Abstract
Novel unsaturated piperazine and homopiperazine derivatives (3a–h) were synthesized in medium to good yields by acylation reactions of piperazine and homopiperazine with methacrylic anhydride (2a) and benzoyl chloride (2b). Piperazine containing dihydrofuran compounds (5a–l) were obtained from radical addition and cyclizations of 3a–h with 1,3-dicarbonyl compounds such as dimedone (4a), ethyl acetoacetate (4b) and acetylacetone (4c) mediated by Mn(OAc)3 for the first time. While the reaction of 3b (1-methacryloylpiperazine) with 4a and 4b gave bis-dihydrofurans (5b and 5d) beside mono-dihydrofurans (5a and 5c), the reaction of 3b–e, 3g, 3h, and 3e with 1,3-dicarbonyl compounds gave mono dihydrofuran compounds (5f–l) in medium to high yields. Structures of all novel compounds were determined by melting point analysis, 1H NMR, 13C NMR, HRMS, and FTIR methods. All piperazine containing dihydrofuran compounds were evaluated for their inhibitory activities toward acetylcholinesterase (AChE) by Ellman method and IC50 values were presented. Compounds 5c, 5d, 5e, 5i, and 5l show highest inhibitory activities with IC50 values of 5.79, 3.89, 5.07, 4.30, and 2.24 µM, respectively. In addition, molecular docking studies were performed on selected structures 5d, 5i, and 5l to investigate ligand–protein interactions. Binding energies were calculated and compared with standart drug donepezil.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer’s disease (AD) is a progressive and chronic neurodegenerative disorder which is one of the main causes of dementia that effects elder people (Anand et al. 2014; Gao et al. 2018). Among the various proposed pathogenesis hyphotesis of AD, cholinergic hyphothesis is the most commonly accepted and the decrease of neurotransmitter acetylcholine levels in the brain is considered the most prominent cause of AD (Akasofu et al. 2008; Dumas and Newhouse 2011; Molinuevo and Gauthier 2013).
Acetylcholinesterase (AChE) is an enzyme belongs to cholinesterase family and the main biological function of AChE is to terminate impulse transmission at cholinergic synapses by catalyzing the rapid hydrolysis of the neurotransmitter acetylcholine (Tougu 2001; Berwaldt et al. 2019). Inhibiting AChE is the most effective and promising way and AChE inhibitors such as donepezil (Sugimoto et al. 1995), rivastigmine (Weintraub et al. 2011) and galantamine (Giacobini 2004) are frequently used for the treatment of AD.
Nitrogen bearing heterocycles have drawn much attention due to their biological properties (Yang et al. 2007; Khan et al. 2014). Piperazine structure is considered as a “privileged structure” in medicinal chemistry owing to their capabilities of binding to multiple receptors with high affinity (Szabo et al. 2014). There are many biological activity studies such as, anticonvulsant (Harish et al. 2013), antibacterial (Chaudhary et al. 2006), antituberculosis (Patel and Telvekar 2014), antiviral (Wang et al. 2009), anticancer (Duan et al. 2013), and antimalarial (Pretorius et al. 2013). Also there are many reports of piperazine derivatives showing acetylcholinesterase inhibition in literature (Meena et al. 2015; Piemontese et al. 2018; Demirayak et al. 2019) which make piperazine containing compounds suitable and potential candidates for AD treatment drugs.
Dihydrofurans gathered much attention due to their biological activities. They have much potential to be used as building blocks for drugs. Sarcophytoxide (Chen et al. 2012), Clerodin (Lallemand et al. 2002), Fercoprolone (Appendino et al. 1998), and Austocystin (Kornsakulkarn et al. 2012) are some natural biologically active compounds that contain dihydrofuran moieties. It is known that dihydrofurans can be synthesized from transition metal salts (Mn3+, Ce4+, Co3+, etc.) capable of transferring single electrons that form α-carbon radicals with enolizable active methylene compounds and the addition of this radical to unsaturated systems are widely used to generate new C–C bonds (Melikyan 1993; Snider 1996; Mondal and Bora 2013). Among these metal salts, cerium(IV) ammonium nitrate (Kobayashi et al. 2003; Chuang and Wu 2004; Nair et al. 2006) and manganese(III) acetate and are widely used in these reaction. There are many works in literature about syntheses mediated by Mn(OAc)3 such as synthesis of spirodiones (Hyunh et al. 2017), azofulvene derivatives from indoles (Lofstrand et al. 2016), ferrocene substituted dihydrofurans (Aslan et al. 2017), spirobenzofurans (Ergüntürk et al. 2017), polysubstituted α-naphtols (He et al. 2020), bicyclic tetrahydrofurans (Chany et al. 2015), macrocyclic compounds with dihydrofuran rings (Ito et al. 2011), and spirodihydrofurans (Yokote et al. 2020). Also there are works about alkene cyanophosphinoylation (Zhang et al. 2017), C–H alkylation of arenes (Castro et al. 2016) and radical alkoxycarbonylation of indoles (Li et al. 2018).
Our research group has reported the radical addition and cyclization of 1,3-dicarbonyl compounds with various unsaturated systems such as aromatic and heteroaromatic conjugated alkenes (Yılmaz et al. 2005, 2008; Yilmaz 2011a, b; Yılmaz and Pekel 2011; Yilmaz et al. 2014; Ustalar and Yilmaz 2017; Özgür et al. 2019), dienes (Hocaoglu, Yilmaz 2019; Yılmaz et al. 2011a, b; Ustalar et al. 2017; Yilmaz and Ustalar 2016) and conjugated amide derivatives (Yılmaz and Pekel, 2001a, b; Burgaz et al. 2007; Yılmaz et al. 2011a, b; Yilmaz et al. 2016) using manganese(III) acetate and cerium(IV) ammonium nitrate (CAN), obtaining functionalized dihydrofuran containing compounds.
In this work we synthesized new piperazine substituted dihydrofuran compounds (5a–l) obtained by the Mn(OAc)3 mediated radical cyclization of enolizable 1,3-dicarbonyls (4a–c) with unsaturated piperazine and homopiperazine derivatives (3a–h) in medium to high yields. There are no detailed works in literature about AChE inhibiton studies of dihydrofuran derivatives. Thus, we evaluated AChE inhibitions of piperazine substituted dihydrofuran compounds in detail. Evaluation of AChE inhibition capabilities of synthesized compounds were performed according to in vitro Ellman method (Ellman et al. 1961). Also in silico docking studies were performed on compounds (5d, 5i, and 5l) to explore ligand–protein interaction and binding affinities with the active site of AChE.
Materials and methods
Experimental
Melting points were determined on a Gallenkamp capillary melting point apparatus. IR spectra (ATR) were obtained with a Bruker Tensor27 spectrophotometer in the 400–4000 cm−1 range with 2 cm−1 resolutions. 1H NMR and 13C NMR spectra were recorded on a Varian Mercury-400 High performance Digital FT-NMR and Varian Oxford NMR300 spectrometers. High resolution mass time-of-flight spectra were measured on an Agilent 1200/6210 LC/MS spectrophotometer. UV absorbance was measured by Rigol Ultra-3000 UV–Vis spectrophotometer. Thin layer chromatography (TLC) was performed on Merck aluminum-packed silica gel plates. Purification of products was performed by column chromatography on silica gel (Merck silica gel 60, 40–60 μm) or preparative TLC on silica gel of Merck (PF254-366 nm). All reagents and solvents were highest purity and were used without further purification. Radical oxidant Mn(OAc)3 was synthesized by electrochemical method (Yılmaz et al. 2011a, b).
General synthesis procedure and spectroscopic data of unsaturated piperazine compounds (3a–h)
The unsaturated piperazine and homopiperazine derivatives (3a–h) were synthesized by acylation reactions of piperazine and homopiperazine with methacrylic anhydride (2a) and benzoyl chloride (2b). Compounds 3a (Korhonen 1995), 3b (Shea et al. 1990), and 3g (Kazuo 1984) were synthesized by modifying the literature method. Corresponding starting piperazine derivative (1a–d) was dissolved in 20 mL chloroform and the solution was stirred in ice bath for 15 min. Then a dilute solution of suitable acylation reagent (2a and 2b) in chloroform was added dropwise. After instillation, reaction was removed from ice bath and allowed to stir overnight. Water (20 mL) was added and crude product was extracted three times with chloroform (3 × 20 mL). Combined organic phases were dried over anhydrous Na2SO4 and evaporated. The crude product (3a–h) was purified by column chromatography on silica using n-hexane/EtOAc (1:1) as eluent.
