Abstract
Hygienic behavior is an economically beneficial, heritable trait, which has evolved to limit the impact of honeybee pathogens. Selecting and breeding colonies with high levels of hygienic behavior has become a feasible and environmentally friendly strategy to control brood diseases in honeybee colonies worldwide. The identification of genes involved in the expression of this character may not only unravel molecular and biochemical pathways underlying hygienic behavior, but also serve as a practical approach to select disease resistance biomarkers useful for honeybee breeding programs. In the present work, we evaluated, at genetic level, Apis mellifera stocks selected for hygienic behavior, widely used for commercial apiculture in Argentina. We analyzed the expression profiles of five genes previously identified as candidates associated with hygienic behavior both in QTL and global gene expression studies in honeybees, more precisely, involved in perception and processing of olfactory information. We validated the differential expression of these genes as potentially responsible for behavioral differences in our selected stocks. Our results indicate that four of them (octopamine receptor, smell-impaired, odorant-binding protein 3, and odorant-binding protein 4) were differentially expressed between hygienic and non-hygienic bees within our highly hygienic colonies. The present findings improve our understanding of the molecular mechanisms underlying the differentiation of middle-age worker bees in their genetic propensity to perform hygienic behavior. This progress towards the genetic characterization of highly hygienic colonies that are commercially used in Argentine apiculture lays the groundwork for future development of targets for marker-assisted selection of disease-resistant honeybee stocks.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Social insects in general and the honeybee Apis mellifera L. (Hymenoptera: Apidae) in particular provide excellent models to study complex behaviors that emerge from their social life. These complex behaviors are clearly affected by genes and the social environment (Hunt 2007). The recent development of powerful genomic tools has set the stage for studying social behaviors of honeybees in molecular terms (Robinson et al. 2005; Smith et al. 2008; Zayed and Robinson 2012).
Hygienic behavior is an example of a complex behavior that has evolved as a general mechanism of resistance to brood diseases in honeybee colonies (Gilliam et al. 1983; Boecking and Drescher 1992; Spivak and Reuter 1998a, b, 2001a; Harbo and Harris 1999). This behavior, performed by middle-age workers, consists in detecting, uncapping, and removing diseased brood from combs before the pathogen is transmissible, thus reducing the spread of the infection in the colony (Rothenbuhler 1964a, b; Arathi et al. 2000).
The genetic basis of this behavior was firstly evidenced by Rothenbuhler (1964) in a classic backcrossing experiment. From the data obtained, the author inferred that the two components of the hygienic behavior, i.e., uncapping of diseased brood and removing diseased brood from the hive, were controlled by independent loci. Later studies expanded Rothenbuhler’s hypothesis to a multilocus model (Moritz 1988), providing evidence that the genetic bases of hygienic behavior are more complex than previously thought (Lapidge et al. 2002; Oxley et al. 2010).
Individual workers bees show large variation in their propensity to perform tasks of overall hygienic behavior (Arathi et al. 2000; Arathi and Spivak 2001; Oxley et al. 2010). The partitioning of hygienic tasks among workers in a hive is the result of stimuli from both the internal and external environment (Momot and Rothenbuhler, 1971) and the different genetic propensities of each worker to perform each task (“response threshold model of task allocation”: Bonabeau et al. 1998; Beshers and Fewell 2001; Oldroyd and Thompson 2007). Previous studies support the hypothesis that a worker’s genetic propensity to engage in hygienic behavior is related to the ability to detect and integrate specific olfactory cues emitted from diseased, parasitized, or dead brood (Masterman et al. 2000; Spivak et al. 2003; Nazzi et al. 2004; Swanson et al. 2009; Schöning et al. 2012; Chakroborty et al. 2015; Mondet et al. 2015). These authors found that bees that express hygienic behavior have higher olfactory sensitivity to odors associated with diseased brood than non-hygienic bees.
In this context, the genes responsible for the expression of hygienic behavior may potentially be those encoding proteins that directly interact with the stimulus (e.g., odorant-binding proteins, odor receptors), or participate in its biochemical pathway (e.g., transcription factors, proteins involved in chemosensory transduction and signal processing and integration). Additionally, genes that influence social interactions among workers and nestmate recognition and genes involved in olfactory learning and memory may also be candidates for hygienic behavior. In support of this hypothesis, a previous study based on QTL analyses reported candidate genes associated with hygienic behavior (Oxley et al. 2010), including some genes involved in olfaction, learning, and social behavior (reviewed by Zakar et al. 2014).
Varroa-sensitive hygiene (VSH), a specific hygienic behavior towards the ectoparasite Varroa destructor, is of particular interest as it interrupts the reproductive cycle of the mite, thereby limiting the number of offspring produced in honeybee colonies (reviewed by Rosenkranz et al. 2010; Nazzi and Le Conte 2016). The identification of genes that influence this behavior would help understand the molecular pathways associated with social immunity in the honeybee and, more importantly, provide putative targets for marker-assisted selection of brood-disease-resistant colonies. Specifically, several genes associated with neuronal sensitivity and olfaction have been proposed as candidates involved in VSH and resistance to Varroa parasitism in previous microarray and QTL analyses (Navajas et al. 2008; Le Conte et al. 2011; Tsuruda et al. 2012; Mondet et al. 2015, Spötter et al. 2016).
