Abstract
The apparent absence of intra-nest signals and communication about food resources (recruitment) among social wasps does not rule out the possibility of information transfer and coordinated foraging among nestmates. In the present study, we tested the hypothesis that the common wasp (Vespula vulgaris) shows nest-based information transfer and foraging activation: an increase in the probability of an individual leaving the nest as a result of information about resources received from successful foragers. We controlled for the possibility of local enhancement, chemical trails at the food source and climatic variation. We found evidence that food choice and discovery of resources in the field by naïve foragers were assisted by information previously or simultaneously provided by experienced nestmates. This information was related to chemical cues associated with the food and possibly to its location. Our observations suggest piloting between common wasp foragers. At the trained nest, there was a change in foraging effort at the colony level when known resources were available. Reactivated, experienced foragers were the main group responsible for the increase in foraging traffic rate observed at the colony level. To our knowledge, this is the first study clearly demonstrating nest-based information transfer about food resources in V. vulgaris and one of the few providing evidence of foraging activation in social wasps. Our data are consistent with the possibility of recruitment in this group of social insects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Information can be acquired by individuals directly (personal information), or indirectly, by gathering it from other individuals (social information) (Dall et al. 2005). Useful information can be available to individuals in the form of cues or signals (Wilson 1971). Cues are passive sources of information, aspects of the physical or social environment that may convey information incidentally, and are considered evolutionarily basal (Nieh 2009; Johnson 2010). Signals are stimuli shaped by natural selection to convey expressly the information, which can be actively targeted to their recipients (Nieh 2009; Johnson 2010). Stimuli can be transmitted through different channels such as visual, chemical, acoustic or thermal (Nieh 2009; Jaffe et al. 2012).
The transfer of information through signals is “communication” (Wilson 1971). In social insects, “recruitment” is defined as a specific type of communication that brings nestmates to a location where work is required (Wilson 1971). In the context of foraging, this communication serves to bring nestmates from the nest to a food source (Wilson 1971). Recruitment can be costly (Dechaume-Moncharmont et al. 2005), situation dependent (Jeanne et al. 1995), and its intensity and efficiency can vary dramatically among species (Aguilar et al. 2005; Jarau et al. 2000). In ants and termites, recruitment is well documented, and is frequently mediated by pheromone trails (Jaffe et al. 2012). Inside their nests, bumble bees and stingless bees use a variety of foraging signals via distinct information channels (Biesmeijer and Slaa 2004; Dornhaus and Chittka 1999, 2001, 2004; Nieh 2004). Some stingless bees show evidence of “piloting” (analogous to the ants’ “tandem running”), in which one individual leads one or more nestmates to a resource (Aguilar et al. 2005; Nieh 2009). Honey bees are well known for their dance communication, codifying resource profitability and location relative to the hive (von Frisch 1967).
Despite some reports (Naumann 1970; Taylor 2012), there is no clear evidence that social wasps (Hymenoptera: Vespidae) use signals to recruit nestmates to food sources (Raveret-Richter 2000; Jeanne and Taylor 2009; Nieh 2009). The giant hornet, Vespa mandarinia, is the only wasp known to utilize a field-based recruitment signal. In this species, individual scouts spotting beehives scent mark them by means of a pheromone. Scouts trigger a group attack, coordinated with a band of nestmates (Ono et al. 1995). Thus, in social wasps, active information transfer seems to be the exception, where individual exploitation of environmental cues has been suggested to be the rule (Raveret-Richter 2000).