2-Methyl-1-(piperazin-1-yl)prop-2-en-1-one (3a)
It was obtained as a yellow oil; yield: 72% (16.5 g, 100 mmol); 1H NMR (400 MHz, CDCl3) δ (ppm): 5.16 (1H, s, –C=CH), 5.00 (1H, s, –C=CH), 3.55 (4H, s, –CH2–), 2.84 (4H, s, –CH2–), 1.93 (3H, s, –CH3) (Korhonen 1995).
1,1′-(Piperazine-1,4-diyl)bis(2-methylprop-2-en-1-one) (3b)
It was obtained as a colorless solid; yield 75% (3.82 g, 10 mmol); mp: 113–115 °C (114–115 °C, Shea et al. 1990); 1H NMR (400 MHz, CDCl3) δ (ppm): 5.24 (2H, s, –C=CH), 5.05 (2H, s, –C=CH) 3.58 (8H, s, –CH2–) 1.96 (6H, s, –CH3) (Shea et al. 1990).
1-(4-Benzoylpiperazin-1-yl)-2-methylprop-2-en-1-one (3c)
It was obtained as a colorless solid; yield 72% (1.36 g, 5 mmol); mp: 101–103 °C; IR (ATR) υmax 3050 (aromatic C–H), 2972 (aliphatic C–H), 2915 (aliphatic C–H), 1635 (amide C=O), 1603 (C=C), 1194 (C–N), 750, 700 (aromatic C–H) cm−1; 1H NMR (400 MHz, CDCl3) δ (ppm): 7.43–7.37 (5H, m, Ar–H), 5.22 (1H, s, –C=CH), 5.04 (1H, s, –C=CH), 3.60 (8H, s, –CH2–), 1.95 (3H, s, –CH3); 13C NMR (100 MHz, CDCl3) δ (ppm): 171.3 (C=O), 170.6 (C=O), 139.9 (C=C), 135.1 (aromatic C), 130.0 (aromatic C), 128.6 (aromatic C), 127.0 (aromatic C), 116.1 (–C=C–), 46.6 (–CH2), 41.6 (–CH2), 20.4 (–CH3); HRMS (ESI) (m/z): Calcd for C15H18N2O2 258.136, found: 258.447 (M + H)+.
2-Methyl-1-(4-(tetrahydrofuran-2-carbonyl)piperazin-1-yl)prop-2-en-1-one (3d)
It was obtained as a yellow oil; yield 82% (1.13 g, 4 mmol); IR (ATR) υmax 2976 (aliphatic C–H), 2918 (aliphatic C–H), 2871 (aliphatic C–H), 1641 (C=C), 1630 (amide C=O), 1611 (C=C), 1195 (C–N), 1080 (C–O) cm−1; 1H NMR (400 MHz, CDCl3) δ (ppm): 5.24 (1H, s, –C=CH), 5.05 (1H, s, –C=CH), 4.60 (1H, t, J = 6.0 Hz, –CH), 3.94–3.82 (2H, m, –CH2), 3.76–3.44 (8H, m, –CH2), 2.06–1.99 (2H, m, –CH2–), 1.92 (3H, s, –CH3), 1.90–1.88 (2H, m, –CH2–); 13C NMR (100 MHz, CDCl3) δ (ppm): 171.3 (C=O), 169.9 (C=O), 139.9 (C=C), 116.0 (C=C), 75.9 (O–C–), 69.0 (–CH2–), 45.4 (–CH2–), 42.2 (–CH2–), 28.0 (–CH2–), 25.7 (–CH2–), 20.4 (–CH3); HRMS (ESI) (m/z) Calcd for C13H20N2O3 253.15467, found: 253.15552 (M + H)+.
1-(4-(Furan-2-carbonyl)piperazin-1-yl)-2-methylprop-2-en-1-one (3e)
It was obtained as a yellow oil; yield 88% (1.21 g, 4.8 mmol); IR (ATR) υmax 3117 (aromatic C–H), 2978 (aliphatic C–H), 2917 (aliphatic C–H), 2862 (aliphatic C–H), 1638 (amide C=O) 1608 (C=C), 1180 (C–N), 1092 (C–O), 750, 700 (aromatic C–H) cm−1; 1H NMR (400 MHz, CDCl3) δ (ppm): 7.51 (1H, s, Ar–H), 7.06 (1H, d, J = 3.2 Hz, Ar–H), 6.51 (1H, dd, J = 3.6, 2.0 Hz, Ar–H), 5.26 (1H, s, –C=CH), 5.08 (1H, s, –C=CH), 3.81 (4H, s, –CH2–), 3.68 (4H, s, –CH2–), 1.98 (3H, s, –CH3); 13C NMR (100 MHz, CDCl3) δ (ppm): 171.3 (C=O), 159.1 (C=O), 147.5 (aromatic C), 143.9 (aromatic C), 139.9 (C=C), 117.1 (aromatic C), 116.1 (C=C), 111.4 (aromatic C), 46.8 (–CH2–), 41.7 (–CH2–), 20.4 (–CH3); HRMS (ESI) (m/z) Calcd for C13H16N2O3 249.12337, found: 249.12382 (M + H)+.
1-(1,4-Diazepan-1-yl)-2-methylprop-2-en-1-one (3f)
It was obtained as a yellow oil; yield 70% (2.53 g, 15 mmol); IR (ATR) υmax 3367 (N–H), 2972 (aliphatic C–H), 1644 (amide C=O) 1615 (C=C), 1201 (C–N) cm−1; 1H-NMR (400 MHz, CDCl3) δ (ppm): 5.16 (1H, s, –C=CH), 5.02 (1H, s, –C=CH), 3.64–3.44 (2H, m, –CH2–), 3.50 (1H, s, –NH), 3.00–2.88 (2H, m, –CH2–), 2.60 (4H, s, –CH2–), 1.97 (3H, s, –CH3), 1.86 (2H, m, –CH2–); 13C NMR (100 MHz, CDCl3) δ (ppm): 172.7 (C=O), 141.0 (C=C), 114.9 (C=C), 58.0 (–CH2–), 50.3 (–CH2–), 47.3 (–CH2–), 31.3 (–CH2–), 18.2 (–CH3); HRMS (ESI) (m/z) Calcd for C9H16N2O 168.125 found: 168.434 (M+H)+.
1,1′-(1,4-Diazepane-1,4-diyl)bis(2-methylprop-2-en-1-one) (3g)
It was obtained as a yellow oil; yield 70% (3.3 g, 12 mmol) 1H NMR (400 MHz, CDCl3) δ (ppm): 5.13 (2H, s, –C=CH), 4.96 (2H, s, –C=CH), 3.68–3.50 (8H, m, –CH2–), 1.92 (6H, s, –CH3), 1.82 (2H, s, –CH2–) (Kazuo 1984).
1-(4-Benzoyl-1,4-diazepan-1-yl)-2-methylprop-2-en-1-one (3h)
It was obtained as a yellow oil; yield 60% (1.95 g, 7 mmol); IR (ATR) 3079 (aromatic C–H), 2948 (aliphatic C–H), 2919 (aliphatic C–H), 2872 (aliphatic C–H), 1630 (amide C=O), 1613 (C=C), 1299 (C–N), 731, 703 (aromatic C–H) cm−1; 1H NMR (400 MHz, CDCl3) δ (ppm): 7.33–7.25 (5H, m, Ar–H), 5.10 (1H, s, –C=CH), 4.96 (1H, s, –C=CH), 3.75–3.33 (8H, m, –CH2–), 2.25 (1H, s, –CH2–), 1.90 (3H, s, –CH3), 1.67 (1H, s, –CH2–); 13C NMR (100 MHz, CDCl3), δ (ppm): 172.8 (C=O), 171.5 (C=O), 140.6 (C=C), 136.0 (aromatic C), 129.5 (aromatic C), 128.5 (aromatic C), 126.5 (aromatic C), 115.4 (C=C), 50.2 (–CH2–), 49.4 (–CH2–), 48.7 (–CH2–), 47.7 (–CH2–), 27.9 (–CH2–), 20.5 (–CH3); HRMS (ESI) (m/z) Calcd for C16H20N2O2 273.15975 found: 273.16014 (M + H)+.
General synthesis procedure and spectroscopic data of piperazine-dihydrofuran compounds (5a–k)
A solution of Mn(OAc)3 (2 mmol, 530 mg) in 15 mL glacial acetic acid was heated to 80 °C until dissolved. After that the solution temperature was set to 65 °C. A solution of corresponding 1,3-dicarbonyl (4a–c) (1 mmol) and related unsaturated piperazine compound (1.2 mmol) in 3 mL of AcOH was added to Mn(OAc)3 solution. The mixture was stirred and disappearance of dark brown color indicated that the reaction was finished. Water (20 mL) was added and crude product was extracted with chloroform (3 × 20 mL). Combined organic phases were neutralized with saturated NaHCO3 solution, dried over anhydrous Na2SO4 and evaporated. The residue was purified with column chromatography using chloroforom–acetone (85:15) as eluent.