The similarities and differences between the two types of hygiene, general hygienic behavior and VSH, are not well understood (Danka et al. 2013), and the genetic and biochemical mechanisms that drive them are poorly resolved (Parker et al. 2012). In particular, previous evidence has shown that selectively breeding bees to enhance the general hygienic behavior would increase the ability of the honeybee population to manage varroosis (Spivak and Reuter 2001b; Harbo and Harris 2005; Büchler et al. 2010; Rinderer et al. 2010). However, a later study found lack of a strong phenotypic association between general hygienic behavior and VSH (Danka et al. 2013). At a genetic level, results from previous microarray and QTL analyses have shown some overlapping between candidate genes for the two behaviors (revised by Mondet et al. 2015; see Parker et al. 2012 for a comparative proteomic analysis of VSH and general hygienic behavior).
The expression profiling of specific genes represents a reliable tool to study the underlying mechanisms of ecologically relevant insect behaviors (Fitzpatrick et al. 2005; Dallacqua et al. 2007; Lockett et al. 2016). Particularly for the hygienic behavior in honeybees, changes in the expression of candidate genes can be used to understand their contribution to behavioral variation among worker bees or colonies, and allow the characterization of commercial honeybee stocks at a genetic level.
We have previously advanced on the behavioral description of hygienic behavior dynamics, and reported specialization and persistence among hygienic honeybees in selected colonies (Palacio et al. 2010; Scannapieco et al. 2016) from the Argentine Bee Breeding Program—MeGA (Palacio et al. 2000). In the present study, we characterized the behavioral differentiation between hygienic and non-hygienic honeybees from these highly hygienic colonies using a comparative expression analysis. We selected five genes previously described in the literature as involved in the perception and processing of olfactory information (smell-impaired 21F, rodgi, odorant-binding protein 3, odorant-binding protein 4) and memory olfaction (octopamine receptor 1), and validated their expression profiles in our selected stocks. These genes have been previously identified as candidates involved in hygienic behavior (Oxley et al. 2010; Boutin et al. 2015) and Varroa tolerance (Navajas et al. 2008; Tsuruda et al. 2012; Mondet et al. 2015) in genetic studies performed in other honeybee stocks. We compared expression profiles of individual workers that were observed performing hygienic behavior [hygienic bees (H)] with those of workers that were not observed performing hygienic behavior (NOP bees). Our study was conducted on worker heads considering that the gene expression in brain and sensorial organs is closely related to the behavioral status in honeybees (Zayed and Robinson 2012; Mondet et al. 2015). We hypothesized that differences in the perception and/or integration of olfactory stimuli at this level may account for the differentiation between H and NOP bees from selected Argentine hygienic colonies.
Materials and methods
Material
The A. mellifera worker bees used in this study came from two honeybee colonies selected for having high hygienic behavior and analyzed in detail at a behavioral level in a previous study (Scannapieco et al. 2016). These colonies belong to the MeGA program (Palacio et al. 2000) and are part of a closed-breeding program at Unidad Integrada INTA Balcarce-Facultad de Ciencias Agrarias, Universidad de Mar del Plata (Province of Buenos Aires, Argentina), which has been carried out for the last 10 years.
Emerging bees from each hygienic colony were individually marked in the thorax with a numbered tag, and placed in observation hives. Two observation hives (one for each hygienic colony), with 1000 tagged bees each, were prepared to study the activities performed by these tagged workers towards cells containing pin-killed brood (described in detail in Scannapieco et al. 2016). Each observation hive was established with a frame containing stored honey and pollen, larvae, pupae, and empty combs, and approximately 1900 untagged bees of various ages, and was provided with additional sugar syrup (sugar:water 2:1) in external feeders.
To monitor the honeybees while performing hygienic behavior, a 10 × 5 cm comb section (experimental comb) was placed in the center of the comb of each observation hive. In order to elicit hygienic behavior, each experimental comb contained 20 cells of pin-killed sealed brood and 20 cells of undisturbed sealed pupae (considered as controls) as described in Palacio et al. (2000) and by Newton and Ostasiewski (1986). This technique is usually used to measure colony-level hygienic behavior (Spivak and Downey 1998; Palacio et al. 2010).
Video recordings of the area with the experimental comb in the observation hives were made on four consecutive days. Recordings began 11 days after the last tagged bees were placed in the observation hives, when they were 11–15 days old. Behaviors of the tagged bees were recorded from the videos, and after the 4-day period, when the observations were concluded, hygienic (H) and non-hygienic (NOP) bees were immediately sampled from the observation hives. These sampled bees were 15–19 days old at the sampling time, were not involved in foraging activities, and only performed in-hive tasks during the experiment. H refers to tagged bees that performed hygienic activities during the filming period, while NOP refers to tagged bees that performed no hygienic behavior on any of the days of the observation period.
For gene expression analysis, a subset of 15-day-old (age at sampling time) H and NOP bees were selected. In particular, among these 15-day-old H sampled bees, those that showed high frequency of visits to pin-killed brood cells (>3 visits per day per bee) for at least 3 days (temporal persistence) and specifically performed olfactory sub-tasks (as inspection and uncapping) were considered for this analysis. This selection was based on previous evidence showing that inspectors and uncappers have higher odor discrimination abilities than removers (Masterman et al. 2000; Gramacho and Spivak 2003; Palacio et al. 2010). Notice that a randomly selected subset of 15-day-old NOP bees was used for gene expression analysis (like all NOP bees, these bees did not perform any sub-task in the observation hives during the experiment). The procedure was repeated for the two colonies analyzed. The sets of selected 15-day-old H and NOP bees were individually frozen and stored at −70 °C for subsequent molecular analysis.