Social wasps are typically generalist and opportunistic foragers that use a variety of mechanisms to locate and choose the resources needed (Raveret-Richter 2000; D’Adamo and Lozada 2009; Lozada and D’Adamo 2011). Individual wasps are influenced by past foraging experience and have the ability to learn landmarks through orientation flights and to promptly associate colours and odours with food rewards (Raveret-Richter 2000). Foragers integrate old and new memories (D’Adamo and Lozada 2009) and are capable of generalizing visual stimuli (Lozada and D’Adamo 2011). In general, foraging wasps utilize a combination of cues to obtain the greatest amount of information (Jeanne and Taylor 2009). Landing responses are elicited mainly by odour cues on both protein and carbohydrate resources (Moreyra et al. 2006). Using their learning abilities, social wasp foragers return to foraging sites where they have been successful and may feed repeatedly on the same kind of resource, thus acting as facultative specialists (Raveret-Richter 2000).
Interesting insights can come from the comparison between social and solitary wasps, showing facultative specialization on a finer scale (e.g., prey genus within the constrained order or family of prey) (Gonzaga and Araújo 2007; Santoro et al. 2011). Individual specialization in solitary wasps is driven by many factors, including predator/prey size relationships (Polidori et al. 2010), prey mobility (Polidori et al. 2013), abundance (Santoro et al. 2011) and nest–nest distance (Polidori et al. 2012). The ultimate availability of the resources and the inter-individual information flow, together with the underlying learning processes involved, can be key for explaining individual foraging patterns both in a solitary and a social context.
Although the traditional view is that foraging in social wasps is an individual activity (Raveret-Richter 2000), several studies have highlighted that cue-mediated forms of information transfer and co-ordination appear to occur amongst foragers (e.g., Overmyer and Jeanne 1998; Schueller et al. 2010; Taylor 2012). In the German wasp, Vespula germanica, and in Polybia occidentalis, scent extracts diluted in sucrose presented in training feeders, or directly inserted in the nest, were associated with food by naïve wasps and used as cues to detect resources outside the nest (Overmyer and Jeanne 1998; Jandt and Jeanne 2005; Taylor et al. 2010; Schueller et al. 2010; Taylor et al. 2011, 2012a, b). This food-related transfer of information may take place in the field (e.g., local enhancement) and at the nest (e.g., foraging activation) (Nieh 2009).
Local enhancement occurs when “an animal’s attention is directed to a particular location or object by the action or presence of conspecifics” (Raveret-Richter 2000). This phenomenon is well known in social wasps: foragers frequently show a non-random, aggregated distribution both on carbohydrate and protein resources (Jeanne and Taylor 2009). Individuals of species such as V. vulgaris and V. germanica clearly show a tendency to aggregate (Raveret-Richter and Tisch 1999). This phenomenon is cue-based, not requiring any active signal (Parrish and Fowler 1983), and is context dependent (Raveret-Richter 2000; Wilson-Rankin 2014). Indeed, wasps of the genus Vespula are not known to scent mark food sources (Jandt et al. 2005; Taylor et al. 2011). Yet, chemical trails (e.g., cuticular hydrocarbons footprints) might still play a role in the context of wasp foraging (Raveret-Richter 2000; Jeanne and Taylor 2009), as they do in individual intra-nest orientation (Steinmetz and Schmolz 2003; Steinmetz et al. 2002), and the search for nest-sites of swarm-founding species (Naumann 1975; Taylor et al. 2011).
Foraging activation consists of “an increase in the probability of an individual leaving the nest as a result of information received (at the nest) from successful foragers” (Nieh 2009). Accumulation of Vespula pensylvanica foragers at baits in the field was greater when repeated visitation by nestmates was allowed (Wilson-Rankin 2014). Hrncir et al. (2007) demonstrated that P. occidentalis foragers only arrived at feeders after nestmates were trained to those feeders. An increase in forager departure rates can be artificially triggered in P. occidentalis and V. germanica colonies by the simple insertion of a sucrose solution inside their nests (Taylor et al. 2012a, b), as is also known for honey bees and bumble bees (Dornhaus and Chittka 2001). Cues alone are hence sufficient to modulate social wasp colonies’ foraging activity.
