidence intervals (CIs) for continuous outcomes (LVEF), and risk ratios (RRs) with 95% CIs for dichotomous outcomes (cardiovascular death, rehospitalization, myocardial infarction, heart failure, and total death). Heterogeneity was tested using the Cochran Q statistic (p < 0.1) and quantified with the I2 statistic, which describes the variation of effect size that is attributable to heterogeneity across studies. An I2 value greater than 50% indicates significant heterogeneity. The value of the I2 statistic is used to select the appropriate pooling method: fixed-effects models are used for I2 < 50% and random-effects models for I2 > 50%. If significant heterogeneity was present, we searched for the potential sources of heterogeneity. Sensitivity analysis was performed to detect the influence of a single study on the overall estimate via omitting one study in turn when necessary. Owing to the limited number (<10) of included studies, publication bias was not assessed. Results were considered statistically significant at p < 0.05. All statistical analyses were performed using Review Manager Version 5.3 (The Cochrane Collaboration, Software Update, Oxford, UK).
Results
Literature search, study characteristics, and quality assessment
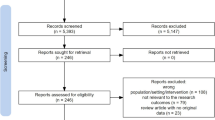
A detailed flowchart of the search and selection results is shown in Fig. 1. In total, 511 potentially relevant articles were identified initially. Finally, six RCTs that met our inclusion criteria were included in the meta-analysis [19,20,21,22, 26, 27].
The main characteristics of the six RCTs included in the meta-analysis are presented in Table 1. The six studies were published between 2013 and 2017, and sample sizes ranged from 30 to 371 with a total of 625. The approaches for MSC application included intravenous infusion, intracoronary transplantation, and intramyocardial injection.
Among the six RCTs, three studies reported cardiovascular death [19, 20, 22], two studies reported LVEF [20, 21], three studies reported rehospitalization [20,21,22], two studies reported myocardial infarction [19, 20], two studies reported the recurrence of heart failure [20, 26], and five studies reported total death [19,20,21, 26, 27]. The Jadad scores of the six included studies vary from 3 to 5, and all six studies are considered to be of high quality according to the quality assessment.
Primary outcome: cardiovascular death
The primary outcome data were analyzed with the fixed-effects model. The pooled estimate of the three included RCTs suggested that compared with control group for heart failure, MSC intervention had no significant influence on cardiovascular death (RR = 0.76; 95% CI = 0.38–1.52; p = 0.43), with low heterogeneity among the studies (I2 = 32%, heterogeneity p = 0.23, Fig. 2).
Sensitivity analysis
Low heterogeneity was observed among the included studies regarding cardiovascular death. Thus, we did not perform sensitivity analysis by omitting one study in turn to detect the source of heterogeneity.
Secondary outcomes
Compared with the control group, MSC treatment was associated with significantly increased LVEF (MD = 9.64; 95% CI = 7.56–11.71; p < 0.00001; Fig. 3) and reduced rehospitalization rates (RR = 0.41; 95% CI = 0.23–0.73; p = 0.003; Fig. 4), but it had no significant impact on the incidence of myocardial infarction (RR = 0.72; 95% CI = 0.10–5.02; p = 0.74; Fig. 5), the recurrence of heart failure (RR = 0.88; 95% CI = 0.28–2.81; p = 0.83; Fig. 6), and total death (RR = 0.68; 95% CI = 0.37–1.25; p = 0.21; Fig. 7).
Discussion
Stem cell therapy has been explored for the treatment for heart failure for more than a decade [20], but different stem cell populations and evaluation methods remain a challenge to fully understanding the efficacy of stem cell administration for clinical treatment [28]. Improvements in cardiac function and regeneration of damaged heart tissue are observed through various mechanisms including transdifferentiation, cell fusion, and paracrine modulation [29, 30]. Stem cell therapy is reported to be safe and to offer patients with heart failure moderate benefits in survival, left ventricular function, and quality of life [31, 32]. Cell-based therapy for chronic ischemic and nonischemic disease involves a range of cellular products and delivery routes including autologous or allogenic bone marrow mononuclear cells and MSC administered by intramyocardial injections, percutaneous intracoronary infusion, and exceptionally peripheral intravenous infusion [31, 33, 34].