2-(4-Methacryloylpiperazine-1-carbonyl)-2,6,6-trimethyl-2,3,6,7-tetrahydrobenzofuran-4(5H)-one (5a)
It was obtained as a colorless solid; yield 77% (277 mg, 0.76 mmol); mp: 139–141 °C; IR (ATR) υmax 2955 (aliphatic C–H), 2922 (aliphatic C–H), 2875 (aliphatic C–H), 1636 (amide C=O), 1620 (C=C), 1194 (C–N), 1016 (C–O); 1H NMR (400 MHz, CDCl3) δ (ppm): 5.23 (1H, s, –C=CH), 5.04 (1H, s, –C=CH), 3.57 (8H, s, –CH2–), 3.47 (1H, d, J = 15.2 Hz, –CH2–), 2.69 (1H, d, J = 15.2 Hz, –CH2–), 2.27 (2H, d, J = 7.2 Hz, –CH2–), 2.23 (2H, s, –CH2–), 1.94 (3H, s, –CH3), 1.60 (3H, s, -CH3), 1.10 (3H, s –CH3), 1.08 (3H, s, –CH3); 13C NMR (100 MHz, CDCl3), δ (ppm): 194.6 (C=O), 172.9 (C=C–O), 171.3 (C=O), 169.7 (C=O), 139.9 (C=C), 116.1 (C=C), 111.4 (C=C–O), 91.9 (O–C–), 50.8 (–CH2–), 46.1 (–CH2–), 43.5 (–CH2–), 37.7 (–CH2–), 37.5 (–CH2–), 32.2 (–CH2–), 28.6 (–CH2–), 26.2 (–CH2–), 20.4 (-CH3), 20.4 (–CH3); HRMS (ESI) (m/z) Calcd for C20H28N2O4 361.21346 found: 361.21282 (M+H)+.
2,2′-(Piperazine-1,4-dicarbonyl)bis(2,6,6-trimethyl-2,3,6,7-tetrahydrobenzofuran-4(5H)-one) (5b)
It was obtained as a colorless solid; yield 40% (192 mg, 0.38 mmol); mp: 205–207 °C; IR (ATR) 2955 (aliphatic C–H), 2904 (aliphatic C–H), 2874 (aliphatic C–H), 2850 (aliphatic C–H), 1628 (amide C=O), 1610 (C=C), 1180 (C–N), 1012 (C–O) cm−1; 1H NMR (400 MHz, CDCl3) δ (ppm): 3.58 (8H, s, –CH2–), 3.46 (2H, d, J = 15.2 Hz, –CH2–), 2.68 (2H, d, J = 15.2 Hz, –CH2–), 2.31 (4H, d, J = 16.0 Hz, –CH2–), 2.24 (4H, d, J = 16.0 Hz, –CH2–), 1.59 (6H, s, –CH3), 1.10 (6H, s, –CH3), 1.07 (6H, s, –CH3); 13C NMR (100 MHz, CDCl3) δ (ppm): 194.7 (C=O), 173.0 (C=C–O–), 169.8 (C=O), 111.4 (C=C–O), 91.9 (O–C–), 50.9 (–CH2–), 46.1 (–CH2–), 43.6 (–CH2–), 37.8 (–CH2–), 37.6 (–CH2–), 34.3 (–CH2–), 28.7 (–CH3–), 26.3 (–CH3); HRMS (ESI) (m/z) Calcd for C28H38N2O6 499.28026 found: 499.28260 (M + H)+.
Ethyl 5-(4-methacryloylpiperazine-1-carbonyl)-2,5-dimethyl-4,5-dihydrofuran-3-carboxylate (5c)
It was obtained as a colorless solid; yield 40% (122 mg, 0.38 mmol); mp: 74–76 °C; IR (ATR) 2974 (aliphatic CH), 2901 (aliphatic CH), 1702 (ester C=O), 1628 (amide C=O), 1610 (C=C), 1246 (C–N), 1195 (C–O) cm−1; 1H NMR (400 MHz, CDCl3), δ (ppm): 5.22 (1H, s, –C=CH), 5.03 (1H, s, –C=CH), 4.15 (2H, q, J = 7.2 Hz, –O–CH2CH3), 3.58 (1H, d, J = 15.6 Hz, –CH2–), 3.56 (8H, s, –CH2–), 2.71 (1H, d, J = 15.6 Hz, –CH2–), 2.17 (3H, s, –CH3), 1.94 (3H, s, –CH3), 1.56 (3H, s, –CH3), 1.25 (3H, t, J = 7.2 Hz, –O–CH2CH3) 13C NMR (100 MHz, CDCl3) δ (ppm): 171.4 (C=C–O), 170.5 (C=O), 165.5 (C=O), 164.5 (C=O), 139.9 (C=C), 116.1 (C=C), 102.3 (C=C–O), 88.3 (O–C), 59.7 (–CH2), 46.4 (–CH2), 43.6 (–CH2), 41.2 (–CH2), 26.0 (CH3), 20.4 (CH3), 14.3 (CH3), 14.0 (CH3); HRMS (ESI) (m/z) Calcd for C17H24N2O4 321.18088 found: 321.18203 (M + H)+.
Diethyl 5,5′-(piperazine-1,4-dicarbonyl)bis(2,5-dimethyl-4,5-dihydrofuran-3-carboxylate) (5d)
It was obtained as a colorless solid; yield 12% (60 mg, 0.12 mmol); mp: 123–125 °C; IR (ATR) 2982 (aliphatic C–H), 2930 (aliphatic C–H), 1794 (ester C=O), 1698 (amide C=O), 1620 (C=C), 1197 (C–N), 1056 (C–O) cm−1; 1H NMR (400 MHz, CDCl3) δ (ppm): 4.16 (4H, q, J = 7.2 Hz, –O–CH2CH3), 3.61 (2H, d, J = 15.2 Hz, –CH2–), 3.60 (8H, s, –CH2–), 2.73 (2H, d, J = 15.2 Hz, –CH2–), 2.19 (6H, s, –CH3), 1.57 (6H, s, –CH3), 1.27 (6H, t, J = 7.2 Hz, –O–CH2CH3); 13C NMR (100 MHz, CDCl3) δ (ppm): 176.4 (C=C–O), 165.6 (C=O), 164.5 (C=O), 102.3 (C=C–O), 88.3 (O–C), 59.7 (–CH2–), 46.4 (–CH2–), 43.6 (–CH2–), 41.2 (–CH2–), 26.03 (–CH3), 14.3 (–CH3), 14.1 (–CH3); HRMS (ESI) (m/z) Calcd for C24H34N2O8 501.2274 found: 501.22171 (M + Na)+.
Ethyl 2,5-dimethyl-5-(4-(2,6,6-trimethyl-4-oxo-2,3,4,5,6,7-hexahydrobenzofuran-2-carbonyl)piperazine-1-carbonyl)-4,5-dihydrofuran-3-carboxylate (5e)
It was obtained as a yellow oil; yield 36% (175 mg, 0.35 mmol) mp: 143–145 °C IR (ATR) 2956 (aliphatic C–H), 2916 (aliphatic C–H), 2850 (aliphatic C–H), 1703 (ester C=O), 1627 (amide C=O), 1615 (C=C), 1141 (C–N), 1014 (C–O) cm−1; 1H NMR (400 MHz, CDCl3) δ (ppm): 4.16 (2H, q, J = 7.2 Hz, –O–CH2CH3), 3.72 (8H, s, –CH2–), 3.59 (1H, dd, J = 15.2, 2.0 Hz, –CH2–), 3.47 (1H, dd, J = 15.2, 2.0 Hz, –CH2–), 2.73 (1H, dd, J = 15.2, 2.0 Hz, –CH2–) 2.70 (1H, dd, J = 15.2, 2.0 Hz, –CH2–), 2.30 (2H, dd, J = 14.0, 1.2 Hz, –CH2–), 2.25 (2H, dd, J = 14.0, 1.2 Hz, –CH2–), 2.18 (3H, d, J = 2.0 Hz, –CH3), 1.60 (3H, s, –CH3), 1.57 (3H, s, –CH3), 1.25 (3H, t, J = 7.2 Hz, –CH3), 1.11 (3H, s, –CH3) 1.08 (3H, s, –CH3); 13C NMR (100 MHz, CDCl3) δ (ppm): 194.5 (C=O), 172.9 (C=C–O), 165.5 (C=O), 164.5 (C=O), 111.42 (C=C–O), 91.8 (O–C), 88.3 (O–C), 59.7 (–CH2–), 50.9 (–CH2–), 46.2 (–CH2–), 43.4 (–CH2–), 41.2 (–CH2–), 37.8 (–CH–), 37.5 (–CH2–), 34.2 (–CH2–), 28.7 (–CH2–), 28.69 (–C–), 28.6 (–CH3), 26.2 (–CH3), 26.0 (–CH3), 14.3 (–CH3), 14.0 (–CH3); HRMS (ESI) (m/z) Calcd for C26H36N2O7 489.25953 found: 489.25956 (M + H)+.