The common wasp Vespula vulgaris, native to Eurasia, has become a notorious pest in countries such as Argentina and New Zealand, attaining high densities and causing major ecological impacts in the invaded range (Lester et al. 2014). In the New Zealand beech forests (Fuscospora and Lophozonia spp.), the common wasp has spectacularly displaced the German wasp within a few years of invasion (Harris et al. 1994). To our knowledge, no experiment has yet demonstrated nest-based foraging information sharing in V. vulgaris. Moreover, no study to date has investigated if social information flow coming from wasps freely foraging in the field can trigger variation in foraging effort measurable at the colony level. Both issues are worth investigating, considering the plasticity of foraging ecology and inter-specific variation known within the genus Vespula (e.g., Parrish and Fowler 1983; Raveret-Richter and Tisch 1999; D’ Adamo et al. 2001; Kim et al. 2007; Grangier and Lester 2011). We have hence designed an experiment to study the possibility of intra-nest information sharing and foraging activation in the common wasp, aiming to answer the following questions:
-
1.
Does V. vulgaris show nest-based social information sharing? Controlling for local enhancement and chemical cues potentially left at the food source is the scent associated with a food resource simultaneously or previously brought into the nest by successful foragers learned and used by nestmates in their foraging choice in the field? Is there any evidence of information transfer concerning the location of food?
-
2.
Can a V. vulgaris colony as a whole change its foraging effort on the basis of intra-nest social information flow? Is the renewed availability of a specific food source related to a change in the foraging activity of a colony containing individuals familiar with that food source? What is the role of individual experience in the eventual increase in the foraging traffic rate at the nest?
Materials and methods
Wasp colonies and study site
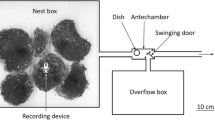
Two underground colonies of V. vulgaris were excavated from Arthur’s Pass, New Zealand (42°59′20.63″S, 171°44′48.79″E), on 10/02/2012. The nests were placed in wooden nest boxes (60 × 38 × 35 cm inside dimensions) with a metal grid 2 × 2 cm wide suspended inside at mid-height. The nest boxes were placed in the grounds of a Plant and Food Research Ltd. Laboratory in Lincoln. The two nests were placed 240 m apart from each other. Most of the site was covered in mown grass, with several tree patches dominated by Quercus spp. Nest box entrances were then opened and wasps were allowed to forage outside of the nest boxes via a clear plastic tube (20 cm length, 2 cm inside diameter). The boxes had a glass wall covered with a wooden sliding door that allowed us to monitor the status of the nests, which grew in size during the season. Colonies were given 3 weeks to recover and acclimate before our experiment began. Nest 1 was used for the experiments, Nest 2 as a control nest.
Individual foraging choice (in the field)
Training protocol
On 1st of March 2012, training was commenced near Nest 1 by allowing wasps to feed from a piece of tissue paper soaked with a 30 % sucrose solution kept directly in front of the nest box entrance. The tissue with the feeding individuals on it was then transported manually to the training feeding station (Fig. 1), initially placed 1 m from the nest. The procedure was repeated several times to encourage the wasps to become familiar with the artificial feeder. The training station was then moved in 5-m steps away from the nest at intervals of approximately 15 min, until the desired final location, the “training site” (Fig. 1), 60 m north-east of the nest. At this point, the sucrose solution was scented with 3 ml/l vanilla extract (Hansells Natural Essence, Hansells Food Group, Auckland) (experimental series I). To ensure that all the individuals directly experiencing the training site and scent were recognizable, all the wasps visiting the feeder were marked with waterproof, non-toxic Fas TM orange paint (Fine Art Supplies Ltd, Auckland) applied with a brush on their thorax and abdomen while they were feeding (referred to as “experienced foragers”). As noted previously (e.g., Wilson-Rankin 2014), marking in situ (without constriction or anaesthesia) does not appear to disturb foragers. Exposure of the scented food and concomitant marking was done for the next 4 h and during 2 and 4 March (an additional 6 h). The station was removed and re-presented in the same location each time. From the 12 March, the training station was re-presented providing a sucrose solution scented with 3 ml/l peppermint extract (Hansells Natural Essence) (experimental series II). Experienced foragers landing on the station were re-marked with Fas TM green paint. The odour switch was made to control for any innate odour preferences potentially biasing choices made at the choice station (see below).