Selection of candidate genes
Five genes putatively involved in neural development and olfaction were chosen to test their involvement in the expression of hygienic behavior. As octopamine appears to be involved in the behavioral differentiation of hygienic and non-hygienic bees (Spivak et al. 2003), we studied the expression profile of its receptor, octopamine receptor 1 (oa1), in our selected colonies. This locus has been previously characterized at molecular and physiological level by Grohmann et al. (2003) and Sinakevitch et al. (2011). We also selected two other genes: smell-impaired 21F (smi21F) and rodgi, based on that reported by Navajas et al. (2008), who found that, in V. destructor-tolerant honeybee pupae, these genes are over-expressed compared to susceptible pupae. Finally, two genes that encode odorant-binding proteins, odorant-binding protein 3 (obp3), and odorant-binding protein 4 (obp4), were chosen as candidate genes based on recent transcriptome analysis data and QTL studies (Oxley et al. 2010; Tsuruda et al. 2012; Boutin et al. 2015; Mondet et al. 2015). The gene names and symbols and the accession numbers of the cDNA sequences from GenBank are listed in Table 1.
RNA isolation and cDNA synthesis
Total RNA was individually extracted from the head of 4–5 worker bees of each behavioral state (H and NOP) and each colony (1, 2) using TRIzol® reagent (Invitrogen). All these workers were 15 days old. Specifically, we used worker heads based on the premise that the differentiation between H and NOP bees would manifest as differences at both the sensory organ and central nervous system levels (see Guarna et al. 2015; Mondet et al. 2015). The resultant RNA was resuspended in 20 μl of DEPC-treated water. The quantity and quality of RNA were assessed using a Nanodrop spectrophotometer (Thermo Scientific Nanodrop 1000) and agarose gel electrophoresis (1% P/V). About 1 µg of total RNA was used as template to synthesize first-strand cDNA using ImProm-II Reverse Transcriptase (Promega) and Oligo (dT) primers (Promega), following the manufacturer’s protocol. The resultant cDNA was diluted 1/5 for further use in Real-Time quantitative PCR (RT-qPCR) experiments.
Transcript quantification by RT-qPCR
The expression of the five candidate genes and the two reference genes (ribosomal protein 49 -rp49- and β actin -act-) was assessed using RT-qPCR (Table 1). These selected reference genes are considered suitable for normalization of qPCR data in A. mellifera since they have shown high expression stability during honeybee development and between different tissues (Lourenço et al. 2008). However, we specifically tested their expression stability level between our two groups of bees (H and NOP) before candidate gene quantification. Two parameters were considered to evaluate the stability of the reference genes: the M value (average expression stability) and the CV value (coefficient of variation on the normalized relative quantities) according to Hellemans et al. (2007). These values can then be compared against empirically determined thresholds for adequate stability, with values of M < 0.5 and CV < 25% being acceptable in gene expression studies. The analysis of the quantification of reference genes evidenced both actin and rp49 as stably expressed genes between H and NOP bees, with M and CV values lower than 0.5 and 25%, respectively (M = 0.21 and CV = 20.93 for act; M = 0.19 and CV = 18.63 for rp49).
The forward and reverse primer sequences for the standard genes were obtained from Lourenço et al. (2008) (Table 1), whereas those for the candidate genes were designed using Primer-BLAST tool (http://blast.ncbi.nlm.nih.gov/Blast.cgi) based on sequences available in the honeybee genome (Assembly scaffolds Amel 4.5; http://hymenopteragenome.org/beebase) and NCBI database (http://www.ncbi.nlm.nih.gov). Prior to quantification, the identity of the amplified regions was verified by sequencing and further comparison with the nucleotide databases, using NCBI BLASTN (Zhang et al. 2000). The efficiency for each set of primers was determined by running a dilution series (1000×, 100×, 10×, 1×) in triplicate. The efficiency values were adequately high and at least 97% (Table 1).
The RT-qPCR assays were performed in a Light Cycler 96 (Roche), using the cDNA as template. The reaction mix consisted of 10 μl of Fast Start Essential DNA Green Master (Roche), 1 μl (50 μM) of forward and reverse primers, 8 μl of dH2O, and 1 μl of cDNA template. The cycling parameters were 95 °C for 5 min followed by 40 cycles of 95 °C for 10 s and 60 °C for 45 s ending with a melting curve product amplification. Three reactions were performed for each biological sample (head of individual worker bee), and means were calculated for each locus, group (H, NOP) and colony (1, 2).
To analyze the expression profiles of the candidate genes, we applied the NRQ model, which consists of the conversion of quantification cycle values (C q) into normalized relative quantities (NRQs), the adjustment for differences in PCR efficiency between the amplicons (Pfaffl 2001), and the normalization of the data using multiple reference genes (Hellemans et al. 2007). We calculated the relative quantities and normalized the data following the formulas detailed in Hellemans et al. (2007).
Statistical analysis
To analyze differences in the gene expression between H and NOP bees, a general linear model (GLM) was applied separately for each candidate gene, using InfoStat software (Di Rienzo et al. 2014). The response variable was NRQ. We considered the behavioral state (H, NOP) as a fixed factor and the colony (1, 2) as a random factor. The Shapiro–Wilks and Levene tests and the residue normality were analyzed in each dataset (each candidate gene). The variance was modeled using Akaike criterion (AIC). To evaluate differences in NRQ between H and NOP bees, we used the LSD Fisher test. The level of significance was set at 5%.