1-(4-(4-Acetyl-2,5-dimethyl-2,3-dihydrofuran-2-carbonyl)piperazin-1-yl)-2-methylprop-2-en-1-one (5f)
It was obtained as a yellow oil; yield 65% (208 mg, 0.64 mmol) IR (ATR) 2982 (aliphatic C–H), 2920 (aliphatic C–H), 2864 (aliphatic C–H), 1630 (amide C=O), 1613 (C=C), 1192 (C–N), 1017 (C–O) cm−1; 1H NMR (400 MHz, CDCl3) δ (ppm): 5.23 (1H, s, –C=CH), 5.03 (1H, s, –C=CH), 3.74 (1H, d, J = 15.2 Hz, –CH2–), 3.56 (8H, s, –CH2–), 2.76 (1H, d, J = 15.2 Hz, –CH2–), 2.19 (3H, s, –CH3), 2.17 (3H, s, –CH3), 1.94 (3H, s, –CH3), 1.57 (3H, s, –CH3); 13C NMR (100 MHz, CDCl3) δ (ppm): 194.8 (C=O), 171.4 (C=C–O), 170.3 (C=O), 164.1 (C=O), 139.8 (C=C), 116.1 (C=C), 111.5 (C=C–O), 88.6 (O–C), 46.4 (–CH2–), 43.5 (–CH2–), 41.9 (–CH2–), 29.6 (–CH3), 26.0 (–CH3), 20.4 (–CH3), 14.8 (-CH3); HRMS (ESI) (m/z) Calcd for C17H24N2O4 321.18088 found: 321.18203 (M + H)+.
2-(4-Benzoylpiperazine-1-carbonyl)-2,6,6-trimethyl-2,3,6,7-tetrahydrobenzofuran-4(5H)-one (5g)
It was obtained as a yellow solid; yield 60% (238 mg, 0.60 mmol); mp:142–144 °C; IR (ATR) 2961 (aliphatic C–H), 2916 (aliphatic C–H) 2869 (aliphatic C–H), 2850 (aliphatic C–H), 1640 (amide C=O), 1627 (C=C), 1230 (C–N), 1008 (C–O), 749, 705 (aromatic C–H) cm−1; 1H NMR (400 MHz, CDCl3) δ (ppm): 7.44–7.38 (5H, m, Ar–H), 3.67 (8H, s, –CH2–), 3.48 (1H, d, J = 15.2 Hz, –CH2–), 2.69 (1H, d, J = 15.2 Hz, –CH2–), 2.27 (2H, s, –CH2–), 2.23 (2H, s, –CH2–) 1.60 (3H, s, –CH3), 1.10 (3H, s, –CH3), 1.08 (3H, s, –CH3); 13C NMR (100 MHz, CDCl3) δ (ppm): 194.6 (C=O), 173 (–C=C–O), 170.7 (C=O), 169.8 (C=O), 135.1 (aromatic C), 130.2 (aromatic C), 128.7 (aromatic C), 127.1 (aromatic C), 111.5 (C=C), 92 (O–C), 50.9 (–CH2–), 46.3 (–CH2–), 43.6 (–CH2–), 37.8 (–CH2–), 37.6 (–C–), 34.3 (–CH2), 28.7 (–CH3), 28.7 (–CH3), 26.3 (–CH3); HRMS (ESI) (m/z) Calcd for C23H28N2O4 397.21218 found: 397.21301 (M + H)+.
2,6,6-Trimethyl-2-(4-(tetrahydrofuran-2-carbonyl)piperazine-1-carbonyl)-2,3,6,7-tetrahydrobenzofuran-4(5H)-one (5h)
It was obtained as a yellow solid; yield 43% (167 mg, 0.42 mmol); mp: 76–78 °C; IR (ATR) 2957 (aliphatic C–H), 2930 (aliphatic C–H) 2872 (aliphatic C–H), 1711 (C=O) 1631 (amide C=O), 1610 (C=C), 1232 (C–N), 1028 (C–O) cm−1; 1H NMR (400 MHz, CDCl3) δ (ppm): 4.56 (1H, t, J = 5.2 Hz, O–CH–), 3.86 (2H, m, –CH2–), 3.66 (8H, s, –CH2–), 3.46 (1H, d, J = 15.2 Hz, –CH2–), 2.68 (1H, d, J = 15.2 Hz, –CH2–), 2.27 (2H, d, J = 6.0 Hz, –CH2–), 2.22 (2H, s, –CH2–), 2.03–1.87 (4H, m, –CH2–), 1.59 (3H, s, –CH3), 1.10 (3H, s, –CH3), 1.08 (3H, s, –CH3); 13C NMR (100 MHz, CDCl3), δ (ppm): 194.5 (C=O), 173 (C=C–O), 171.2 (C=O), 169.6 (C=O), 111.4 (C=C–O), 91.9 (O–C), 75.9 (–C–), 69.0 (–CH2–), 50.8 (–CH2–), 45.3 (–CH2–), 42.1 (–CH2–), 37.7 (–CH2–), 37.5 (–CH2–), 34.2 (–C–), 29.6 (–CH2–), 28.6 (–CH2–), 28.0 (–CH3), 26.2 (–CH3), 25.7 (–CH3); HRMS (ESI) (m/z) Calcd for C21H30N2O5 391.22275 found: 391.22301 (M + H)+.
2-(4-(Furan-2-carbonyl)piperazine-1-carbonyl)-2,6,6-trimethyl-2,3,6,7-tetrahydrobenzofuran-4(5H)-one (5i)
It was obtained as a yellow oil; yield 40% (154 mg, 0.40 mmol) IR (ATR) 3115 (aromatic C–H), 2955 (aliphatic C–H), 2926 (aliphatic C–H), 2870 (aliphatic C–H), 1626 (amide C=O), 1610 (C=C), 1232 (C–N), 1026 (C–O), 746, 700 (aromatic C–H) cm−1; 1H NMR (400 MHz, CDCl3) δ (ppm): 7.50 (1H, d, J = 0.8 Hz, Ar–H), 7.06 (1H, d, J = 3.6 Hz, Ar–H), 6.50 (1H, dd, J = 3.6, 0.8 Hz, Ar–H), 3.71 (8H, s, –CH2–), 3.50 (1H, d, J = 15.2, –CH2–), 2.71 (1H, d, J = 15.2, –CH2–), 2.30 (2H, d, J = 3.2 Hz, –CH2–), 2.25 (2H, s, –CH2–), 1.62 (3H, s, –CH3), 1.12 (3H, s, –CH3), 1.10 (3H, s, –CH3); 13C NMR (100 MHz, CDCl3) δ (ppm): 194.6 (–C=O), 172.9 (C=C–O), 169.7 (C=O), 159.2 (C=O), 147.5 (aromatic C), 143.9 (aromatic C), 117.2 (aromatic C), 111.5 (aromatic C), 111.4 (C=C–O), 91.9 (–C–), 50.9 (–CH2), 46.3 (–CH2–), 43.6 (–CH2–), 37.8 (–CH2–), 37.5 (–C–), 34.2 (–CH2–), 28.7 (–CH3), 28.6 (–CH3), 26.2 (–CH3); HRMS (ESI) (m/z) Calcd for C21H26N2O5 387.19145 found: 387.19210 (M + H)+.
2-(4-Methacryloyl-1,4-diazepane-1-carbonyl)-2,6,6-trimethyl-2,3,6,7-tetrahydrobenzofuran-4(5H)-one (5j)
It was obtained as a colorless oil; yield 41% (153 mg, 0.40 mmol); IR (ATR) 2955 (aliphatic C–H), 2872 (aliphatic C–H), 1630 (amide C=O), 1615 (C=C), 1232 (C–N), 1028 (C–O) cm−1; 1H NMR (400 MHz, CDCl3) δ (ppm): 5.14 (1H, s, –C=CH), 4.96 (1H, d, J = 15.2 Hz, –CH2–), 3.92–3.36 (8H, m, –CH2–), 3.43 (1H, d, J = 15.6 Hz, –CH2–), 2.68 (1H, d, J = 15.2 Hz, –CH2–), 2.29 (2H, s, –CH2–), 2.22 (2H, s, –CH2), 1.93 (3H, s, –CH3), 1.82 (2H, s, –CH2–), 1.58 (3H, d, J = 8.4 Hz, –CH3), 1.08 (6H, d, J = 8.8 Hz, –CH3); 13C NMR (100 MHz, CDCl3) δ (ppm): 194.6 (C=O), 173.3 (C=C–O), 172.3 (C=O), 170.6 (C=O), 140.5 (C=C), 115.4 (C=C), 111.3 (C=C–O), 92.1 (–C–), 50.9 (–CH2–), 49.7 (–CH2–), 49.9 (–CH2–), 47.5 (–CH2–), 47.0 (–CH2–), 37.7 (–CH2–), 34.2 (–C–), 29.6 (–CH2–), 28.7 (–CH3), 28.5 (–CH3), 28.15 (–CH2–), 26.3 (–CH3), 20.5 (–CH3); HRMS (ESI) (m/z) Calcd for C21H30N2O4 375.22783 found: 375.22850 (M + H)+.
