Abstract
Objective
The purpose of this work was to compare fluoride release from three orthodontic adhesives and fluoride penetration into the enamel surface.
Materials and methods
A total of 156 extracted human premolar teeth were randomly assigned to three experimental groups and one control group (without bonding) with 39 teeth per group. Brackets were bonded to teeth using Fuji Ortho LC®, Illuminate®, or Light Bond®. The amount of fluoride released (ppm) into artificial saliva was measured by a fluoride ion-selective electrode connected to an ion analyzer on days 1, 3, 7, and 30. Fluoride penetration was investigated after 1, 2, and 3 months; 13 teeth of each group were randomly selected at every period of study and sectioned across the center of the bracket. The surface of the cross-section was studied under the scanning electron microscope, and the fluoride concentration (weight%) at 1, 2, and 3 µm below the outer enamel surface was determined by energy-dispersive X-ray microanalysis.
Results
On days 1, 3, 7, and 30, the mean cumulative fluoride release from the three orthodontic adhesives were significantly different (p < 0.05). Illuminate® released the greatest fluoride, followed by Fuji Ortho LC® and Light Bond®. After 1, 2, and 3 months, fluoride penetration into enamel was only found from Fuji Ortho LC®. The fluoride concentration decreased with depth but there were no significant differences (p > 0.05) over time at all depths.
Conclusions
The in vitro study indicated that fluoride release is a common property of the three fluoride-releasing orthodontic adhesives: Illuminate®, Fuji Ortho LC®, and Light Bond®. However, detectable fluoride penetration is a specific property of Fuji Ortho LC®. Further clinical studies should be undertaken to investigate the benefit of the two adhesives Illuminate® and Fuji Ortho LC® on protection of enamel demineralization.
Zusammenfassung
Zielsetzung
Ziel der Arbeit war der Vergleich der Fluoridfreisetzung und Penetration in die Zahnschmelzoberfläche bei 3 verschiedenen kieferorthopädischen Adhäsiven.
Material und Methoden
Insgesamt 156 extrahierte humane Prämolaren wurden randomisiert einer von 3 experimentellen Gruppen bzw. einer Kontrollgruppe (ohne Bonding) zugeordnet (jeweils n = 39). Das Bonding der Brackets erfolgte mittels Fuji Ortho LC®, Illuminate® oder Light Bond®. Die Menge des freigesetzten Fluorids (ppm) wurde in Kunstspeichel mittels einer Fluor-selektiven Elektrode und per Ionenanalyse an den Tagen 1, 3, 7 und 30 bestimmt. Die Penetration wurde nach 1, 2 und 3 Monaten ermittelt. Zu jedem Zeitabschnitt der Untersuchung wurden aus jeder Gruppe je 13 Zähne randomisiert ausgewählt. Es wurden jeweils auf Höhe der Bracketmitte Schnitte hergestellt. Die Oberfläche des Querschnitts wurde rasterelektronenmikroskopisch untersucht, und die Fluoridkonzentration (Gewichtsprozent) 1, 2 und 3 µm unter der Zahnschmelzoberfläche wurde mit Hilfe energiedispersiver Röntgenmikroanalyse bestimmt.
Ergebnisse
Die durchschnittliche kumulative Fluoridfreisetzung aus den 3 Adhäsiven war an den Tagen 1, 3, 7 und 30 signifikant unterschiedlich (p < 0,05). Von Illuminate® wurde die höchste Fluoridmenge freigesetzt, gefolgt von Fuji Ortho LC® und Light Bond®. Zu den Zeitpunkten 1, 2 und 3 Monate nach Bonding zeigte sich eine Penetration in den Zahnschmelz nur bei Fuji Ortho LC®. Die Fluoridkonzentration nahm mit der Tiefe ab, über die Zeit dagegen wurden zwischen den Tiefen keine signifikanten Unterschiede (p > 0,05) nachgewiesen.
