Abstract
Background
Transesophageal echocardiography (TEE) plays a unique role in transcatheter closure of atrial septal defects (ASD) and patent foramen ovale (PFO). However, problems such as the need for general anesthesia, possible trauma from endotracheal intubation, presence of “blind spots,” and occasional inadequate imaging of some cardiac structures have necessitated better imaging techniques. Our study aimed to compare the findings of TEE during the initial diagnostic examination with those from intracardiac echocardiography (ICE) acquired during the interventional procedure.
Methods
A total of 65 patients in whom TEE was used for the diagnosis of ASD or PFO were included. Of these, 40 patients (61.5%) had ASD with significant left to right shunt and 25 (38.5%) patients had PFO associated with transient ischemic attack or stroke. ICE imaging was performed under local anesthesia in all patients to guide interatrial communication closure.
Results
ICE provided adequate views of the defects and surrounding structures during the various stages of device deployment. In eight patients (12.3%) an additional anatomical variation was detected. All patients had successful device implantation and were discharged 1 day after the procedure.
Conclusion
ICE is a safe and high-quality imaging technique for guiding transcatheter ASD and PFO occlusion. Additionally, ICE can both facilitate device implantation and detect cardiac abnormalities that are not identified with TEE during the initial diagnostic investigation.
Zusammenfassung
Hintergrund
Die transösophageale Echokardiographie (TEE) ist von besonderer Bedeutung beim kathetergestützten Verschluss eines Vorhofseptumdefekts („atrial septal defect“, ASD) oder eines offenen Foramen ovale („patent foramen ovale“, PFO). Jedoch wurden durch Probleme wie die Notwendigkeit einer Allgemeinnarkose, mögliche Verletzungen durch die endotracheale Intubation, das Vorhandensein „blinder Flecke“ und die gelegentlich unzureichende Darstellung einiger kardialer Strukturen bessere Bildgebungsverfahren erforderlich. Die vorliegende Studie zielte darauf ab, die Befunde der TEE während der diagnostischen Eingangsuntersuchung mit den Befunden der während der Intervention durchgeführten intrakardialen Echokardiographie (ICE) zu vergleichen.
Methoden
Insgesamt wurden 65 Patienten, bei denen die TEE zur Diagnosestellung eines ASD oder PFO verwendet wurden, in die Studie aufgenommen. Davon wiesen 40 Patienten (61,5%) einen ASD mit erheblichem Links-rechts-Shunt auf, bei 25 (38,5%) Patienten bestand ein PFO, das mit transienten ischämischen Attacken oder einem Schlaganfall einherging. Die ICE-Bildgebung wurde bei allen Patienten unter Lokalanästhesie durchgeführt, um den Verschluss der Verbindung zwischen den beiden Vorhöfen zu steuern.
Ergebnisse
Die ICE-Bildgebung lieferte ausreichende Ansichten der Defekte und der umgebenden Strukturen während der verschiedenen Stadien der Intervention. Bei 8 Patienten (12,3%) wurde eine zusätzliche anatomische Variante entdeckt. Bei allen Patienten war die Implantation des Systems erfolgreich, sie wurden einen Tag nach der Intervention entlassen.
Schlussfolgerung
Die ICE ist ein sicheres Bildgebungsverfahren von hoher Qualität für den kathetergestützten Verschluss eines ASD oder eines PFO.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Percutaneous transcatheter occlusion of atrial septal defects (ASD) and patent foramen ovale (PFO) is less invasive than the gold standard of surgical closure [1]. Indications for this emerging therapeutic option are recurrent cryptogenic stroke due to presumed paradoxical embolism through the PFO after failure of conventional drug therapy [2]. The indications for surgical ASD closure are significant right chamber enlargement with hemodynamically significant shunts (Qp/Qs >1.5) in patients with normal pulmonary resistance, and a residual tissue rim of interatrial septum surrounding the defect greater than 5 mm [3].
Different devices have been used for the invasive management of these conditions. Among these, the Amplatzer Occluder is most widely used. This is a self-expanding wire mesh with double discs. It contains inner polyester fabric patches that, along with the wire mesh, cause the formation and accumulation of a blood clot, which seals the opening. Following successful deployment of the device, tissue grows over it and the device becomes incorporated into the atrial septum [4].