2-(4-Benzoyl-1,4-diazepane-1-carbonyl)-2,6,6-trimethyl-2,3,6,7-tetrahydrobenzofuran-4(5H)-one (5k)
It was obtained as a colorless oil; yield 50% (205 mg, 0.50 mmol); IR (ATR) 3079 (aromatic C–H), 2956 (aliphatic C–H), 2926 (aliphatic C–H), 2870 (aliphatic C–H), 1626 (amide C=O), 1610 (C=C), 1230 (C–N), 1006 (C–O), 746, 704 (aromatic C–H) cm−1; 1H-NMR (400 MHz, CDCl3) δ (ppm): 7.37v7.19 (5H, m, Ar–H), 4.00–3.26 (8H, m, –CH2–), 3.46 (1H, d, J = 15.2 Hz, –CH2–), 2.68 (1H, d, J = 15.2 Hz, –CH2–), 2.29 (2H, s, –CH2–), 2.21 (2H, s, –CH2–) 1.97 (1H, s, –CH2–), 1.71 (1H, s, –CH2–), 1.60 (3H, s, –CH3), 1.08 (6H, d, J = 15.2 Hz, –CH3); 13C NMR (100 MHz, CDCl3) δ (ppm): 194.7 (C=O), 173.3 (C=C–O), 171.5 (C=O), 171.0 (C=O), 136.0 (aromatic C), 129.6 (aromatic C), 128.5 (aromatic C), 126.4 (aromatic C), 111.4 (C=C–O), 92.1 (–C–), 50.9 (–CH2–), 50 (–CH2–), 48.0 (–CH2–), 47.4 (–CH2–), 45.8 (–CH2–), 37.8 (–CH2–), 29.6 (–C–), 28.7 (–CH2–), 28.5 (–CH3–) 28.4 (–CH3), 27.3 (–CH3), 26.3 (–CH2–); HRMS (ESI) (m/z) Calcd for C24H30N2O4 411.22783 found: 411.22854 (M + H)+.
Ethyl 5-(4-(furan-2-carbonyl)piperazine-1-carbonyl)-2,5-dimethyl-4,5-dihydrofuran-3-carboxylate (5l)
It was obtained as yellow oil; yield 20% (75 mg, 0.20 mmol); IR (ATR) 3100 (aromatic C–H), 2960 (aliphatic C–H), 2920 (aliphatic C–H), 1710 (ester C=O), 1630 (amide C=O), 1615 (C=C), 1230 (C–N), 1100 (C–O), 750, 700 (aromatic C–H) cm−1; 1H NMR (400 MHz, CDCl3) δ (ppm): 7.48 (1H, d, J = 1.2 Hz, Ar–H), 7.03 (1H, d, J = 3.2 Hz, Ar–H), 6.48 (1H, dd, J = 3.2, 1.2 Hz, Ar–H), 4.14 (2H, q, J = 7.6 Hz, –O–CH2CH3), 3.81 (8H, s, –CH2–), 3.59 (1H, dd, J = 15.6, 1.6 Hz, –CH2–), 2.72 (1H, dd, J = 15.6, 1.6 Hz, –CH2–), 2.18 (3H, s, –CH3), 1.57 (3H, s, –CH3), 1.25 (3H, t, J = 7.6 Hz, O–CH2CH3); 13C NMR (100 MHz, CDCl3) δ (ppm): 170.6 (C=C–O), 165.6 (C=O), 164.6 (C=O), 159.2 (C=O), 147.6 (aromatic C), 143.9 (aromatic C), 117.1 (aromatic C), 111.5 (aromatic C), 102.3 (C=C–O), 88.3 (–C–), 59.7 (–CH2–), 46.3 (–CH2–), 43.5 (–CH2–), 41.2 (–CH2–), 26.0 (–CH3), 14.3 (–CH2–), 14.0 (–CH3); HRMS (ESI) (m/z) Calcd for C19H24N2O6 377.17071 found: 377.17199 (M + H)+.
Results and discussion
Chemistry
The starting reagents (3a–h), unsaturated piperazines and homo piperazines, were obtained from the reaction of piperazine (1a), piperazine derivatives (1c, 1d, and 3a) and homo piperazines (1b and 3f), with acylation reagents (methacrylic anhydride (2a) and benzoyl chloride (2b)) in medium to high yields (Table 1). All acylated products bear methacryloyl moeities and their FTIR spectra show amide stretchings between 1672–1620 cm−1 and alkene stretchings at 1620–1600. Also, 1H NMR spectra of 3a–h show two terminal alkene protons between 5.3–5.0 ppm as singlet for each proton and also aromatic protons of 3c, 3e, and 3h are observed between 7.43–6.50 ppm. Moreover, 13C NMR spectra of novel unsaturated piperazine derivatives show C=O carbon peaks between 172–169 ppm and C=C peaks belonging to methacryl moeity between 141–139 ppm and 116–114 ppm. All HRMS analysis of novel unsaturated piperazine derivatives were performed with a ±5 ppm maximum margin or error.
Radical cyclization of dimethacryloyl piperazine (3b) with dimedone (4a) mediated by Mn(OAc)3 gave mono dihydrofuran (5a, 77%) and bis-dihydrofuran (5b, 10%). These compounds were diferentiated by their 1H NMR spectra. Although terminal alkene protons of 5a resonated at 5.23 ppm and 5.04 ppm, absence of these peaks in spectrum of 5b indicates that product 5b is bis-dihydrofuran. In addition, geminal proton peaks of these dihydrofuran ring are observed between 3.50–3.46 and 2.73–2.68 ppm as doublets (J = 15.2 Hz) for each proton. Also, the treatment of 3b with ethyl acetoacetate (4b) formed dihydrofurans 5c (40%) and 5d (12%) and these compounds were differentiated by 1H NMR similarly to 5a and 5b.
To further increase the formation yield of bis-dihydrofuran compound (5b), reaction of mono dihydrofuran (5a) with dimedone (4a) was performed and compound 5b was obtained in 40% yield. In addition, bis-dihydrofuran compound (5e) was obtained from the radical cyclization of 5a with ethyl acetoacetate (4b) in 36% yield (Scheme 1).
As can be seen on Table 2, radical cyclization of 3b with acetylacetone (4c) gave 5f in 65% yield. In addition, piperazine-dihydrofuran compounds 5g (60%), 5h (43%), and 5i (40%) were obtained by the reactions of 3c–e with dimedone (4a), in medium yields, respectively. Radical cyclizations of homopiperazine derivatives (3g and 3h) were also performed with dimedone (4a) and as a result 5j (41%) and 5k (50%) were obtained in medium yields. Also compound 5l (20%) was obtained from the reaction of 3e with 4b in low yield.
The proposed mechanism for the formation of dihydrofurans is explained in Scheme 2. According to this mechanism, the enol form of dimedone (A) reacts with Mn(OAc)3 and an alpha carbon radical B is formed, while Mn3+ reduces to Mn2+. An electron from alkene is added to this α-carbon radical and produces the radical carbon intermediate C. Intermediate C oxidizes to carbocation D with Mn(OAc)3 and intramolecular cyclization of D forms the product E (5a).
Biological activity
In vitro inhibition experiments of AChE
In vitro AChE inhibitory activities of test compounds were determined by slightly modified Ellman method (Ellman et al. 1961). AChE (from electric eel, type V-S), acetylthiocholine iodide (ATCI), 5,5′-Dithiobis(2-nitrobenzoic acid) (DTNB) were obtained from Sigma Aldrich.
The assay solution contained 1480 µL of phosphate buffer (pH = 8.0, 0.1 M), 50 µL of DTNB solution (prepared with pH 7 phosphate buffer), 20 µL of different concentrations of test compounds in ethanol-deionized water (1:1), 10 µL of substrate solution (ATCI, prepared with deionized water), and 25 µL of AChE solution (prepared with deionized water and 1% gelatin). All test compounds were incubated for 10 min at 30 °C and absorbances at 412 nm were determined. A control reaction containing all ingredients except inhibitory test compounds was performed same as above and the absorbance at 412 nm was considered 100% enzyme activity. The percentage activity of AChE for any tested compound was calculated with the formula:
The concentration of each test compound was tested in triplicate and IC50 values were calculated graphically using GraphPad Prism 8.0.3 software. IC50 value is defined as the concentration of sample which performs 50% inhibition towards AChE.