Plan view of the set-up for a experiment 1, b experiment 2, c experiment 3 and d detail of the feeding stations (above, side view; below, plan view). a Experiment 1—foragers visiting the training site were free to go back and forth from the nest (no capture at the training site: social information allowed—see arrows). b Experiment 2—foragers visiting the training site were unable to go back and forth from the nest (capture at the training site: social information not allowed—see arrows). c Experiment 3—foragers visiting the training site were alternatively captured or not (alternate social information—see arrows). d The training station had one feeder consisting of a glass jar (18 cm high, 10 cm diameter) containing a 30 % sucrose solution scented with vanilla (V, series I) or peppermint (P, series II) placed inverted over a white plastic dish (26 cm diameter) containing four layers of folded tissue paper. The feeder was placed on the top of a white PVC cylinder (40 cm high, 30 cm diameter). The choice station had a rotatable array with three feeders separated by 10 cm from each other. One feeder was scented with vanilla (V), another with peppermint (P) and a third one had no scent (C). Sugar and scent extract concentration were the same as the training station. The position of the choice station was variable: it was placed in the area between Nest 1 and the training station (locations marked by “×”, experiment 1) or instead of the training station at the training site (experiment 2). The control station was built as the training one, but the feeder was characterized by the presence of 60 painted pins (effective wasp dummies—Parrish and Fowler 1983) on the plastic dish. The cylinder sustaining the feeder had a transparent band and four holes (8 cm diameter), each one covered by a thin metal mesh. Inside the cylinder, a visible and variable number of wasps (15–37) kept in individual transparent cages were feeding on pads imbibed with sucrose solution
Experimental protocol
Experiment 1: Newcomers’ arrival and choice with simultaneous intra-nest social information
In this experiment, the training and the choice station were presented simultaneously (Fig. 1a), with two observers sitting 1 m from each station. The training station was presented at the training site (see above). The position of the choice station, always closer to Nest 1 than the training one, was changed every hour and was determined randomly from among 19 intersections of a 10 × 10 m grid (Fig. 1a, see “×”).
At the training station (Fig. 1d), wasps were not captured and were free to fly back and forth between the training site and the nest (“social information allowed”). All unmarked, naïve individuals landing on the training station (referred to as “newcomers”) were marked with Fas water-based paint on thorax and abdomen while feeding (orange in series I; green in series II) and the number of newcomers was recorded at 10-min intervals.
The choice station (Fig. 1d) was designed to test whether the choice of the visiting wasps was influenced by the scent associated with resources brought home by nestmates and hence represented a test for nest-based information transfer. On this station, the exact arrival time and the choice of each wasp (first feeder on which each individual landed and fed) were recorded. To avoid experimental artefacts due to a side preference of the individuals (Scheiner et al. 2013) or wind direction (Overmyer and Jeanne 1998), the feeders’ position relative to the choice station was changed randomly by rotating the station’s array every 10 min. Both experienced foragers and newcomers arriving at the choice station were captured with 45-ml specimen jars. Experienced foragers (whose choice was not included in the analysis) were released at the training location at the end of each trial. Newcomers were later anesthetized with carbon dioxide, individually marked and, once they recovered, released in front of the Nest 1 entrance. Marking was achieved by means of a colour and position code of paint spots applied with water-based, soft felt-tip pens (Unipaint marker; Mitsubishi Pencil Co., Tokyo, Japan) on the thorax and one white Fas paint spot on the abdomen. If the newcomers were seen entering the nest box immediately or during the following days, they were considered part of the trained colony and included in the analysis on the feeders’ choice (see below). Experimental trials (n = 11) lasted between 60 and 200 min, encompassing a total of 31 h.