Several mechanisms may explain the clinical benefit of MSCs treatment for patients with heart failure, including reductions in myocardial cell apoptosis, modulation of inflammation, myocardial fibrosis, neovascularization, and increased cell differentiation [35]. Incorporation of MSCs into tissues involves multiple processes consisting of cell recruitment, migration, and adhesion [36]. Umbilical cord MSCs have a high migration capacity and have shown a good response to serum in heart failure patients, thus this cell type might sense biological cues mediating the therapeutic effect by systemic delivery [20]. Our meta-analysis suggests that compared with control interventions for heart failure, MSC treatment is associated with significantly improved LVEF and reduced rehospitalization rates, but with no significant influence on cardiovascular death.
Clinical trials have validated the safety of MSC-based therapies, with no increase observed in acute infusion toxicity, organ system complications, infection, death, or malignancy in treated patients [37, 38]. A phase 2 study confirmed the safety of intravenous administration of allogeneic MSCs (up to 5 × 106 cells/kg) in patients with acute myocardial infarction, as evidenced by no increase in adverse event rate and a decrease in hospitalization rate and arrhythmic events at the 6‑month follow-up [38]. In addition, the safety of the intravenous administration of ischemia-tolerant allogeneic MSCs has been evaluated in patients with nonischemic cardiomyopathy, and the results revealed no increase in death, hospitalizations, and serious adverse events at the 90-day follow-up [39]. There is no statistically significant difference in the incidence of myocardial infarction, the recurrence of heart failure, and total death between MSC treatment and control intervention for heart failure patients based on the results of this meta-analysis.
Limitations
This meta-analysis has several potential limitations that should be taken into account. First, our analysis is based on only six RCTs and five of them have a modest sample size (n < 100). Overestimation of the treatment effect is more likely in smaller trials compared with larger samples. Next, the trials involved allogeneic and autologous MSCs as well as umbilical cord MSCs administered by intravenous infusion, intracoronary transplantation, and intramyocardial injection in included RCTs, and these different cellular products and delivery routes may have some impact on the pooled results. Finally, the optimal cell sources and doses as well as the delivery routes remain undefined and future studies should focus on these issues.
Practical conclusion
MSC treatment can provide some benefits for patients with heart failure.
References
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS et al (2016) ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Eur Heart J 2016(37):2129–2200 (Developed with the special contribution of the Heart Failure Association (HFA) of the ESC)
Anand I (2018) Stable but progressive nature of heart failure: considerations for primary care physicians. Am J Cardiovasc Drugs. https://doi.org/10.1007/s40256-018-0277-0
O’Meara E, Prescott MF, Claggett B, Rouleau JL, Chiang LM, Solomon SD et al (2018) Independent Prognostic Value of Serum Soluble ST2 Measurements in Patients With Heart Failure and a Reduced Ejection Fraction in the PARADIGM-HF Trial. Circ Heart Fail 11. https://doi.org/10.1161/CIRCHEARTFAILURE.117.004446 (Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure)
Writing Group M, Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ et al (2016) Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation 133:e38–e360