Transesophageal echocardiography (TEE) has been successfully employed to guide transcatheter device closure of ASD and PFO [5]. Over the past few years, specially designed ultrasound-tipped catheters have made intracardiac imaging possible. Specifically, intracardiac echocardiography (ICE) provides increased patient comfort and improved imaging quality of the interatrial septum during percutaneous occlusion of PFO and ASD. Other advantages include clear visualization of the inner chamber cardiac wall and imaging of cardiac structures in adequate depth [6]. The recently designed ultrasound-tipped catheters have also incorporated Doppler imaging. As a result, ICE is preferred over TEE for imaging guidance during intra-atrial septal defect closure [7].
In this study, we report our experience with ICE during PFO and ASD closure procedures by comparing the imaging findings of ICE with those of TEE during the initial diagnostic investigation.
Methods
Study population
A total of 66 consecutive patients who were admitted to our hospital for percutaneous closure of ASD and PFO were enrolled from November 2008 to November 2010. Of these, one patient was excluded owing to a recent intracerebral hemorrhage and thus the final analysis included 65 patients. Our inclusion criteria were: (a) significant secundum ASD (large-diameter defect [>10 mm] with significant left-to-right shunting [Qp/Qs >1.5]); (b) PFO associated with previous cryptogenic stroke due to presumed paradoxical embolism through the PFO with contraindications to anticoagulant treatment; (c) PFO associated with coexisting atrial septal aneurysm; and (d) PFO in professional divers. The diagnostic criteria for atrial septal aneurysm encompassed the detection of a sacculation or deformity in the interatrial septum or the foramen ovale region with an excursion of 10 mm into the right or left atrium or with the sum of bilateral excursions measuring more than 10 mm. All patients underwent TEE with a multiplane probe (GE Vivid-7) to confirm the diagnosis, to define the size, the location, and the number of ASDs as well as to rule out other associated abnormalities (anomalous pulmonary vein drainage). Patients with left atrial or left ventricular thrombus were excluded from our study. All patients underwent TEE evaluation at the referring center by experienced operators.
Closure device
The Amplatzer ASD or PFO occluder (AGA Medical, Golden Valley, Minn.), which consists of two self-expanding Nitinol discs joined together by a central waist, was used. Dacron patches were embedded within the two discs to promote internal thrombus formation and eventual endocardialization. The device was sized so that the central waist completely occluded the defect. The diameter of the device used for patients with ASD was selected according to the size of the defect, which was measured by an occlusion balloon.
Imaging technique
ICE imaging was performed using the AcuNav, an 8‑Fr ultrasound-tipped catheter (Siemens Medical Solutions, Erlangen, Germany). AcuNav offers 90o sector imaging and it has a changeable ultrasound frequency (5.5, 7.5, 8.5, 10 MHz) depending on the applied console, and a tissue penetration capacity of 15 cm. The catheter can be externally manipulated and is capable of a four-way tip articulation inside the cardiac chambers. The tension control knob secures the catheter in the desired position. Moreover, AcuNav has Doppler capabilities for further information on blood flow and velocity. Additional features of the AcuNav are continuous wave (CW) Doppler for quantification of flow with an imaging frequency of 5.0 MHz and pulsed wave (PW) Doppler for targeted blood flow examination. PW imaging frequencies range between 5.0 MHz and 4.0 MHz.
Procedure
The AcuNav catheter was advanced through an 8‑Fr, 25-mm sheath in the mid-right atrium under fluoroscopic guidance. In all patients with ASD, the defect size and location, the pulmonary veins, the adequacy of the septal rims, and the adjacent structures were assessed. In patients with PFO, the interatrial shunt was confirmed by the presence of contrast in the left atrium during the Valsalva maneuver. Specifically, 10 ml of agitated normal saline was injected through a 7-Fr sheath in the femoral vein. Thereafter, color Doppler examination of the septum was performed for further scanning and potential detection of additional defects.
Wire insertion across the defect was facilitated by ICE. Finally, the wire was placed into the left pulmonary vein with the use of a multipurpose catheter. The Amplatzer guiding catheter device was introduced via the right or left femoral vein through a long sheath. For ASD closure, the diameter of the stop flow-balloon across the defect was measured by ICE and the size of the closure device was selected accordingly. Closure of the defect was performed according to the standard protocol. During device deployment, alignment of the device, its relation to neighboring structures, especially atrioventricular valves, and capture of the septum by the device were closely observed in different planes. The procedure was completed with contrast injection and color Doppler interrogation to confirm the absence of periprosthetic leak and to verify the successful closure of the defect.
During the procedure, all patients received heparin. Postprocedurally, patients received dual antiplatelet therapy with aspirin and clopidogrel for 3 months and aspirin only for another 3 months. All patients were followed up at 3‑ and 6‑month timepoints. Prophylactic antibiotic treatment was initiated prior to the intervention and was continued for 6 months after the procedure.