Schlussfolgerungen
Die In-vitro-Studie zeigt, dass alle 3 verwendeten Adhäsive—Illuminate®, Fuji Ortho LC®, and Light Bond®—Fluorid freisetzen. Eine messbare Penetration von Fluor in den Schmelz dagegen ist eine spezifische Eigenschaft von Fuji Ortho LC®. Zur Erforschung eines möglichen Benefits der beiden Adhäsive Illuminate® und Fuji Ortho LC® für den Schutz vor Demineralisation des Zahnschmelzes sollten weitere klinische Studien durchgeführt werden.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A common problem during fixed orthodontic appliance treatment is enamel demineralization, associated with plaque accumulation and a poor natural self- cleaning mechanism due to surface irregularity of the appliances [4, 30]. Several studies [17, 23, 24] have reported a significant increase in the prevalence and severity of demineralization after fixed appliance therapy: when compared with controls, the overall prevalence of demineralization among orthodontic patients ranged from 2 to 96%.
Protective measures such as oral hygiene instruction, mechanical removal of the plaque, and application of topical fluoride agents are prescribed, but their effectiveness in prevention of demineralization was limited [18]. Geiger et al. [16] found that only 42% of patients rinsed with a sodium fluoride mouth-rinse at least every other day. To solve this problem, fluoride-releasing orthodontic adhesives comprising glass ionomer cement (GIC) and resin composite are suggested, assuming that delivery of the fluoride from these materials to the area close to the bracket can prevent enamel demineralization without patient cooperation.
Glass ionomer cements (GICs) have been shown to be effective for fluoride release and minimizing demineralization, but their bond strength was less than that of composite resins [21, 25]. Resin-modified GICs (RMGICs) can overcome the problem of GICs bond strength. The bond strength of RMGICs, in response to shear and tensile forces, was almost double that of conventional GICs and four times the minimum bond strength (8.5 MPa) suggested for successful orthodontic bonding [19, 20]. In addition, RMGICs could release fluoride over the long term; the amount of released fluoride was comparable with conventional GICs [33]. The GICs and RMGICs can be also recharged with fluoride from the oral environment and subsequently release it. This fluoride recharge property is clinically useful because it can occur after periodic use of fluoride, such as from fluoridated toothpaste or fluoride mouthwash, thus, promoting clinical peribracket protection [10].
Composite resins are the most common adhesive used for direct orthodontic bonding because of their acceptable bond strength and ease of application, but enamel demineralization surrounding the bracket is a significant problem. To solve this, attempts have been made to incorporate fluoride into the composite resins but the quantity and duration of fluoride release are poor [6, 15]. Polyacid-modified resin composites, known as compomers, have intermediate composition and properties compared to those of GICs/RMGICs and composite resins [1].
Previous studies have presented evidence of fluoride release from some fluoride-releasing orthodontic adhesives. However, the evidence of fluoride penetration into human enamel from these adhesives is limited. Therefore, further studies to determine both fluoride release from orthodontic adhesives and fluoride penetration into enamel are needed to clarify their potential protection against enamel demineralization. Thus, the objectives of this laboratory study were to investigate the amount of fluoride release from three fluoride-releasing orthodontic adhesives up to 1 month and to examine fluoride penetration into the enamel at 1, 2, and 3 months after bonding.
Materials and methods
A total of 156 extracted human permanent premolar teeth were collected after the approval by Chulalongkorn University’s Ethical Committee. The teeth were macroscopically free of caries, enamel defects (white spot lesions) and restorations. Before the experiment they were kept in 0.1% thymol at 25 °C. The experiment started with cleaning the teeth, cutting them at 5 mm below the cemento-enamel junction (CEJ) with a low speed cutting machine (ISOMET 1000, Buehler, Lake Bluff, IL, USA), and polished with nonfluoride pumice. The teeth were divided randomly into four groups (39 teeth per group) for bonding with Fuji Ortho LC® (GC Corporation, Tokyo, Japan), Illuminate® (Ortho Organizer, Carlsbad, CA, USA), Light Bond® (Reliance Orthodontic Products, Itasca, IL, USA) (Table 1), and a control group (without bonding).