In vitro evaluation of inhibitory activities of piperazine-dihydrofuran compounds (5a–k) towards AChE
Over recent years there are some works in literature about AChE inhibition of piperazine containing compounds. Aliabadi et al. (2017) described the synthesis of benzamide-piperazine derivatives and evaluate their inhibition capabilities against AChE and reported IC50 values between 0.44–27 µM (Aliabadi et al. 2017). In addition, Chaves and coworkers developed hydroxybenzimidazole-donepezil hybrids and evaluated them for AChE inhibition. They reported IC50 values between 1.67–21 µM (Chaves et al. 2020). Moreover, benzothiazole-piperazine hybrids were developed by Mishra et al. and evaluated for their AChE inhibition. They reported IC50 values between 2.31 and 26.43 µM (Mishra et al. 2020).
Although AChE inhibition of many piperazine derivatives were reported in the literature, acylated piperazine derivatives (3b–h) were tested against AChE and it was found that they have almost no inhibition effect (IC50 > 100 µM). However, piperazine substituted dihydrofuran compounds (5a–l) were tested against AChE and it was confirmed that they show significantly higher inhibitions. All results were compared to standard drugs Donepezil and Galanthamine (Table 3).
IC50 values of piperazine containing dihydrofurans (5a–5d) formed by the reaction of dimethacryloyl piperazine (3b) with dimedone (4a) and ethyl acetoacetate (4b) were calculated as 17.93, 11.17, 5.79, and 3.89 µM, respectively. Also, compound (5e) has AChE inhibition effect with an IC50 value of 5.07 µM. In addition compound 5f shows medium inhibition (IC50 = 11.87 µM). It can be seen that carbethoxy carrying compound (5d) show best inhibition effect (IC50 = 3.89 µM) against AChE among them. These results show that bis-dihydrofurans (5b, 5d, and 5e) possess higher inhibiton power than mono-dihydrofurans and carbethoxy carrying piperazine-dihydrofuran compounds (5c, 5d, and 5e) are much more potent than the other dihydrofuran compounds (5a and 5b).
By comparing piperazine substituted dihydrofuran (5a) with homopiperazine-dihydrofuran (5k), it can be seen that, while 5a has inhibition effect on AChE with an IC50 value of 17.93 µM, compound 5k has almost no inhibiton effect (IC50 > 100 µM). Similar result was achieved from the comparison of compounds 5g and 5k. On the other hand, the IC50 values of piperazine substituted dihydrofuran (5g–i) were calculated as 14.34 µM for benzoyl substituted compound 5g, 8.55 µM for tetrahydrofuroyl substituted compound 5h and 4.30 µM for furoyl substituted compound 5i. According to these results, it can be seen that tetrahydrofuroyl and furoyl substituted piperazine-dihydrofuran compounds have significantly more inhibiton effect.
Summarily, in the light of these results, it can be concluded that both carbethoxy and furoyl substitutions have significant positive effect on AChE inhibiton capabilities of piperazine-dihydrofuran compounds. For this reason, compound 5l was designed and it’s inhibition effect was tested and show much higher inhibiton than other piperazine-dihydrofuran compounds (IC50 = 2.24 µM), as expected.
In silico molecular docking experiments
Three dimensional structure of recombinant human AChE complexed with donepezil was obtained from Protein Data Bank (PDB code: 4EY7) (Cheung et al. 2012). All water molecules, detergents and B-chain of enzyme were removed. Conformational analysis was performed with Avogadro software and most stable conformations were optimized with semiempirical PM6 method in Gaussian 09 Software. All ligand–protein docking calculations were performed as flexible ligand in rigid protein using AutoDock Vina software (Trott and Olson 2010). Best docking mod of ligand in terms of binding energy (kcal/mol) was selected and used. Docking results of ligands were compared with standart drug donepezil.
Molecular docking results of selected piperazine-dihydrofuran compounds (5d, 5i, and 5l)
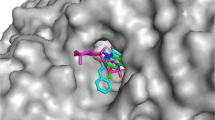
To investigate the ligand–protein interactions, molecular docking studies were performed on the three most potent inhibitor compounds 5d, 5i, 5l and standart drug donepezil. Firstly, to validate the docking procedure, co-crystallized ligand donepezil was re-docked to target AChE enzyme. Validation result shows near perfect alignment with original ligand with RMSD of 0.340 and −12.2 kcal/mol binding score. Overlapping of native ligand donepezil and re-docked donepezil was given at Fig. 1.
Top docking poses of ligands in terms of free energy were used to evaluate interactions with protein. Binding energies for donepezil, 5d, 5i, and 5l are −12.2, −8.9, −8.7, and −9.6 kcal/mol, respectively. AChE active site cavity with docked donepezil and ligand 5l were given at Fig. 2 to compare binding poses of donepezil and 5l.
As can be seen on Fig. 3 donepezil N-benzyl moeity interacts with aromatic groups of HIS447 and TRP86 through π–π interactions. Also, piperidine ring forms π–alkyl and π–π interactions with aromatic moeities of TYR341, TYR337, and PHE338. In addition carbonyl oxygen forms a conventional C–H interaction with PHE295. TRP286 interacts with benzene and methoxy group through π–π and π–σ bonds, respectively. Similarly TYR341 forms π–π and π–σ interactions with benzene and CH2 group, respectively. Ligand–protein interactions of top binding poses of ligands 5d, 5i, and 5l were given in Fig. 4.
By investigating the interactions between top docking mod of 5i and AChE it can be seen that, dimedone methyls form interactions like donepezil with aromatic groups of HIS447 at catalytic triad and TRP86 at anionic site through π–alkyl bonds. In addition dihydrofuran methyl and piperazine ring interacts with TYR341 and TYR 124 at peripheral anionic site of AChE through π–alkyl interactions. Lastly, aromatic furan ring forms a π–π interaction with TYR341.
By investigating the interactions of bis carbethoxy bearing piperazine-dihydrofuran compound 5d, it can be seen that amide carbonyl forms a conventional C–H interaction with PHE295, similar to donepezil carbonyl moeity. In addition, methyl on the dihydrofuran ring interacts through π–alkyl bonds with peripheral anionic site residues TRP286, TYR124, and TYR341. The other methyl on the dihydrofuran forms π–alkyl interactions with PHE297 and PHE338 at acyl pocket. In addition ester group of 5d interacts with HIS287 with conventional C–H interaction.
By looking at ligand–protein interactions of 5l, the most potent inhibitor in this work, it can be seen that both methyl moeities interacts with TRP286 with π–σ and π–alkyl interactions. Also, two dihydrofuran methyls form π–alkyl interactions with TYR341, TYR124, PHE297, and PHE338. In addition, piperazine ring forms π–alkyl interactions with PHE338 and TYR337 similar to piperidine ring of donepezil. Moreover, furan ring interacts with TRP86 through a π–π interaction.
By considering the ligand–protein interactions of the most potent inhibitors (5d, 5i, and 5l) on this work, it can be seen that they show similar mode of actions like standart drug donepezil and docking results support in vitro experimental results.
Conclusion
In the presented work, potential AChE inhibitors, new piperazin, and homopiperazine containing dihydrofurans (5a–l), were synthesized by Mn(OAc)3 mediated radical cyclization and their AChE inhibition capabilities were tested. Although, all piperazine-dihydrofuran compounds (except 5j and 5k) showed high inhibitory activities (IC50 values of ranging from 2.24 to 17.93 µM), all starting unsaturated piperazine derivatives (3a–h) show weak inhibitions (IC50 > 100 µM). These results indicated that dihydrofuran moeity has positive effect on inhibitions. Carbethoxy and furoyl carrying dihydrofuran compounds show high inhibition than other dihydrofuran compounds. For this reason, compound 5l, which contains both furoyl and carbethoxy moieties was synthesized and show highest inhibition of all (IC50 = 2.24 µM).
In addition molecular docking studies were performed on three most potent inhibitors (5d, 5i, and 5l), to investigate ligand–protein interactions and binding energies. Ligand–protein interactions show similar mode of actions to standart drug donepezil. Binding energies are −12.2 kcal/mol for donepezil and −8.9, −8.7, and −9.6 kcal/mol for 5d, 5i, and 5l, respectively. All these results are promising and obtained piperazine-dihydrofuran compounds may be potential AChE inhibitors.