Results
The study enrolled 65 patients (44 [67.7%] males; mean age, 45.5 years). Of these patients, 40 (61.5%) had ASD while 25 (38.5%) had PFO. The clinical characteristics of each group are presented in Tables 1 and 2, respectively. Patients with ASD were older and had more comorbidities compared with patients in the PFO group. The mean Qp/Qs ratio for patients with secundum ASD was 1.5. More than 60% of the patients with PFO also had an atrial septal aneurysm.
TEE and ICE imaging
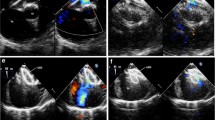
The mean two-dimensional size of secundum defects as detected by TEE was 18.3 ± 7.5 mm while its measurement by ICE showed a mean size of 20 ± 3.4 mm (p = 0.003). Additionally, ICE revealed a Chiari network with thrombus in one patient (1.5%) and additional septal defects in four patients (6.2%)—two (8%) with PFO and two (5%) with ASD—that were not seen with TEE. Interestingly, in three patients, after microbubble infusion, ICE did not reveal PFO as was suggested by TEE imaging. No ICE-related complications were observed in our cohort.
Patients with PFO
The 25-mm device was used in 21 (84%) patients while the 30-mm cribriform device was used in three patients with a large atrial septal aneurysm. Finally, the 18-mm device was employed in one patient with a small PFO.
Patients with ASD
The mean balloon-stretched diameter ranged from 22 to 34 mm while the size of the Amplatzer device that was used ranged from 25 to 38 mm. In two patients with ASD, a small residual shunt remained. Mean procedural time was 44 ± 23 min. There were no procedure-related complications. The mean length of hospital stay was 1 day. At the 6‑month follow-up, TEE did not reveal any complications.
Discussion
In our study, we describe our experience with ICE for guiding percutaneous closure of ASD and PFO in patients with TEE evaluation. Furthermore, we report the findings that were observed by using exclusively ICE for imaging guidance during the procedure. ICE was superior in identifying defects that were not detectable by TEE. Its use also modified and facilitated the technique for the deployment and positioning of the device. An advantage of ICE is that no general anesthesia is needed, thereby reducing the risk associated with the procedure.
Both TEE and ICE are safe and efficient for guiding PFO or ASD closure, each with their own advantages and disadvantages. For example, TEE has no risk of intracardiac injury and does not require additional venous access, thereby minimizing the risk of bleeding at the site of vascular access. Although TEE is an economical approach, it needs specialized staff with good rapport between the echocardiographer and the interventionalist. It also requires sedation and intubation of the patients. By contrast, in ICE there is no need for a multi-member team to obtain and interpret the images. The interventionalist has control over the images, which makes the procedure easier and less time consuming. Additionally, ICE offers the opportunity to visualize the atrial septum from the superior vena cava entrance to the inferior portion of the septum, the pulmonary veins, the left ventricle, and the mitral valve (Table 3). For atrial defects located in the inferior portion of the septum, ICE is superior to TEE in terms of visualization [8]. Highly detailed images from the area of implantation and information on the relationship between the device and the septum cannot be easily obtained with TEE [9]. Despite the fact that TEE is equipped with multiplane imaging, it fails to depict the complete interatrial septum from another point of view. Although additional maneuverability is possible, the images obtained are less detailed than those obtained by ICE [10]. By contrast, although ICE does not allow for multiplane imaging, its flexible probe facilitates viewing the septum from different angles [11]. This feature of ICE enables imaging of the inferior portion of the interatrial septum, which is a crucial part in interatrial communication procedures [12]. Moreover, a limitation of the TEE are the artifacts produced by the air in the esophagus, trachea, and stomach. Another important advantage of ICE is that percutaneous interatrial septal defect closure under ICE guidance results in shorter procedural time, faster patient turnaround time, and reduced radiation exposure time [13].
Regarding complications related to the imaging techniques, TEE appears to be associated with more trauma complications as well as the need for sedation. This is the main reason for its contraindication in patients with a history of dysphagia or esophageal disease. The risks of ICE imaging, by contrast, have been reported to be smaller and suggested to be similar to the risk of electrophysiological studies. Nevertheless, a concern of ICE imaging is the possibility of vascular injury caused by placing an 8‑Fr sheath to allow for ICE catheter access. This is especially the case in younger children.