Results
Behavioral characteristics of hygienic honeybees
From the 1000 tagged bees of each observation hive, 27% were seen performing some hygiene sub-tasks (H bees), while the remaining bees (73%) were not observed to be engaged in any hygiene activities (NOP bees) through the entire observation period (Fig. 1a). The two colonies (observation hives) showed no differences in the proportion of H and NOP bees (Chi-square test, P > 0.05). The median age of H bees was 14 days, with a high proportion of bees performing hygienic activities early in life (11–13 days old). Three types of sub-tasks associated with hygienic behavior were observed: inspecting the cells containing abnormal brood, opening capped-brood cells, and removing the brood from the cells (Fig. 1a). Also, 29 or 30% of H bees were involved in either inspecting or uncapping sub-tasks and 40% were removers (performed the removing sub-task) (Fig. 1a). Almost 60% of the H bees performed only one sub-task of the hygienic behavior (sub-task specialists) throughout the entire observation period, and a small proportion (13%) were observed performing the three activities of the hygienic behavior (Fig. 1b). Among the H bees that performed any two hygiene sub-tasks, 10.5% were observed performing the two sub-tasks associated with abnormal brood detection (inspecting and uncapping) (Fig. 1b). From this subset of H bees, 15-day-old highly hygienic and persistent bees were selected for gene expression analysis.
Distribution of hygienic activities among middle-age worker bees. a Proportion of bees that were involved in hygienic activities (H bees) and proportion of bees that were not observed performing hygienic behavior (NOP bees). Hygienic bee activities were inspecting the cells containing abnormal brood (I), uncapping the brood cells (U), and removing the brood from the cells (R). Values represent the mean of the two colonies analyzed. b Proportion of H bees that performed one, two, or three hygienic sub-tasks (inspecting, uncapping, and removing). The specific activities and their percentages are shown for the H subset that performed two out of three hygiene sub-tasks. Values represent the mean of the two colonies analyzed
Differential gene expression in the honeybee brain
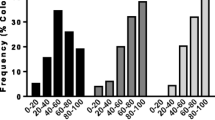
Significant differences in the expression profiles of four out of the five genes analyzed were detected between H and NOP bees (GLM analysis, Fig. 2). For all loci, the behavioral groups showed the same expression pattern in the two colonies analyzed. Means of the two colonies are plotted in Fig. 2. In particular, oa1 showed higher expression levels in H bees than in NOP bees (GLM: F 1,15 = 21.36; P = 0.0005). The other genes smi21F (GLM: F 1,13 = 21.70; P = 0.0004), obp3 (GLM: F 1,13 = 23.0; P = 0.0003), and obp4 (GLM: F 1,13 = 142.03; P < 0.0001) were down-regulated in H bees compared to NOP bees. The rodgi gene also tended to show lower expression levels in H bees than in NOP bees, with only a marginally significant difference (GLM: F 1,13 = 3.89; P = 0.0701).
Comparative expression profiles obtained by qPCR between hygienic bees (H) vs bees not observed performing hygienic behavior (NOP). Relative gene expression level (NRQ) for the candidate genes in H and NOP bees. β-actin (act) and ribosomal protein 49 (rp49) were used as reference genes. Values correspond to the means of the two colonies analyzed since both colonies showed the same expression pattern for all loci. Significant comparisons (P < 0.05) were indicated with an asterisk (*). Mean of each bar is based on 8–10 samples
Discussion
Hygienic behavior, a well-studied social trait, is an important component of social immunity in honeybees (reviewed by Wilson-Rich et al. 2009; Le Conte et al. 2011). Recent studies have shown that some variation in hygienic behavior performance as well as in Varroa tolerance could be explained by differences in the expression of genes involved in odor perception and neuronal sensitivity (Navajas et al. 2008; Oxley et al. 2010; Boutin et al. 2015; Mondet et al. 2015). In our study, in selected Argentine honeybee stocks provided by the MeGA program (Palacio et al. 2000), we validated a set of putative candidate genes that could specifically be associated with the expression of hygienic behavior. Our results indicate that the five genes analyzed here seem to be involved in the genetic propensity of worker bees of our stocks to perform hygienic behavior. Four of them (oa1, smi21F, obp3, and obp4) showed significant differences in their expression levels between bees that actively performed hygienic tasks (H) and bees that were not involved in hygienic activities (NOP). While the present results do not allow inferring a causal relationship between the gene expression pattern and hygienic behavior, they are in line with previous evidence that correlates changes in the expression of specific genes in the brain with honeybee behavior (reviewed by Zayed and Robinson 2012), and support the importance of specific metabolic routes of perception, processing, and integration of olfactory stimuli for the hygienic behavior performance.