In three experimental groups, a metal bracket (universal bracket, upper bicuspid; Ormco Corporation, Orange, CA, USA) was randomly bonded to the middle of the buccal enamel surface of each tooth following the manufacturer’s instruction. The amount of adhesive was indirectly controlled by using a stress and tension gauge (GreenDentalOrtho, Guangdong, China) applying pressure of 300 g force [2, 3]; the excess adhesive around the bracket base was removed before polymerization with a LED Curing Light (Kerr Corporation, Orange, CA, USA). After bonding, each tooth was stored individually in 2 ml of non-fluoride artificial saliva in a plastic container at 37 °C. (artificial saliva formula: KCl 0.75 g, MgCl2 0.07 g, CaCl2 0.199 g, K2HPO4 0.965 g, KH2PO4 0.439 g, sorbitol 70% BP 36 g, deionized water 1200 ml).
Prior to measuring fluoride release, an ion analyzer (QI518C, Q-I-S, the Netherlands) and fluoride ion-selective electrode (Model SL518, Select Bioscience, England) were calibrated with a series of standard fluoride solutions (0.01, 0.1, 1, 10 ppm). At every period of study (days 1, 3, 7, and 30), 2 ml of artificial saliva in each container was taken and total ionic strength adjustment buffer (TISAB III, Thermo Fisher Scientific, Chelmsford, MA, USA) was added before measuring fluoride release (in ppm). After measurement, the old artificial saliva in each container was changed and renewed with 2 ml of fresh artificial saliva.
The fluoride penetration into the enamel was investigated from thirteen teeth, randomly selected at each of 1, 2, and 3 months. Each tooth with a bonded bracket was thoroughly rinsed with deionized water and embedded in a resin block (25 × 25 × 25 mm). Then, sectioned buccolingually and occlusocervically at the center of the bracket with a low speed cutting machine (Isomet 1000, Buehler, Lake Bluff, IL, USA) and polished with a polishing machine (Nano 2000, Pace Technology, Tucson, AZ, USA). All samples were kept in a desiccator for at least 2 days before coating with carbon in a vacuum evaporator.
The surface of the cross-section was studied under the scanning electron microscope (JSM-5410LV, JEOL, Tokyo, Japan), and the fluoride composition was determined by energy-dispersive X-ray microanalysis with a silicon detector at 15 kV accelerating voltage, 43 µA beam current, and 100-s acquisition time operating in an in-line scanning mode. For each sample, three spectra were collected under the middle of bracket at depths of 1, 2, and 3 µm below the outer enamel surface. The quantitative analysis of element (weight %) was performed by Link ISIS software version 3.0 (Oxford Instruments Limited, Buckinghamshire, England) with a non-standard analysis mode by using the cobalt element as a reference standard.
Statistical analysis
The fluoride release was analyzed with Kruskal–Wallis H test and Mann–Whitney U test. The fluoride penetration was analyzed with one-way ANOVA and post hoc multiple comparisons. All statistics were tested at 95% confidence intervals (α = 0.05) with SPSS statistics 17.0 (IBM Corporation, New York, NY, USA).
Results
Fluoride release
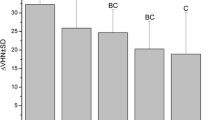
The pattern of fluoride release from each adhesive was the same. The most fluoride was released on day 1 and then decreased sharply to almost half on day 3, except for that from Light Bond® which was non-detectable on day 3 (Table 2; Fig. 1). Fuji Ortho LC® and Illuminate® showed the same pattern, fluoride release decreased markedly from day 3 to day 7 and slightly increased on day 30 (Table 2; Fig. 1). There were significant decreases in the mean cumulative fluoride release between day 1 and day 3, day 1 and day 7, day 1 and day 30 (p < 0.05). In addition, there were significant increases of cumulative fluoride release between day 3 and day 30, and day 7 and day 30 (p < 0.05). The control group (tooth without bonding) showed no detectable fluoride release (Table 2).
When comparing the fluoride release from the three adhesives at the same time point, the results indicated that Illuminate® released the greatest amount of fluoride, followed by Fuji Ortho LC® and Light Bond® (Table 2; Fig. 1). The amount of fluoride release from Illuminate® was significant—almost double that of Fuji Ortho LC® at every observation period (Table 2; Fig. 1) (p < 0.05).