Experiment 2: Newcomers’ arrival and choice with past intra-nest social information
In this experiment, the choice and the control station were presented simultaneously, at the same distance from Nest 1 (Fig. 1b). The choice station was placed at the training site, the control station 60 m north of Nest 1 and 30 m west of the training station.
The control station (Fig. 1d) was designed to give wasps all the cues provided by a group of other foraging individuals to control for potential local enhancement biasing the number of newcomers arriving at the two stations (Fig. 1b). All the wasps visiting both stations were captured as soon as they landed on the feeders (“social information not allowed”). At the choice station, the choice of each individual was recorded. Experienced foragers were captured and released at the end of the trial. Newcomers were captured, individually marked and released after the trial, following the same protocol described in experiment 1 for the wasps arriving at the choice station. The choice station’s array (Fig. 1d) was rotated every 10 min. Experimental trials (n = 3) lasted between 120 and 240 min, encompassing a total of 10 h.
All the experiment 1 and 2 trials were run between 14.00 and 1.00 h. Data in series I (training scent = vanilla) were gathered between 5 and 12 March (12 h), while data in series II (training scent = peppermint) were gathered between 12 and 26 March (29 h).
Experiment 3: Newcomers’ arrival with alternate social information
In this experiment, the training station was placed at the training site and incoming wasps were alternatively captured or not, following a random sequence in the trials (Fig. 1c). Counts and marking of newcomers were performed following the same protocol described in experiment 1 for the wasps arriving at the training station. Data were collected between 26 March and 12 April. Experimental trials (n = 11) lasted between 60 and 180 min, encompassing a total of 28 h (capture, 17 h; no-capture, 11 h).
Control experiment 1: Innate odour preferences, eventual food-site marking substances
To control for innate odour preferences, the training scent and control scent utilized during series I were swapped during series II. The plastic dishes and the tissue paper of the feeding stations were changed daily. To control for eventual food-site marking substances, the plastic dish and tissue paper were changed between trials and every day. Moreover, one feeder with non-scented sucrose solution was kept in front of Nest 2. The feeder was visited by foragers from the untrained colony (Nest 2) and later removed and replaced for four 30-min intervals with the experimental feeder soon after experiment 2 trials (and other wasps’ visits). All the wasps landing on the feeders were captured and their choice recorded. Individuals were kept in small cages and used in the control station without being released, to ensure that only individuals naïve to the scents were counted during each trial.
Control experiment 2: Detection of the feeder by individual search
Before the beginning of the training phase, we placed the training station (unscented) in its final location, for four consecutive days. During each day (7 a.m. to 9 p.m.), we observed hourly the feeding station to see if wasps were foraging on it.
Colony foraging effort (at the nest)
To measure colony foraging effort and assess colony size during the experiment, we measured the foraging traffic rate (FTR) of the nests. FTR, here defined as the total number of wasps entering and exiting the nest in a 10-min interval, was recorded daily by means of direct observations and video analysis. During the previously described experiments, two cameras were set on the top of Nest 1 and Nest 2 tube entrances. Video recordings also allowed determining the provenience of the wasps individually marked at the feeding stations. For 8 days, simultaneously to experiment 1 trials, Nest 1 and Nest 2 FTR was video-recorded. On each day, the cameras filmed the nest entrances for the first 10-min interval of each hour starting when the feeding stations were first placed in the field (Interval I) and then during the trials, after one (Interval II) and 2 h (Interval III), respectively. The estimated number of workers in the two colonies was obtained adapting Malham et al. (1991) predictive equation based on foraging traffic rate (number of workers = 3.2243× FTR). For each colony, an average FTR value was obtained from observations over the entire duration of the experiments.