Octopamine acts as a neuromodulator in the honeybee brain and plays a major role in olfactory learning and memory formation, thus affecting complex behavioral responses (Menzel 1999; Schulz and Robinson 2001; Spivak et al. 2003; Rein et al. 2013; reviewed by Verlinden et al. 2010). This biogenic amine exerts its effects by binding to specific receptor proteins that belong to the superfamily of the G protein-coupled receptors. The octopamine receptor 1 (oa1) characterized from the honeybee (Grohmann et al. 2003) is abundant in many somata of the brain (mushroom bodies, the antennal lobes, and the optic lobes) (Sinakevitch et al. 2011), which is consistent with its involvement in the processing of sensory inputs, antennal motor outputs, and high-order brain functions (Grohmann et al. 2003). In the present study, we detected an over-expression of oa1 in H bee heads, which is in line with previous results found by Spivak et al. (2003). These authors found higher staining intensity of octopamine-immunoreactive neurons in the brain of hygienic bees than in that of non-hygienic bees. The up-regulation of octopamine production (Spivak et al. 2003) and the up-regulation of the expression of its receptor (our study) in bees performing hygienic behavior indicate that octopamine signaling via oa1 is involved in shaping the performance of hygienic behavior in middle-age worker bees. As octopamine can enhance the response of bees to olfactory stimuli (Mercer and Menzel 1982; Spivak et al. 2003), higher levels of oa1 in the nervous system of hygienic bees could increase neural olfactory sensitivity, and hence contribute to the detection of odor cues emitted from abnormal brood at low stimulus level. Despite our results suggesting that octopamine signaling would modulate the performance of hygienic behavior, more information is required about whether H bees show increased levels of octopamine and receptor sites, and if this increase is basal or induced in H bees by exposure to abnormal brood. Behavioral studies have demonstrated that RNAi-mediated down-regulation of oa1 expression leads to a reduction in olfactory associative learning in the honeybee (Farooqui et al. 2003). Perhaps, oa1 action both increases olfactory sensitivity in H middle-age bees (which allows them to rapidly initiate the behavior) and has a role in olfactory learning through repeated experience with abnormal brood odors. The H bees analyzed in the present study are specialists in the detection sub-task and show increased temporal persistence in this activity (performance of the task through days) (Scannapieco et al. 2016), suggesting that learning could lead H bees to a better detection of the stimulus with continued exposure (Masterman et al. 2000). Overall, our results are consistent with the role of octopamine and its receptor in experience-dependent behaviors, as hygienic behavior. The specific role of oa1 in improving the behavior performance of our H bees (e.g., modulating neural sensitivity or improving olfactory learning) requires further analysis.
We found that smi21F was also differently regulated between H and NOP bees. In particular, H bees showed lower levels of the smi21F transcript than NOP bees. The same tendency was found for the rodgi gene, but with only marginally significant differences. The present results are consistent with those of Navajas et al. (2008), who detected both rodgi and smi21F as candidate genes involved in behavioral tolerance against V. destructor. These authors found a slight up-regulation of these genes in V. destructor-tolerant pupae compared to susceptible pupae. The difference in the regulation pattern between the present (down-regulation) and previous (up-regulation) results may be due to the analysis of different tissues and stages (whole pupae in Navajas et al. vs worker heads in the present study), different levels (colony level in Navajas et al. vs individual level in the present study), or different traits (Varroa tolerance in Navajas et al. vs hygienic behavior in the present study). Irrespectively of the regulation pattern, the fact that smi21F and rodgi are differentially regulated in different bee genetic resources and life stages supports the idea that they are putative genes involved in both hygienic behavior and V. destructor tolerance.
Odorant-binding proteins are water-soluble proteins that facilitate the delivery of hydrophobic odorants and pheromones to olfactory receptors located in the dendritic membrane of insect chemosensory neurons, and so are involved in chemosensory perception (reviewed by Vieira and Rozas 2011; see Forêt and Maleszka 2006). The two members of the Obp family analyzed here, obp3 and obp4, were down-regulated in our H bees compared to NOP bees. In particular, obp3 has been previously identified as a gene of interest in a transcriptomic study investigating the central nervous system of honeybees and its contribution to V. destructor-sensitive hygiene (VSH) (Le Conte et al. 2011) and, recently, in colony survival to Varroa infestation (Jiang et al. 2016). Interestingly, at brain level, this gene is regulated in a direction opposite to that of antennae; that is, while it is expressed at higher levels in the antennae of VSH+ bees (Mondet et al. 2015) than in VSH- bees, the opposite is true in the brain (Le Conte et al. 2011). This differential expression between the antennae and the brain may explain the down-regulation of obp3 in H bees compared with NOP bees in our study, as we did not discriminate between these two tissues for RNA extraction. Meanwhile, obp4 has been found in a QTL region associated with general hygienic behavior (Oxley et al. 2010), and over-expressed in non-hygienic bees in a transcriptomic study of brains of hygienic and non-hygienic worker bees (Boutin et al. 2015). Moreover, recent studies have reported obp4 as a putative gene involved both in VSH in A. mellifera (Tsuruda et al. 2012) and in resistance to the mite in the eastern honeybee, Apis cerana (Fabricius) (Ji et al. 2014). In agreement with these previous studies, our results provide evidence for a general pattern of down-regulation of the whole set (antennae plus brain), and highlight obp3 and obp4 as strong candidates for the involvement in the molecular pathways of hygienic behavior in A. mellifera (regardless of the source of the stimulus: pin-killed, freeze-killed, or V. destructor-infested brood). However, the extent to which Obps are critical for olfactory discrimination in H bees remains unclear.
The importance of using genetic tools to improve the conservation management of local bees has been shown by Zayed (2009). Our study analyzed transcriptional profiles of genes putatively involved in the expression of hygienic behavior in selected Argentine honey bee stocks, and yielded valuable information that can be implemented in breeding efforts to improve the selection strategy of genetic resources used by commercial apiculture. We envision that the information reported here could be useful to develop a molecular diagnosis technique that could complement the time-consuming field behavioral assays usually used to evaluate and identify disease-tolerant resources.