References
Akasofu S, Kimura M, Kosasa T, Sawada K, Ogura H (2008) Study of neuroprotection of donepezil, a therapy for Alzheimer’s disease. Chem Biol Interact 175:222–226
Aliabadi A, Mohammadi-Farani A, Bistouni JR (2017) Synthesis and acetylcholinesterase inhibitory assessment of benzamide derivatives incorporated piperazine moiety as potential anti-Alzheimer agents. J Pharm Sci Res 9:1598–1603
Anand R, Gill KD, Mahdi AA (2014) Therapeutics of Alzheimer’s disease: past, present and future. Neuropharmacology 76:27–50
Appendino G, Cravotto G, Palmisano G, Annunziata R (1998) Synthesis of fercoprolone, a degraded prenylated coumarin. Tetrahedron 54:10819–10826
Aslan H, Öktemer A, Dal H, Hökelek T (2017) Synthesis of ferrocene substituted dihydrofuran derivatives via manganese(III) acetate mediated radical addition-cyclization reactions. Tetrahedron 73:7223–7232
Berwaldt GA, Gouvêa DP, da Silva DS, das Neves AM, Soares MSP, Azambuja JH, Siqueira GM, Spanevello RM, Cunico W (2019) Synthesis and biological evaluation of benzothiazin-4-ones: a possible new class of acetylcholinesterase inhibitors. J Enzym Inhib Med Chem 34:197–203
Burgaz EV, Yılmaz M, Pekel AT, Öktemer A (2007) Oxidative cyclization of 3-oxopropanenitriles with a,b-unsaturated amides by manganese(III) acetate. Regio- and stereoselective synthesis of 4-cyano-2,3-dihydrofuran-3-carboxamides. Tetrahedron 63:7229–7239
Castro S, Fernandez JJ, Fananas FJ, Vicente R, Rodriguez F (2016) Manganese‐mediated C–H alkylation of unbiased arenes using alkylboronic acids. Chem Eur J 22:9068–9071
Chany AC, Marx LB, Burton JW (2015) Synthesis of bicyclic tetrahydrofurans from linear precursors using manganese(III) acetate. Org Biomol Chem 13:9190
Chaudhary P, Kumar R, Verma AK, Singh D, Yadav V, Chhillar AK, Sharma GL, Chandra R (2006) Synthesis and antimicrobial activity of N-alkyl and N-aryl piperazine derivatives. Bioorg Med Chem 14:1819–1826
Chaves S, Resta S, Rinaldo F, Costa M, Josselin R, Gwizdala K, Piemontese L, Capriati V, Pereira Santos AR, Cardoso SM, Santos MA (2020) Design, synthesis, and in vitro evaluation of hydroxybenzimidazole-donepezil analogues as multitarget-directed ligands for the treatment of Alzheimer’s disease. Molecules 25:985
Chen SP, Chen BW, Dai CF, Sung PJ, Wu YC, Sheu JH (2012) Sarcophytonins F and G, new dihydrofuranocembranoids from a dongsha atoll soft coral sarcophyton sp. Bull Chem Soc Jpn 85:920–922
Cheung J, Rudolph MJ, Burshteyn F, Cassidy MS, Gary EN, Love J, Franklin MC, Height JJ (2012) Structures of human acetylcholinesterase in complex with pharmacologically important ligands. J Med Chem 55:10282–10286
Chuang CP, Wu YL (2004) Oxidative free radical reactions of enamino esters. Tetrahedron 60:1841–1847
Demirayak Ş, Şahin Z, Ertaş M, Bülbül EF, Bender C, Biltekin SN, Berk B, Sağlık BN, Levent S, Yurttaş L (2019) Novel thiazole‐piperazine derivatives as potential cholinesterase inhibitors. J Heterocycl Chem 56:3370–3386
Duan YC, Ma YC, Zhang E, Shi XJ, Wang MM, Ye XW, Liu HM (2013) Design and synthesis of novel 1,2,3-triazole-dithiocarbamate hybrids as potential anticancer agents. Eur J Med Chem 62:11–19
Dumas JA, Newhouse PA (2011) The cholinergic hypothesis of cognitive aging revisited again: cholinergic functional compensation. Pharmacol Biochem Behav 99:254–61
Ellman GL, Courtney KD, Andres Jr V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:90–95
Ergüntürk D, Gürdere MB, Budak Y, Ceylan M (2017) Synthesis, characterization, and investigations ofantimicrobial activity of 6,6-dimethyl-3-aryl-30,40,6,7-tetrahydro-10H,3H-spiro [benzofuran-2,20-naphthalene]-10,4(5H)-dione. Synth Commun 47:1501–1206
Fu J, Bao F, Gu M, Liu J, Zhang Z, Ding J, Xie S, Ding J (2020) Design, synthesis and evaluation of quinolinone derivatives containing dithiocarbamate moiety as multifunctional AChE inhibitors for the treatment of Alzheimer’s disease. J Enzym Inhib 35:118–128
Gao X, Liu L, Liu H, Tang J, Kang L, Wu H, Cui P, Yan J (2018) Structure–activity relationship investigation of benzamide and picolinamide derivatives containing dimethylamine side chain as acetylcholinesterase inhibitors. J Enzym Inhib Med Chem 33:110–114
Giacobini E (2004) Cholinesterase inhibitors: new roles and therapeutic alternatives. Pharm Res 50:433–440
Harish KP, Mohana KN, Mallesha L, Kumar BNP (2013) Synthesis of novel 1-[5-(4-methoxy-phenyl)-[1,3,4]oxadiazol-2-yl]-piperazine derivatives and evaluation of the irinvivoanticonvulsant activity. Eur J Med Chem 65:276–283
He L, Yang Y, Li F, Liu X, Pan W (2020) Mn(III)-mediated facile access to polysubstituted α-naphthols from β-keto ester derivatives and terminal alkynes. Synthesis 52:A–J
Hocaoglu B, Yilmaz M (2019) Regioselective radical addition of 3- oxopropanenitriles with terminal dienes promoted by cerium(IV) ammonium nitrate and manganese(III) acetate. Synth Commun 49:1938–1946
Hyunh TT, Nguyen VH, Nishino H (2017) One-pot synthesis of 2-oxa-7-azaspiro[4.4]nonane-8,9-diones using Mn(III)-based oxidation of 4-acylpyrrolidine-2,3-diones. Tetrahedron Lett 58:3619–3622
Ito Y, Tomiyasu Y, Kawanabe T, Uemura K, Ushimizu Y, Nishino H (2011) One-pot synthesis of cyclophane-type macrocycles using manganese(III)-mediated oxidative radical cyclization. Org Biomol Chem 9:1491
Kazuo S (1984) Synthesis of poly(diketamide sulfides) containing a piperazine group in main chain. Kinki Daigaku Kogakubu Kenkyu Hokoku 18:19–26
Khan I, Ibrar N, Abbas N, Saeed A (2014) Recent advances in the structural library of functionalized quinazoline and quinazolinone scaffolds: synthetic approaches and multifarious applications. Eur J Med Chem 76:193–244
Kobayashi K, Nagase K, Morikawa O, Konishi H (2003) Convenient synthesis of furopyranopyrandione derivatives by the CAN-mediated Furan ring formation. Heterocycles 60:939–946
Korhonen, P (1995) Novel crosslinking agents highly soluble in water, their preparation and use. PCT Int. Appl. WO 9501347
Kornsakulkarn J, Saepua S, Srichomthong K, Supothina S, Thongpanchang C (2012) New mycotoxins from the scale insect fungus Aschersonia coffeae Henn. BCC 28712. Tetrahedron 68:8480–8486
Lallemand JY, Six Y, Ricard LA (2002) Concise synthesis of an advanced clerodin intermediate through a vaultier tandem reaction. Eur J Org Chem 3:503–513
Li CK, Zhang DL, Olamiji OO, Zhang PZ, Shoberu A, Zou JP, Zhang W (2018) Mn(OAc)3-mediated regioselective radical alkoxycarbonylation of indoles, pyrimidinones, and pyridinones. Synthesis 50:2968–2973
Lofstrand VA, Matsuura BS, Furst L, Narayanam JMR, Stephenson JRC (2016) Formation and trapping of azafulvene intermediates derived from manganese-mediated oxidative malonate coupling. Tetrahedron 72:3775–3780
Meena P, Nemaysh V, Khatri M, Manral A, Luthra PM, Tiwari M (2015) Synthesis, biological evaluation and molecular docking study of novel piperidine and piperazine derivatives as multi-targeted agents to treat Alzheimer’s disease. Bioorg Med Chem 23:1135–1148
Melikyan GG (1993) Manganese(III) mediated reactions of unsaturated systems. Synthesis 9:833–850