Fluoride penetration
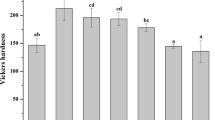
Fluoride penetration could be detected at 1, 2, and 3 µm below the outer enamel surface bonded with Fuji Ortho LC® during 1–3 months (Table 3; Fig. 2). The enamel bonded with the other adhesives and the control did not show any fluoride penetration (Table 3).
During the same observation period, the fluoride concentration (weight %) beneath Fuji Ortho LC® adhesive decreased with depth (from 1 to 3 µm) (Table 3; Fig. 2) at 1 month (1.91, 1.36, and 0.41%, respectively), at 2 months (2.13, 1.64, and 0.88%, respectively), and at 3 months (2.52, 1.23, and 0.39%, respectively). There were significant decreases in the fluoride concentration between the depths of 2 and 3 µm (at 1 month), 1 and 2 µm (at 3 months), 2 and 3 µm (at 3 months), and 1 and 3 µm (at 3 months) (Table 3; Fig. 2; p < 0.05).
When comparing the fluoride concentrations at the same depth with time, the results indicated that at 1 µm depth, the fluoride concentrations increased from 1 to 3 months. At 2 and 3 µm depths, the fluoride concentrations increased only from 1 to 2 months but decreased after 3 months (Table 3; Fig. 2). However, there were no statistically significant differences (p > 0.05) of fluoride concentrations during 1–3 months between all depths.
Discussion
All studied adhesives showed the classic profile of fluoride release, with an initial “burst effect” on the first day and decreasing with time as shown by previous studies [1, 8, 10]. However, fluoride release increased significantly from day 7 to day 30 because it was the cumulative fluoride release for a long-term observation period. Fluoride released from Fuji Ortho LC® was significantly greater than that from Light Bond® at all observation periods. The result corresponded with previous studies [1, 10, 11]. The in vitro study of Chin et al. [9] also supported the fluoride releasing properties of Fuji Ortho LC® and Light Bond®. They concluded that fluoride released from Light Bond® was only 18% of that of Fuji Ortho LC®. Our study also found new knowledge that Illuminate® released the greatest amount of fluoride. Unfortunately, there are no previous data about fluoride release from Illuminate® which was a new product and its composition has not been published. The manipulation of Illuminate®, a single-component cement, is the same as for Light Bond®; thus, we may assume that Illuminate® is a compomer.
It has been accepted that the release of fluoride ions from GICs and RMGICs (Fuji Ortho LC®) results from the acid–base setting reaction between the fluoride-containing aluminosilicate glass powder and the polyacid liquid [5, 22]. The initial profound fluoride release is partly due to surface wash-off as the material sets and the majority of the glass species reacts with the polyacid [32]. The plateau phase after the initial burst has been explained by diffusion of fluoride ions through pores and cracks, and the diffusion through the bulk of the adhesives represents a long-term continuing reaction [31]. It has also been shown that the incorporation of the hydrophilic monomer hydroxyethyl methacrylate (HEMA) into RMGICs increases water absorption to the polymer matrix and thereby facilitates fluoride ion release. It seems that HEMA significantly influences the initial short-term elution, but its effects decrease over the long term, when diffusion from the glass-filler particles predominates [7]. The fluoride ion release from the fluoride-releasing composite resins was significantly lower than that from RMGICs because the ion release is mainly the result of the diffusion of water soluble fluoride ion from the composite into the local environment [5]. Fluoride ion released from the compomers Light Bond® and Illuminate® can be explained by their intermediate composition compared to those of GICs/RMGICs and composite resins [1].
The amounts of fluoride released from orthodontic adhesives varies because of different protocols, with wide variations in the size, shape, and surface area of the sample, type and amount of medium, frequency of medium changes, timing of fluoride measurement, length of the observation period, and unit of measurement [28]. Cranfield et al. [12] found a relationship between the volume of glass ionomer restorative cements and the amount of fluoride released, but Creanor et al. [13] found that reducing the surface area of the sample did not reduce the total cumulative fluoride release. Different mediums (deionized water, distilled water, and artificial saliva) showed different levels of fluoride release. Fluoride released into deionized water and distilled water were significantly greater than that into artificial saliva [14, 26]. The present in vitro study tried to imitate oral conditions by using artificial saliva as the medium. Although the artificial saliva is similar to natural saliva, the organic components of the artificial saliva may interfere with the sensitivity of the lanthanum fluoride membrane of the fluoride electrode [35], thus, affecting the analysis of fluoride content.