Analysis
Fisher’s exact test was used to analyse the wasps’ choice on the choice station, i.e., the differences in frequency between the numbers of newcomers from Nest 1 landing on the training scent vs the control scent dish and the control scent versus the unscented dish (experiments 1 and 2). We further tested whether individuals from Nest 2 (where no foragers were trained with scented sucrose) choose the feeders in equal proportions, as expected for control trials.
To test for significant variations in the FTR of Nest 1 and Nest 2 during experiment 1, linear mixed effects models were used. We tested the effect of the time interval on the number of wasps (all wasps from Nest 1 and 2, experienced and naïve individuals from Nest 1), with date as a random effect and climatic parameters (temperature, wind speed and direction) as covariates.
Linear mixed effects models were also used to test for differences in the number of newcomers arriving at the training site vs other sites during experiment 1 and experiment 2 and newcomers arriving at the training site during experiment 3 trials. We tested the effect of site and trial on the number of newcomers arriving at the stations in 1-h periods, with the date as a random effect and climatic parameters (temperature, wind speed and direction) as covariates.
Ten-minute interval climate data were obtained from the NIWA/Plant and Food Research meteorological station (id: 17603), 1 km north from the experimental area (data accessed from the National Climate Database; http://cliflo.niwa.co.nz/). A summary of the climatic parameters measured during the trials and considered in the analysis is available in the supplementary material (Table S1).
Data analysis was performed using R 3.0.2 (R Development Core Team 2012).
Results
During the experimental trials, Nest 1 had an estimated average number of 1636 (±357) workers, Nest 2 1383 (±323) workers. The unscented training station, placed at the training site for 48 h before the training phase, was not discovered by any wasp. At the end of the first training phase, 180 individuals (experienced foragers) were marked at the training site. No marked individuals were seen entering Nest 2 during the experiment. During the experimental trials, a maximum of 95 individuals were simultaneously present on the training station. At the choice station, during experiments 1 and 2, 81 newcomers from Nest 1 arrived (see below). We could not ascertain the provenience of eight individuals, which were not included in the analysis. No marked wasps were seen departing or arriving from Nest 2.
Individual foraging choice (in the field)
Newcomers’ choice with intra-nest social information Our first hypothesis was that naïve foragers from Nest 1 would show a preference for the feeder scented the same as the most recent resource brought into the nest by experienced foragers (i.e., the training scent) and that more newcomers would arrive when experienced nestmates were not captured at the training site. We expected no preference from naïve foragers of Nest 2. For Nest 1, there were highly significant differences (P < 0.001) in the numbers of wasps choosing the three feeders on the choice station, with naïve foragers preferentially choosing the training scent simultaneously (experiment 1) or more recently presented (experiment 2) at the training site, regardless of whether the training scent was vanilla and the control scent peppermint (as in series I) or vice versa (series II) (Table 1). No difference was found among individuals from Nest 2 (P = 0.339; Table 1). There was no difference between the number of wasps at feeders with control scent versus no added scent (P ≥ 0.335; Table 1).
Newcomers’ arrivals at the feeding stations During experiment 1, a total of 263 naïve individuals arrived at the training station. At the choice station, 58 newcomers arrived from Nest 1 (Table 1), five were of unknown provenience. During experiment 2, a total of 23 newcomers arrived at the choice station (Table 1), three of which were of unknown provenience. No newcomer arrived at the control station. More naïve wasps arrived at the training site compared to other sites (Fig. 2), both during experiment 1 (t = 5.710, df = 47, P < 0.0001) and experiment 2 (t = 4.532, df = 9, P < 0.01). None of the climatic parameters that we measured significantly influenced the number of unmarked wasps arriving at the stations during these experiments. During experiment 3, a total of 94 naïve individuals arrived at the training site. Most of them arrived when experienced foragers were not captured (t = 8.230, df = 13, P < 0.0001; Fig. 3). Wind speed had an effect on their arrival (t = 3.800, df = 13, P = 0.01).