Mishra CB, Shalini S, Gusain S, Prakash A, Kumari J, Kumari S, Yadav AK, Lynn AM, Tiwari M (2020) Development of novel N-(6-methanesulfonylbenzothiazol-2-yl)-3-(4-substituted-piperazin-1-yl)-propionamides with cholinesterase inhibition, anti-b-amyloid aggregation, neuroprotection and cognition enhancing properties for the therapy of Alzheimer’s disease. RSC Adv 10:17602
Molinuevo JL, Gauthier S (2013) Benefits of combined cholinesterase inhibitor and memantine treatment in moderate–severe Alzheimer’s disease. Alzheimers Dement 9:326–331
Mondal M, Bora U (2013) Recent advances in manganese(iii) acetate mediated organic synthesis. RSC Adv 3:18716–18754
Nair V, Mohanan K, Suja TD, Suresh E (2006) Stereoselective synthesis of 3,4-trans-disubstituted pyrrolidines and cyclopentanes via intramolecular radical cyclizations mediated by CAN. Tetrahedron Lett 47:2803–2806
Özgür M, Yılmaz M, Nishino H, Avar EÇ, Dal H, Pekel AT, Hökelek T (2019) Efficient syntheses and antimicrobial activities of new thiophene containing pyranone and quinolinone derivatives using manganese(III) acetate: the effect of thiophene on ring closure–opening reactions. N J Chem 43:5737–5751
Patel KN, Telvekar VN (2014) Design, synthesis and antitubercular evaluation of novel series of N-[4-(piperazin-1-yl)phenyl]cinnamamide derivatives Eur J Med Chem 75:43–56
Piemontese L, Tomás D, Hiremathad A, Capriati VI, Candeias E, Cardoso SM, Chaves S, Santos MA (2018) Donepezil structurebased hybrids as potential multifunctional anti-Alzheimer’s drug candidates. J Enzym Inhib Med Chem 33:1212–1224
Pretorius SI, Breytenbach WJ, de Kock C, Smith PJ, N’Da DD (2013) Synthesis, characterization and antimalarial activity of quinoline–pyrimidine hybrids. Bioorg Med Chem 21:269–277
Shea KJ, Stoddard GJ, Shavelle DM, Wakui F, Choate RM (1990) Synthesis and characterization of highly crosslinked poly(acrylamides) and poly(methacrylamides). A new class of macroporous polyamides. Macromolecules 23:4497–4507
Snider BB (1996) Manganese(III)-based oxidative free-radical cyclizations. Chem Rev 96:339–363
Sugimoto H, Iimura Y, Yamanishi Y, Yamatsu K (1995) Synthesis and structure-activity relationships of acetylcholinesterase inhibitors: 1-benzyl-4-[(5,6-dimethoxy-1-oxoindan-2-yl) methyl] piperidine hydrochloride and related compounds. J Med Chem 38:4821–4829
Tougu V (2001) Acetylcholinesterase: mechanism of catalysis and inhibition. Curr Med Chem 1:155–170
Szabo M, Herenbrink CK, Christopoulos A, Lane JR, Capuano B (2014) Structure–activity relationships of privileged structures lead to the discovery of novel biased ligands at the dopamine D2 receptor. J Med Chem 57:4924–4939
Trott O, Olson AJ (2010) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J Comput Chem 31:455–461
Ustalar A, Yilmaz M (2017) Microwave assisted synthesis of 2,3-dihydro-4H-benzo[4,5]thiazolo[3,2-a]furo[2,3-d]pyrimidin-4-ones and 6,7-dihydro-5H-furo[2,3-d]thiazolo[3,2-a]pyrimidin-5-ones using Mn(OAc)3. Tetrahedron Lett 58:516–519
Ustalar A, Yılmaz M, Osmani A, Keçeli SA (2017) Synthesis and antifungal activity of new dihydrofurocoumarins and dihydrofuroquinolines. Turk J Chem 41:80–88
Wang T, Kadow JF, Zhang Z, Yin Z, Gao Q, Wu D, Parker DD, Yang Z, Zadjura L, Robinson BA, Gong YF, Spicer TP, Blair WS, Shi PY, Yamanaka G, Lin PF, Meanwell NA (2009) Inhibitors of HIV-1 attachment. Part 4: a study of the effect of piperazine substitution patterns on antiviral potency in the context of indole-based derivatives. Bioorg Med Chem Lett 19:5140–5145
Weintraub D, Somogyi M, Meng X (2011) Rivastigmine in Alzheimer’s disease and Parkinson’s disease dementia: an ADAS-cog factor analysis. Am J Alzheimers Dis Other Demen 26:443–449
Yang SM, Malaviya R, Wilson LJ, Argentieri R, Chen X, Yang C, Wang B, Cavender D, Murray WV (2007) Simplified staurosporine analogs as potent JAK3 inhibitors. Bioorg Med Chem Lett 17:326–331
Yılmaz EVB, Yılmaz M, Öktemer A (2011a) Radical cyclizations of conjugated esters and amides with 3-oxopropanenitriles mediated by manganese(III) acetate. Arkivoc ii:363–376
Yılmaz M (2011a) Studies on the radical cyclization of 3-oxopropanenitriles and alkenes with cerium(IV) ammonium nitrate in ether solvents. Helv Chim Acta 94:1335–1342
Yilmaz M (2011b) Synthesis of dihydrofurans containing trifluoromethyl ketone and heterocycles by radical cyclization of fluorinated 1,3-dicarbonyl compounds with 2-thienyl and 2-furyl substituted alkenes. Tetrahedron 67:8255–8263
Yilmaz M, Bicer E, Ustalar A, Pekel AT (2014) Synthesis of furan-substituted dihydrofuran compounds by radicalcyclization reactions mediated by manganese(III) acetate. Arkivoc v:225–236
Yilmaz M, Pekel AT (2001a) Regioselective synthesis of 5-carbamoyl-dihydrofurans mediated manganese (III) acetate in acetic acid. Synth Commun 31:2189–2194
Yilmaz M, Pekel AT (2001b) Synthesis of benzofuran derivatives using Manganese (III) Acetate mediated addition of β-dicarbonyl compounds to alkyne and alkenes—a comparative study. Synth Commun 31:3871–3876
Yılmaz M, Pekel AT (2011) Synthesis of fluoroacylated 4,5-dihydrofurans and fluoroalkylated tetrahydrofurans by the radical cyclization using manganese(III) acetate. Part II. J Fluor Chem 132:628–635
Yilmaz M, Ustalar A (2016) Synthesis of 2-(2-phenylethenyl) substituted 4,5-dihydrofurans by regioselective addition of 1,3-dicarbonyl compounds to dienes promoted by cerium(IV) ammonium nitrate. Arkivoc iii:202–213
Yilmaz M, Ustalar A, Uçan B, Pekel AT (2016) Regio- and diastereoselective synthesis of trans-dihydrofuran-3-carboxamides by radical addition of 1,3-dicarbonyl compounds to acrylamides using manganese(III) acetate and determination of exact configuration by X-ray crystallography. Arkivoc vi:79–91
Yılmaz M, Uzunalioglu N, Pekel AT (2005) Manganese(III) acetate based oxidative cyclizations of 3-oxopropanenitriles with conjugated alkenes and synthesis of 4,5-dihydrofuran-3-carbonitriles containing heterocycles. Tetrahedron 61:8860–8867
Yılmaz M, Yakut M, Pekel AT (2008) Synthesis of 2,3-dihydro-4H-furo[3,2-c] chromen-4-ones and 2,3-dihydronaphtho[2,3-b]furan-4,9-diones by the radical cyclizations of hydroxyenones with electron-rich alkenes using manganese(III) acetate. Synth Commun 38:914–927
Yılmaz M, Yılmaz EVB, Pekel AT (2011b) Radical cyclization of fluorinated 1,3‐dicarbonyl compounds with dienes using manganese(III) acetate and synthesis of fluoroacylated 4,5‐dihydrofurans. Helv Chim Acta 94:2027–2038
Yokote S, Nishikawa S, Shibuya K, Hisano K, Nishino H (2020) Selective synthesis of spiro and dispiro compounds using Mn(III)-based oxidation of tetracarbonyl compounds. Tetrahedron 76:131165
Zhang PZ, Zhang L, Li JA, Shoberu A, Zou JP, Zhang W (2017) Phosphinoyl radical initiated vicinal cyanophosphinoylation of alkenes. Org Lett 19:5537–5540
Acknowledgements
This study was financially supported by the Scientific and Technical Research Council of Turkey (TÜBİTAK) (TBAG-116Z455) and Kocaeli University BAP (2019/031). SS thanks to TÜBİTAK for doctoral fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sari, S., Yilmaz, M. Synthesis, characterization, acetylcholinesterase inhibition, and molecular docking studies of new piperazine substituted dihydrofuran compounds. Med Chem Res 29, 1804–1818 (2020). https://doi.org/10.1007/s00044-020-02599-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-020-02599-0