It has been reported that fluoride-releasing adhesives could take up fluoride ions from the oral environment as a means of replacing fluoride loss [1, 10]. Conventional GICs have the greatest fluoride recharge capacity, followed by RMGICs and compomers, whereas fluoride-releasing composite resins have little fluoride recharge capacity. The recharge of fluoride may contribute to the ability of these materials to provide a long-term inhibitory effect on enamel demineralization because the recharged fluoride is released and presumably contributes to continuous prevention of enamel demineralization. Fluoride-releasing adhesives could be of benefit especially in high-caries risk patients who also require oral hygiene instruction, diet modification, and fluoride mouth rinses [1], which are recommended nightly to maintain long-term fluoride release from orthodontic adhesives.
Only few studies [8, 34] have reported fluoride penetration after bonding or banding with fluoride-releasing orthodontic adhesives by investigating the enamel surface beneath the bracket or band as it was the primary area that had direct contact with the adhesive. Therefore, whether remarkable fluoride penetration occurs should be part of a further study to investigate fluoride penetration into the peribracket area, which is frequently the area sensitive for plaque accumulation and demineralization [25]. The capacity of fluoride release and penetration of fluoride-releasing orthodontic adhesives should be beneficial for reduction of enamel demineralization. However, fluoride penetration could be found only with Fuji Ortho LC®. The result corresponded with a previous study of Chatzistavrou et al. [8] who investigated Fuji I®, a conventional GIC. Our study found that Illuminate® released the greatest amount of fluoride but fluoride penetration was undetectable. This may be due to the effect of the primer layer that prevents penetration of fluoride into the enamel surface. A similar argument can be made for Light Bond®, which is also used with a primer layer.
Our study showed statistically significant differences (p < 0.05) in fluoride concentrations at 1, 2, and 3 µm below the outer enamel surface when bonded with the Fuji Ortho LC® at all observation periods (1, 2, and 3 months). The fluoride concentration decreased with depth and increased with time from 1 to 2 months. This supported the study of Wagner et al. [34] who found that fluoride ions from Fuji Ortho SC® could be incorporated into the surface layer of the enamel and the depth of fluoride penetration reached 4.8–5.7 µm. The fluoride concentration decreased as the depth increased, and the concentration of the fluoride increased from 6 to 12 weeks.
Fluoride is incorporated into apatite crystals during tooth formation and fluoride absorption from the oral cavity can occur lifelong. Enamel of recently erupted teeth absorbs more fluoride than mature teeth. Our study showed the possibility of fluoride being released from orthodontic adhesives and fluoride penetration into the enamel of mature teeth. Further studies should be carried out to test whether the amount of released fluoride obtained from this study is adequate for remineralization of demineralized enamel.
Other factors that affect the amount of fluoride release are pH and quantity of adhesive. It has been reported that when the pH decreases, fluoride released from glass ionomers increases due to chemical erosion and solubility of the cement in an acidic environment [27]. Ogaard et al. [26] found that orthodontic cement VP862® (Vivadent, Schaan, Liechtenstein) released significantly less fluoride in saliva than in distilled water at neutral pH. However, when the salivary pH was lowered to a value of 4, to mimic a severe caries challenge, the amount of fluoride increased up to the level measured in distilled water [26]. Furthermore, it has been suggested that calcium fluoride that deposits on the enamel surface after the application of topical fluoride may serve as a source of ionic fluoride whenever the pH falls to very low levels, therefore, playing an important role in the demineralization and remineralization of the enamel. During a cariogenic challenge, the calcium fluoride releases fluoride ions that could incorporate into enamel as fluoridated hydroxyapatite (FHAP) or fluorapatite (FAP) [29]. Our pilot study indicated that the pH of artificial saliva, in the plastic containers at 37 °C without tooth specimens, changed significantly from the beginning (pH 6.65 ± 0.01) to 1 month of observation (pH 7.25 ± 0.03). Further studies should be undertaken to investigate the effect of pH on fluoride release from orthodontic adhesives.