Kramer DG, Trikalinos TA, Kent DM, Antonopoulos GV, Konstam MA, Udelson JE (2010) Quantitative evaluation of drug or device effects on ventricular remodeling as predictors of therapeutic effects on mortality in patients with heart failure and reduced ejection fraction: a meta-analytic approach. J Am Coll Cardiol 56:392–406
McCutcheon K, Manga P (2018) Left ventricular remodelling in chronic primary mitral regurgitation: implications for medical therapy. Cardiovasc J Afr 29:51–65
Cvijic M, Duchenne J, Unlu S, Michalski B, Aarones M, Winter S et al (2017) Timing of myocardial shortening determines left ventricular regional myocardial work and regional remodelling in hearts with conduction delays. Eur Heart J Cardiovasc Imaging 19(8):941–949. https://doi.org/10.1093/ehjci/jex325
Braunwald E (2015) The war against heart failure: the Lancet lecture. Lancet 385:812–824
Menasche P (2018) Cell therapy trials for heart regeneration—lessons learned and future directions. Nat Rev Cardiol. https://doi.org/10.1038/s41569-018-0013-0
Galvez-Monton C, Soler-Botija C, Iborra-Egea O, Diaz-Guemes I, Marti M, Iglesias-Garcia O et al (2017) Preclinical safety evaluation of allogeneic induced pluripotent stem cell-based therapy in a swine model of myocardial infarction. Tissue Eng Part C Methods 23:736–744
Le TY, Thavapalachandran S, Kizana E, Chong JJ (2017) New developments in cardiac regeneration. Heart Lung Circ 26:316–322
Terzic A, Behfar A (2014) Regenerative heart failure therapy headed for optimization. Eur Heart J 35:1231–1234
Assmus B, Walter DH, Seeger FH, Leistner DM, Steiner J, Ziegler I et al (2013) Effect of shock wave-facilitated intracoronary cell therapy on LVEF in patients with chronic heart failure: the CELLWAVE randomized clinical trial. JAMA 309:1622–1631
Malliaras K, Makkar RR, Smith RR, Cheng K, Wu E, Bonow RO et al (2014) Intracoronary cardiosphere-derived cells after myocardial infarction: evidence of therapeutic regeneration in the final 1‑year results of the CADUCEUS trial (CArdiosphere-Derived aUtologous stem CElls to reverse ventricUlar dySfunction). J Am Coll Cardiol 63:110–122
Heldman AW, DiFede DL, Fishman JE, Zambrano JP, Trachtenberg BH, Karantalis V et al (2014) Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: the TAC-HFT randomized trial. JAMA 311:62–73
Terzic A, Behfar A (2016) Stem cell therapy for heart failure: ensuring regenerative proficiency. Trends Cardiovasc Med 26:395–404
Behfar A, Latere JP, Bartunek J, Homsy C, Daro D, Crespo-Diaz RJ et al (2013) Optimized delivery system achieves enhanced endomyocardial stem cell retention. Circ Cardiovasc Interv 6:710–718
Bartunek J, Davison B, Sherman W, Povsic T, Henry TD, Gersh B et al (2016) Congestive heart failure cardiopoietic regenerative therapy (CHART-1) trial design. Eur J Heart Fail 18:160–168
Bartunek J, Terzic A, Davison BA, Filippatos GS, Radovanovic S, Beleslin B et al (2017) Cardiopoietic cell therapy for advanced ischaemic heart failure: results at 39 weeks of the prospective, randomized, double blind, sham-controlled CHART-1 clinical trial. Eur Heart J 38:648–660
Bartolucci J, Verdugo FJ, Gonzalez PL, Larrea RE, Abarzua E, Goset C et al (2017) Safety and Efficacy of the Intravenous Infusion of Umbilical Cord Mesenchymal Stem Cells in Patients With Heart Failure: A Phase 1/2 Randomized Controlled Trial. Circ Res 121:1192–1204 (RIMECARD Trial [Randomized Clinical Trial of Intravenous Infusion Umbilical Cord Mesenchymal Stem Cells on Cardiopathy])
Zhao XF, Xu Y, Zhu ZY, Gao CY, Shi YN (2015) Clinical observation of umbilical cord mesenchymal stem cell treatment of severe systolic heart failure. Genet Mol Res 14:3010–3017