Since similar neurological proteins that correlated with hygienic behavior have been found in hygienic colonies from different geographical origins (Guarna et al. 2015), and since the genes analyzed here are involved in the expression of this character in different honeybee stocks irrespectively of variations in selection environments (discussed by Zakar et al. 2014), it would be possible to find repeatable and stable markers for marker-assisted selection of bee stocks in Argentina, or even worldwide. In fact, a preliminary analysis of oa1 expression in V. destructor-tolerant Argentine stocks has shown the same expression pattern for this gene, with an up-regulation both in hygienic (present study) and V. destructor-tolerant bees (A.C. Scannapieco, work in progress).
Overall, the analysis of expression patterns of genes putatively involved in the hygienic behavior presented here allowed the molecular characterization of selected and highly hygienic honeybee stocks of extreme importance for Argentine commercial apiculture. Our results support the idea that certain genes involved in different points of the olfactory system (perception and processing) may enable honeybees to respond to chemical cues, facilitating the performance of the various behavioral tasks involved in hygienic behavior. The identification of differentially regulated genes in H and NOP MeGa bee stocks performed in this work constitutes a starting point for an in-depth study regarding the mechanism of regulation of these genes and their contribution to behavioral differentiation between worker bees inside hygienic colonies. Our approach provides the basis for further broadening the spectrum of genes and pathways associated with the hygienic behavior in selected stocks of Argentine honeybees.
References
Arathi HS, Spivak M (2001) Influence of colony genotypic composition on the performance of hygienic behaviour in the honeybee, Apis mellifera L. Anim Behav 62:57–66
Arathi HS, Burns I, Spivak M (2000) Ethology of hygienic behaviour in the honey bee Apis mellifera L. (Hymenoptera: Apidae): behavioural repertoire of hygienic bees. Ethology 106:365–379
Beshers SN, Fewell JH (2001) Models of division of labor in social insects. Annu Rev Entomol 46:413–440
Boecking O, Drescher W (1992) The removal response of Apis mellifera L. colonies to brood in wax and plastic cells after experimental and natural infestation with Varroa jacobsoni Oud. and to freeze-killed brood. Exp Appl Acarol 16:321–329
Bonabeau E, Theraulaz G, Deneubourg JL (1998) Fixed response thresholds and the regulation of division of labour in insect societies. Bull Math Biol 60:753–807
Boutin S, Alburaki M, Mercier PL, Giovenazzo P, Derome N (2015) Differential gene expression between hygienic and non-hygienic honeybee (Apis mellifera L.) hives. BMC Genom 16:500
Büchler R, Berg S, Le Conte Y (2010) Breeding for resistance to Varroa destructor in Europe. Apidologie 41:393–408
Chakroborty NK, Bienefeld K, Menzel R (2015) Odor learning and odor discrimination of bees selected for enhanced hygienic behavior. Apidologie 46:499–514
Dallacqua RP, Simões ZLP, Bitondi MMG (2007) Vitellogenin gene expression in stingless bee workers differing in egg-laying behavior. Insect Soc 54:70–76
Danka RG, Harris JW, Villa JD, Dodds GE (2013) Varying congruence of hygienic responses to Varroa destructor and freeze-killed brood among different types of honeybees. Apidologie 44:447–457
Di Rienzo JA, Casanoves F, Balzarini MG, Gonzalez L, Tablada M, Robledo CW (2014) InfoStat versión 2014. Grupo InfoStat, FCA, Universidad Nacional de Córdoba, Argentina. http://www.infostat.com.ar
Farooqui T, Robinson K, Vaessin H, Smith BH (2003) Modulation of early olfactory processing by an octopaminergic reinforcement pathway in the honeybee. J Neurosci 23:5370–5380
Fitzpatrick MJ, Ben-Shahar Y, Smid HM, Vet LE, Robinson GE, Sokolowski MB (2005) Candidate genes for behavioural ecology. Trends Ecol Evol 20:96–104
Forêt S, Maleszka R (2006) Function and evolution of a gene family encoding odorant binding-like proteins in a social insect, the honey bee (Apis mellifera). Genome Res 16:1404–1413
Gilliam M, Taber S III, Richardson GV (1983) Hygienic behavior of honeybees en relation to chalkbrood disease. Apidologie 14:29–39
Grohmann L, Blenau W, Erber J, Ebert PR, Strünker T, Baumann A (2003) Molecular and functional characterization of an octopamine receptor from honeybee (Apis mellifera) brain. J Neurochem 86:725–735
Gramacho KP, Spivak M (2003) Differences in olfactory sensitivity and behavioral responses among honey bees bred for hygienic behavior. Behav Ecol Sociobiol 54:472–479
Guarna MM, Melathopoulos AP, Huxter E, Iovinella I, Parker R, Stoynov N, Tam A, Moon KM, Chan QWT, Pelosi P, White R, Pernal S, Foster LJ (2015) A search for protein biomarkers links olfactory signal transduction to social immunity. BMC Genom 16:63