Regarding the quantity of adhesive, evaluated by the thickness of the adhesive beneath the bracket base, our pilot study found that the amount of adhesive after a bonding procedure as used in clinical practice varied. The average thickness of each adhesive presented by mean ± SD and coefficient of variation (CV) was as follows: Fuji Ortho LC® = 123.6 ± 76.5 µm (61.9%); Illuminate® = 139.8 ± 57.7 µm (41.3%); Light Bond® = 95.0 ± 43.4 µm (45.7%). However, the mean thickness between the three adhesives was not significantly different (p > 0.05). The coefficient of variation (CV) of the mean thickness of Fuji Ortho LC® was the highest (61.9%), which might be due to the variations in the powder:liquid (P:L) ratio during mixing. If the P:L is high, the mixed cement may not flow as the bracket is forced onto the enamel, resulting in a thick cement layer. However, if the P:L is low, a more fluid cement will result which can be easily displaced under the pressure of bracket placement. The other two adhesives Illuminate® and Light Bond® are a single-paste light-cured adhesives (i.e., no mixing is involved), thus, explaining the lower CV (41.3 and 45.7%, respectively).
Conclusion
The fluoride-releasing orthodontic adhesives Fuji Ortho LC®, Illuminate®, and Light Bond® showed an initial “burst effect” of fluoride release on the first day and then decreased to a low level. Illuminate® released the most fluoride, followed by Fuji Ortho LC® and Light Bond®. Fluoride penetration into enamel could be detected only for Fuji Ortho LC®.
References
Ahn SJ, Lee SJ, Lee DY et al (2011) Effects of different fluoride recharging protocols on fluoride ion release from various orthodontic adhesives. J Dent 39:196–201
Bishara SE, Laffoon JF, Vonwald L et al (2002) The effect of repeated bonding on the shear bond strength of different orthodontic adhesives. Am J Orthod Dentofac Orthop 121:521–525
Bishara SE, VonWald L, Laffoon JF et al (2000) The effect of repeated bonding on the shear bond strength of a composite resin orthodontic adhesive. Angle Orthod 70:435–441
Boersma JG, van der Veen MH, Lagerweij MD et al (2005) Caries prevalence measured with QLF after treatment with fixed orthodontic appliances: influencing factors. Caries Res 39:41–47
Cacciafesta V, Sfondrini MF, Tagliani P et al (2007) In-vitro fluoride release rates from 9 orthodontic bonding adhesives. Am J Orthod Dentofac Orthop 132:656–662
Chadwick SM, Gordon PH (1995) An investigation into the fluoride release of a variety of orthodontic bonding agents. Br J Orthod 22:29–33
Chan WD, Yang L, Wan W et al (2006) Fluoride release from dental cements and composites: a mechanistic study. Dent Mater 22:366–373
Chatzistavrou E, Eliades T, Zinelis S et al (2010) Fluoride release from an orthodontic glass ionomer adhesive in vitro and enamel fluoride uptake in vivo. Am J Orthod Dentofac Orthop 137:458–459
Chin MY, Sandham A, Rumachik EN et al (2009) Fluoride release and cariostatic potential of orthodontic adhesives with and without daily fluoride rinsing. Am J Orthod Dentofac Orthop 136:547–553
Cohen WJ, Wiltshire WA, Dawes C et al (2003) Long-term in vitro fluoride release and rerelease from orthodontic bonding materials containing fluoride. Am J Orthod Dentofac Orthop 124:571–576
Coonar AK, Jones SP, Pearson GJ (2001) An ex vivo investigation into the fluoride release and absorption profiles of three orthodontic adhesives. Eur J Orthod 23:417–424
Cranfield M, Kuhn AT, Winter GB (1982) Factors relating to the rate of fluoride-ion release from glass-ionomer cement. J Dent 10:333–341
Creanor SL, Al-Harthy NS, Gilmour WH et al (2003) Fluoride release from orthodontic cements-effect of specimen surface area and depth. J Dent 31:25–32