Perin EC, Borow KM, Silva GV, DeMaria AN, Marroquin OC, Huang PP et al (2015) A phase II dose-escalation study of allogeneic mesenchymal precursor cells in patients with ischemic or nonischemic heart failure. Circ Res 117:576–584
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ et al (1996) Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials 17:1–12
Kjaergard LL, Villumsen J, Gluud C (2001) Reported methodologic quality and discrepancies between large and small randomized trials in meta-analyses. Ann Intern Med 135:982–989
Mathiasen AB, Qayyum AA, Jorgensen E, Helqvist S, Fischer-Nielsen A, Kofoed KF et al (2015) Bone marrow-derived mesenchymal stromal cell treatment in patients with severe ischaemic heart failure: a randomized placebo-controlled trial (MSC-HF trial). Eur Heart J 36:1744–1753
Bartunek J, Behfar A, Dolatabadi D, Vanderheyden M, Ostojic M, Dens J et al (2013) Cardiopoietic stem cell therapy in heart failure: the C‑CURE (Cardiopoietic stem Cell therapy in heart failURE) multicenter randomized trial with lineage-specified biologics. J Am Coll Cardiol 61:2329–2338
Nesselmann C, Kaminski A, Steinhoff G (2011) Cardiac stem cell therapy. Registered trials and a pilot study in patients with dilated cardiomyopathy. Herz 36:121–134
Williams AR, Hare JM (2011) Mesenchymal stem cells: biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ Res 109:923–940
Nazari-Shafti TZ, Kempfert J, Falk V, Roll W, Stamm C (2018) Regenerative medicine/cardiac cell therapy: adult/somatic progenitor cells. Thorac Cardiovasc Surg 66:42–52
Jeevanantham V, Butler M, Saad A, Abdel-Latif A, Zuba-Surma EK, Dawn B (2012) Adult bone marrow cell therapy improves survival and induces long-term improvement in cardiac parameters: a systematic review and meta-analysis. Circulation 126:551–568
Gho JM, Kummeling GJ, Koudstaal S, Jansen Of Lorkeers SJ, Doevendans PA, Asselbergs FW et al (2013) Cell therapy, a novel remedy for dilated cardiomyopathy? A systematic review. J Card Fail 19:494–502
Kandala J, Upadhyay GA, Pokushalov E, Wu S, Drachman DE, Singh JP (2013) Meta-analysis of stem cell therapy in chronic ischemic cardiomyopathy. Am J Cardiol 112:217–225
Fisher SA, Doree C, Mathur A, Martin-Rendon E (2015) Meta-analysis of cell therapy trials for patients with heart failure. Circ Res 116:1361–1377
Gong X, Wang P, Wu Q, Wang S, Yu L, Wang G (2016) Human umbilical cord blood derived mesenchymal stem cells improve cardiac function in cTnT(R141W) transgenic mouse of dilated cardiomyopathy. Eur J Cell Biol 95:57–67
Huang J, Zhang Z, Guo J, Ni A, Deb A, Zhang L et al (2010) Genetic modification of mesenchymal stem cells overexpressing CCR1 increases cell viability, migration, engraftment, and capillary density in the injured myocardium. Circ Res 106:1753–1762
Lalu MM, McIntyre L, Pugliese C, Fergusson D, Winston BW, Marshall JC et al (2012) Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. PLoS ONE 7:e47559
Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP et al (2009) A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol 54:2277–2286
Butler J, Epstein SE, Greene SJ, Quyyumi AA, Sikora S, Kim RJ et al (2017) Intravenous allogeneic mesenchymal stem cells for nonischemic cardiomyopathy: safety and efficacy results of a phase II-a randomized trial. Circ Res 120:332–340
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
H. Fu and Q. Chen declare that they have no competing interests.
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Fu, H., Chen, Q. Mesenchymal stem cell therapy for heart failure: a meta-analysis. Herz 45, 557–563 (2020). https://doi.org/10.1007/s00059-018-4762-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00059-018-4762-7