Although the equipment used for ICE and TEE share similar characteristics such as similar basic ultrasound platforms, not all ultrasound platforms are commercially available in ICE. The frequency of TEE probes is 4–7 MHz using a Philips OmniPlan TEE probe. The frequency of the ICE probe ranges from 6 to 7, 5 to 8, and 5.5 to 10 MHz using the Siemens Cypress, Aspen, and Sequoia imaging platforms respectively. Thus, ICE imaging appears to be of higher resolution.
Despite the superior role of ICE in guiding the interatrial communication procedures, it is less adequate for visualization of aspects of the cardiac anatomy other than the atrial septum and immediate surrounding structures. Thus, TEE is probably a more suitable method for assessing the cardiac anatomy preprocedurally. As a result, TEE remains a valuable diagnostic imaging tool.
Study limitations
Our study is a single-center, retrospective study with a small sample size.
Conclusion
ICE is an excellent guide in ASD and PFO occluding procedures as it facilitates a detailed investigation of the site of device implantation. However, ICE is not a simple tool and can require a definite learning curve to be used with confidence.
References
Hijazi Z, Wang Z, Cao Q, Koenig P, Waight D, Lang R (2001) Transcatheter closure of atrial septal defects and patent foramen ovale under intracardiac echocardiographic guidance: feasibility and comparison with transesophageal echocardiography. Catheter Cardiovasc Interv 52:194–199
Qureshi AM, Latson LA (2010) Recent advances in closure of atrial septal defects and patent foramen ovale. F1000 Med Rep 2. https://doi.org/10.3410/m2-8
Ebeid MR (2002) Percutaneous catheter closure of secundum atrial septal defects: a review. J Invasive Cardiol 14:25–31
Masura J, Gavora P, Formanek A, Hijazi ZM (1997) Transcatheter closure of secundum atrial septal defects using the new self-centering amplatzer septal occluder: initial human experience. Cathet Cardiovasc Diagn 42:388–393
Daniel WG, Erbel R, Kasper W et al (1991) Safety of transesophageal echocardiography. A multicenter survey of 10,419 examinations. Circulation 83:817–821
Boccalandro F, Baptista E, Muench A, Carter C, Smalling RW (2004) Comparison of intracardiac echocardiography versus transesophageal echocardiography guidance for percutaneous transcatheter closure of atrial septal defect. Am J Cardiol 93:437–440
Bruce CJ, Nishimura RA, Rihal CS et al (2002) Intracardiac echocardiography in the interventional catheterization laboratory: preliminary experience with a novel, phased-array transducer. Am J Cardiol 89:635–640
Koenig PR, Abdulla RI, Cao QL, Hijazi ZM (2003) Use of intracardiac echocardiography to guide catheter closure of atrial communications. Echocardiography 20:781–787
Mullen MJ, Dias BF, Walker F, Siu SC, Benson LN, McLaughlin PR (2003) Intracardiac echocardiography guided device closure of atrial septal defects. J Am Coll Cardiol 41:285–292
Seward JB, Khandheria BK, Oh JK, Freeman WK, Tajik AJ (1992) Critical appraisal of transesophageal echocardiography: limitations, pitfalls, and complications. J Am Soc Echocardiogr 5:288–305
Zanchetta M, Rigatelli G, Pedon L, Zennaro M, Maiolino P, Onorato E (2003) Role of intracardiac echocardiography in atrial septal abnormalities. J Interv Cardiol 16:63–77
Bartel T, Konorza T, Arjumand J et al (2003) Intracardiac echocardiography is superior to conventional monitoring for guiding device closure of interatrial communications. Circulation 107:795–797
Alboliras ET, Hijazi ZM (2004) Comparison of costs of intracardiac echocardiography and transesophageal echocardiography in monitoring percutaneous device closure of atrial septal defect in children and adults. Am J Cardiol 94:690–692
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
C. Kavvouras, M. Vavuranakis, S. Vaina, K. Lampropoulos, G. Bazoukis, G. Tse, and D. Tousoulis declare that they have no competing interests.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975 (in its most recently amended version). Informed consent was obtained from all patients included in the study.
Rights and permissions
About this article
Cite this article
Kavvouras, C., Vavuranakis, M., Vaina, S. et al. Intracardiac echocardiography for percutaneous patent foramen ovale and atrial septal defect occlusion. Herz 44, 445–449 (2019). https://doi.org/10.1007/s00059-017-4678-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00059-017-4678-7
Keywords
- Cardiac septal defects
- Patent foramen ovale
- Intracardiac imaging techniques
- Transesophageal echocardiography
- Percutaneous coronary intervention