Number of newcomers arriving at the feeding stations during experiments 1 (31 h) and 2 (10 h). Boxes represent the lower and upper quartile, the bold line is the median and whiskers represent extreme values, with the circles identifying outliers. We tested the difference in numbers of newcomers arriving at the training site vs other sites in experiment 1 and 2. ***P < 0.0001, **P < 0.01
Number of newcomers arriving at the training site during experiment 3 (28 h). Boxes represent the lower and upper quartile, the bold line is the median and whiskers represent extreme values, with the circles identifying outliers. We tested the difference in numbers of newcomers arriving at the feeding station between trials in which social information was not allowed (experienced foragers were captured) vs allowed (experienced foragers were not captured). ***P < 0.0001
Colony foraging effort (at the nest)
Our second hypothesis was that only Nest 1, with trained, experienced foragers, would show an increase in the foraging traffic rate when the feeding stations were placed in the field.
During experiment 1 trials, FTR of all individuals varied significantly in Nest 1, but not in Nest 2 (Fig. 4). Comparing all the individuals from Nest 1, there was an increase in traffic 1 h after the positioning of the stations in the field (time interval II vs I), with FTR returning to the initial intensity after 2 h (time interval III vs I) (Fig. 4). Among the climatic parameters, wind speed influenced overall FTR of the colony (t = 2.825, df = 11, P = 0.017). Considering naïve individuals from Nest 1, there was a marginal increase in FTR measured in interval II and a significant decrease in interval III (Fig. 4). The linear mixed effects model highlighted a negative effect of wind speed on the traffic rate of these individuals (t = 3.167, df = 11, P = 0.009). Considering experienced individuals from Nest 1, FTR values were higher both during interval II and III than during interval I (Fig. 4). Details of the analysis are available in the supplementary material (Table S2).
Foraging traffic rate (FTR) measured over time during experiment 1 trials for NEST 1 (trained) and NEST 2 (untrained, control). The FTR of NAÏVE and experienced (EXP) foragers from the trained colony are shown separately and summed together (NEST 1). FTR values are mean ± SE. During time interval I, the foraging stations were placed in the field. P values refer to the difference in FTR at interval II and III compared to interval I. ***P < 0.001, **P = 0.002, *P = 0.048 (please see supplementary material, Table S2 for further detail)
Discussion
Our study provides, for the first time, evidence of intra-nest social information sharing in the common wasp, V. vulgaris. Our two experiments (1, 2) for food choice in the field, similarly to Overmyer and Jeanne (1998) study on V. germanica, eliminated the confounding effect of local enhancement, which potentially biased Maschwitz et al. (1974) food choice results for V. vulgaris and V. germanica. The newcomers from the experimental colony were choosing resources matching those brought into the nest by successful foragers (the experienced individuals) (Table 1). Hence, these individuals must have learned inside the nest to associate the scent added to the sucrose at the training station to the food shared by their nestmates and used this information, a chemical cue, to find food resources in the field. Our results on the food choice of naïve V. vulgaris foragers parallel similar results on V. germanica (Overmyer and Jeanne 1998). Trophallaxis represents one possible mechanism for achieving this transfer of information, as discussed for other wasp species such as V. germanica (Overmyer and Jeanne 1998; Jandt and Jeanne 2005; Taylor et al. 2010, 2011, 2012a) and P. occidentalis (Schueller et al. 2010). The role of trophallactic exchanges in foraging information transfer and associative learning was demonstrated in bees and ants (Farina et al. 2005; Farina 1996; Provecho and Josens 2009).