Harbo JR, Harris JW (1999) Heritability in honey bees (Hymenoptera: Apidae) of characteristics associated with resistance to Varroa jacobsoni (Mesostigmata: Varroidae). J Econ Entomol 92:261–265
Harbo JR, Harris JW (2005) Suppressed mite reproduction explained by the behaviour of adult bees. J Apic Res 44:21–23
Hellemans J, Mortier G, De Paepe A, Speleman F, Vandesompele J (2007) qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol 8:R19
Hunt GJ (2007) Flight and fight: a comparative view of the neurophysiology and genetics of honey bee defensive behavior. J Insect Physiol 53:399–410
Ji T, Yin L, Liu Z, Shen F, Shen J (2014) High-throughput sequencing identification of genes involved with Varroa destructor resistance in the eastern honeybee, Apis cerana. Genet Mol Res 13:9086–9096
Jiang S, Robertson T, Mostajeran M, Robertson AJ, Qiu X (2016) Differential gene expression of two extreme honey bee (Apis mellifera) colonies showing Varroa tolerance and susceptibility. Insect Mol Biol 25:272–282
Lapidge KL, Oldroyd BP, Spivak M (2002) Seven suggestive quantitative trait loci influence hygienic behavior of honey bees. Naturwissenschaften 89:565–568
Le Conte Y, Alaux C, Martin JF, Harbo JR, Harris JW, Dantec C, Séverac D, Cros-Arteil S, Navajas M (2011) Social immunity in honeybees (Apis mellifera): transcriptome analysis of varroa-hygienic behaviour. Insect Mol Biol 20:399–408
Lockett GA, Almond EJ, Huggins TJ, Parker JD, Bourke AF (2016) Gene expression differences in relation to age and social environment in queen and worker bumble bees. Exp Gerontol 77:52–61
Lourenço AP, Mackert A, Dos Santos Cristino A, Simões ZLP (2008) Validation of reference genes for gene expression studies in the honey bee, Apis mellifera, by quantitative real-time RT-PCR. Apidologie 39:372–385
Masterman R, Smith BH, Spivak M (2000) Brood odor discrimination abilities in hygienic honey bees (Apis mellifera L.) using proboscis extension reflex conditioning. J Insect Behav 13:87–101
Menzel R (1999) Memory dynamics in the honeybee. J Comp Physiol A 185:323–340
Mercer A, Menzel R (1982) The effects of biogenic amines on conditioned and unconditioned responses to olfactory stimuli in the honeybee Apis mellifera. J Comp Physiol A 145:363–368
Momot JP, Rothenbuhler WC (1971) Behaviour genetics of nest cleaning in honeybees. VI. Interactions of age and genotype of bees, and nectar flow. J Apic Res 10:11–21
Mondet F, Alaux C, Severac D, Rohmer M, Mercer AR, Le Conte Y (2015) Antennae hold a key to Varroa-sensitive hygiene behaviour in honey bees. Sci Rep 5:10454
Moritz R (1988) Selection of group characters in honey bees (Apis mellifera). In: Needham GR, Page RE, Delfinado-Baker M, Bowman CE (eds) Africanized honey bees and bee mites. Ellis Horwood, Chichester, pp 118–124
Navajas M, Migeon A, Alaux C, Martin-Magniette ML, Robinson GE, Evans JD, Cros-Arteil S, Crauser D, Le Conte Y (2008) Differential gene expression of the honey bee Apis mellifera associated with Varroa destructor infection. BMC Genom 9:301
Nazzi F, Le Conte Y (2016) Ecology of Varroa destructor, the major ectoparasite of the western honey bee, Apis mellifera. Annu Rev Entomol 61:417–432
Nazzi F, DellaVedova G, D’Agaro M (2004) A semiochemical from brood cells infested by Varroa destructor triggers hygienic behaviour in Apis mellifera. Apidologie 35:65–70
Newton DC, Ostasiewski NJA (1986) A simplified bioassay for behavioral resistance to American Foulbrood in honey bees (Apis mellifera L). Am Bee J 126:278–281
Oldroyd BP, Thompson GJ (2007) Behavioural genetics of the honey bee Apis mellifera. In: Simpson SJ (ed) Advances in insect physiology, vol. 33. Elsevier, Amsterdam, pp 1–49
Oxley P, Spivak M, Oldroyd BP (2010) Six quantitative trait loci influence task thresholds for hygienic behaviour in honeybees (Apis mellifera). Mol Ecol 19:1453–1461
Palacio MA, Figini EE, Ruffinengo SR, Rodriguez EM, Del Hoyo ML, Bedascarrasbure EL (2000) Changes in a population of Apis mellifera L. selected for hygienic behaviour and its relation to brood disease tolerance. Apidologie 31:471–478
Palacio MA, Rodriguez EM, Goncalves L, Bedascarrasbure EL, Spivak M (2010) Hygienic behaviors of honey bees in response to brood experimentally pin-killed or infected with Ascosphaera apis. Apidologie 41:602–612
Parker R, Guarna MM, Melathopoulos AP, Moon KM, White R, Huxter E, Pernal SF, Foster LJ (2012) Correlation of proteome-wide changes with social immunity behaviors provides insight into resistance to the parasitic mite, Varroa destructor, in the honey bee (Apis mellifera). Genome Biol 13:R81
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res 29:e45
Rein J, Mustard JA, Strauch M, Smith BH, Galizia CG (2013) Octopamine modulates activity of neural networks in the honey bee antennal lobe. J Comp Physiol A 199:947–962
Rinderer TE, Harris JW, Hunt G, De Guzman LI (2010) Breeding for resistance to Varroa destructor in North America. Apidologie 41:409–424