el Mallakh BF, Sarkar NK (1990) Fluoride release from glass-ionomer cements in de-ionized water and artificial saliva. Dent Mater 6:118–122
Fox NA (1990) Fluoride release from orthodontic bonding materials. An in vitro study. Br J Orthod 17:293–298
Geiger AM, Gorelick L, Gwinnett AJ et al (1992) Reducing white spot lesions in orthodontic populations with fluoride rinsing. Am J Orthod Dentofac Orthop 101:403–407
Gorelick L, Geiger AM, Gwinnett AJ (1982) Incidence of white spot formation after bonding and banding. Am J Orthod 81:93–98
Kajander KC, Uhland R, Ophaug RH et al (1987) Topical fluoride in orthodontic bonding. Angle Orthod 57:70–76
Komori A, Ishikawa H (1997) Evaluation of a resin-reinforced glass ionomer cement for use as an orthodontic bonding agent. Angle Orthod 67:189–195
Maijer R, Smith DC (1981) Variables influencing the bond strength of metal orthodontic bracket bases. Am J Orthod 79:20–34
Marcusson A, Norevall LI, Persson M (1997) White spot reduction when using glass ionomer cement for bonding in orthodontics: a longitudinal and comparative study. Eur J Orthod 19:233–242
McNeill CJ, Wiltshire WA, Dawes C et al (2001) Fluoride release from new light-cured orthodontic bonding agents. Am J Orthod Dentofac Orthop 120:392–397
Mitchell L (1992) Decalcification during orthodontic treatment with fixed appliances–an overview. Br J Orthod 19:199–205
Mizrahi E (1982) Enamel demineralization following orthodontic treatment. Am J Orthod 82:62–67
O’Reilly MM, Featherstone JD (1987) Demineralization and remineralization around orthodontic appliances: an in vivo study. Am J Orthod Dentofac Orthop 92:33–40
Ogaard B, Rezk-Lega F, Ruben J et al (1992) Cariostatic effect and fluoride release from a visible light-curing adhesive for bonding of orthodontic brackets. Am J Orthod Dentofac Orthop 101:303–307
Rezk-Lega F, Ogaard B, Arends J (1991) An in vivo study on the merits of two glass ionomers for the cementation of orthodontic bands. Am J Orthod Dentofac Orthop 99:162–167
Rix D, Foley TF, Banting D et al (2001) A comparison of fluoride release by resin-modified GIC and polyacid-modified composite resin. Am J Orthod Dentofac Orthop 120:398–405
Saxegaard E, Rolla G (1989) Kinetics of acquisition and loss of calcium fluoride by enamel in vivo. Caries Res 23:406–411
Sudjalim TR, Woods MG, Manton DJ et al (2007) Prevention of demineralization around orthodontic brackets in vitro. Am J Orthod Dentofac Orthop 131(705):e1–e9
Swartz ML, Phillips RW, Clark HE (1984) Long-term F release from glass ionomer cements. J Dent Res 63:158–160
Tay WM, Braden M (1988) Fluoride ion diffusion from polyalkenoate (glass-ionomer) cements. Biomaterials 9:454–456
Vorhies AB, Donly KJ, Staley RN et al (1998) Enamel demineralization adjacent to orthodontic brackets bonded with hybrid glass ionomer cements: an in vitro study. Am J Orthod Dentofac Orthop 114:668–674
Wagner L, Szepietowska M (2013) Fluoride penetration from three orthodontic adhesives: an experimental study. Korean J Orthod 43:29–34
Wiltshire WA, Janse van Rensburg SD (1995) Fluoride release from four visible light-cured orthodontic adhesive resins. Am J Orthod Dentofac Orthop 108:278–283
Author information
Authors and Affiliations
Corresponding author
Additional information
Smorntree Viteporn: Professor.
Rights and permissions
About this article
Cite this article
Suebsureekul, P., Viteporn, S. Release of fluoride from orthodontic adhesives and penetration into enamel. J Orofac Orthop 78, 185–192 (2017). https://doi.org/10.1007/s00056-016-0072-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00056-016-0072-y