Robinson GE, Grozinger CM, Whitfield CW (2005) Sociogenomics: social life in molecular terms. Nat Rev Genet 6:257–270
Rosenkranz P, Aumeier P, Ziegelmann B (2010) Biology and control of Varroa destructor. J Invertebr Pathol 103:96–119
Rothenbuhler W (1964a) Behavior genetics of nest cleaning in honeybees. I. Responses of four inbred lines to disease killed brood. Anim Behav 12:578–583
Rothenbuhler W (1964b) Behavior genetics of nest cleaning in honey bees. IV. Responses of F1 and backcross generations to disease-killed brood. Am Zool 4:11–123
Scannapieco AC, Lanzavecchia SB, Parreño MA, Liendo MC, Cladera JL, Spivak M, Palacio MA (2016) Individual precocity, temporal persistence and task-specialization of hygienic bees from selected colonies of Apis mellifera. J Apic Sci 60:49–60
Schöning C, Gisder S, Geiselhardt S, Kretschmann I, Bienefeld K, Hilker M, Genersch E (2012) Evidence for damage-dependent hygienic behaviour towards Varroa destructor-parasitised brood in the western honey bee, Apis mellifera. J Exp Biol 215:264–271
Schulz DJ, Robinson GE (2001) Octopamine influences division of labor in honey bee colonies. J Comp Physiol A 187:53–61
Sinakevitch I, Mustard JA, Smith BH (2011) Distribution of the octopamine receptor AmOA1 in the honey bee brain. PLoS One 6:14536
Smith CR, Toth AL, Suarez AV, Robinson GE (2008) Genetic and genomic analyses of the division of labour in insect societies. Nat Rev Genet 9:735–748
Spivak M, Downey DL (1998) Field assays for hygienic behavior in honey bees (Hymenoptera: Apidae). J Econ Entomol 91:64–70
Spivak M, Reuter G (1998a) Honey bee hygienic behavior. Am Bee J 138:283–286
Spivak M, Reuter G (1998b) Performance of hygienic honey bee colonies in a commercial apiary. Apidologie 29:291–302
Spivak M, Reuter G (2001a) Resistance to American foulbrood disease by honey bee colonies Apis mellifera bred for hygienic behavior. Apidologie 32:555–565
Spivak M, Reuter G (2001b) Varroa destructor infestation in untreated honey bee (Hymenoptera: Apidae) colonies selected for hygienic behavior. J Econ Entomol 94:326–331
Spivak M, Masterman R, Ross R, Mesce KA (2003) Hygienic behavior in the honey bee (Apis mellifera L.) and the modulatory role of octopamine. J Neurobiol 55:341–354
Spötter A, Gupta P, Mayer M, Reinsch N, Bienefeld K (2016) Genome-wide association study of a Varroa-specific defense behavior in honeybees (Apis mellifera). J Hered 107:220–227
Swanson J, Torto B, Kells S, Mesce K, Tumlinson J, Spivak M (2009) Volatile compounds from chalkbrood Ascosphaera apis infected larvae elict honey bee (Apis mellifera) hygienic behavior. J Chem Ecol 35:1088–1116
Tsuruda JM, Harris JW, Bourgeois L, Danka RG, Hunt GJ (2012) High-resolution linkage analyses to identify genes that influence Varroa sensitive hygiene behavior in honey bees. PLoS One 7:48276
Verlinden H, Vleugels R, Marchal E, Badisco L, Pflüger H-J, Blenau W, Broeck JV (2010) The role of octopamine in locusts and other arthropods. J Insect Physiol 56:854–867
Vieira FG, Rozas J (2011) Comparative genomics of the odorant-binding and chemosensory protein gene families across the Arthropoda: origin and evolutionary history of the chemosensory system. Genome Biol Evol 3:476–490
Wilson-Rich N, Spivak M, Fefferman N, Starks P (2009) Genetic, individual, and group facilitation of disease resistance in insect societies. Annu Rev Entomol 54:405–423
Zakar E, Jávor A, Kusza S (2014) Genetic bases of tolerance to Varroa destructor in honey bees (Apis mellifera L.). Insect Sci 61:207–215
Zayed A (2009) Bee genetics and conservation. Apidologie 40:237–262
Zayed A, Robinson GE (2012) Understanding the relationship between brain gene expression and social behavior: lessons from the honey bee. Annu Rev Genet 46:591–615
Zhang Y, Liu X, Zhang W, Han R (2010) Differential gene expression of the honey bees Apis mellifera and A. cerana induced by Varroa destructor infection. J Insect Physiol 56:1207–1218
Acknowledgements
We are grateful to Marla Spivak (Department of Entomology, University of Minnesota, USA) for her valuable comments on the manuscript and Analía Martinez (Unidad Integrada INTA Balcarce, FCA-Universidad de Mar del Plata, Buenos Aires Argentina) for helping at various stages of the experiments and for her technical support with hygienic breeding material. This research was supported by the National Agency for Promotion Science and Technology (ANPCyT, through PICT 0133-2011 Project) to ACS and by the National Apicultural Program—National Institute of Agricultural Technology (INTA) to MAP and SBL.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Scannapieco, A.C., Mannino, M.C., Soto, G. et al. Expression analysis of genes putatively associated with hygienic behavior in selected stocks of Apis mellifera L. from Argentina. Insect. Soc. 64, 485–494 (2017). https://doi.org/10.1007/s00040-017-0567-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00040-017-0567-6