Interestingly, the sucrose with the training scent was preferentially chosen by naïve foragers from the experimental colony both when the social information flow was concurrent (experiment 1) or up to several days prior (experiment 2). This finding is consistent with the fact that scents of rewarding foods are stored in long-term memory by foraging hymenopterans (Jandt and Jeanne 2005). Indeed, wasps are well known for their prompt associative learning (Raveret-Richter 2000). Remarkably, vespid foragers are able to integrate old and new experiences after one or very few learning episodes (Weiss et al. 2004; Lozada and D’Adamo 2011). Our set-up also provides evidence that additional odours, unless linked to previous experience, are meaningless to vespulids (Table 1), supporting Taylor et al. (2012a) findings on V. germanica.
During experiments 1 and 2 (Fig. 1a, b), significantly more unmarked individuals arrived at the feeding station placed at the training site compared to the other station simultaneously presented in the field (Fig. 2). This was the case even if the latter was closer to the training colony (experiment 1, Figs. 1a, 2). During experiment 3 (Fig. 1c), more naïve foragers arrived at the same site when experienced foragers were free to travel back and forth from the experimental colony and social information was allowed (Fig. 3), ruling out the possibility of site biases (Nieh 2004). These results differ from work with V. germanica (Overmyer and Jeanne 1998) but are similar to Wilson-Rankin (2014) findings on V. pensylvanica. Most excitingly, our data are consistent with the hypothesis of a mechanism of food location information transfer in the common wasp.
A proportion of the experienced foragers (36 %) and newcomers (17 %) were observed arriving at the training site almost simultaneously (within 3 s) with another individual. When the choice station was located at the training site (experiment 2), the prompt trapping of the first individual arriving (n = 8) appeared to impede the landing of the following one. These tandem arrivals, neither observed nor described in other wasps (e.g., Overmyer and Jeanne 1998; Raveret-Richter 2000; Wilson-Rankin 2014), suggest pilot flights, as for other flying hymenopterans (Nieh 2004), with newcomers possibly finding their way to the training site by following experienced nestmates. Most newcomers arrived at the training site when experienced foragers were not captured (experiments 1 and 3) and the appearance of tandem arrivals could result from the high number of departing and arriving foragers at the feeding station. In fact, piloting is difficult to prove, but has often been suggested in stingless bees, on the basis of the temporal synchrony in the arrival times of foragers and recruits (e.g., Aguilar et al. 2005; Kajobe and Echazarreta 2005; Nieh 2009). We propose it as a possible mechanism partially explaining the observed patterns.
From this study, we temper our conclusions and urge caution regarding food location communication behaviours in this wasp species, for three main reasons. Firstly, training to different sites would ideally be required to confirm our results (Nieh 2004). Secondly, when not captured, a number of experienced foragers (most of them arriving during the first hour) were present on the feeder and could themselves have provided an additional, predominant cue to naïve foragers. Even if the experiment 2 control station was designed to control as much as possible for this potential local enhancement bias on the newcomers’ arrival, no formal test for local enhancement has been standardized yet (Schueller et al. 2010). Thirdly, newcomers arriving at the training site during experiment 1 and 3 were not individually marked and we do not know how many of them were from the experimental colony (Nieh 2004), although we suspect it to be the vast majority. The disentangling of any mechanism underlying the observed patterns, such as piloting, was beyond the scope of the present study.
We also demonstrated that there can be significant adjustments in the foraging effort of one wasp colony in response to social information about one food source (Fig. 4). Given the difficulties encountered in the training of large numbers of vespulid foragers, our data are limited to one experimental colony and one control colony. Nonetheless, the experimental, trained colony as a whole repeatedly and consistently peaked in its foraging effort after the feeding stations were placed in the field (Fig. 4, interval II), while the control colony did not. We therefore interpret our results as evidence for a conditional, plastic response at the colony level to the availability of resources in the field. The rapid increase in foraging traffic rate of the trained colony, corresponding to an increase in the food brought back to the nest, is similar to that recorded by Taylor et al. (2012a) when inserting food in V. germanica nests. To our knowledge, we have demonstrated for the first time that a measurable colony response can be triggered by the activity of free-flying, trained wasps, without the insertion of the resource inside the nest.
Interesting dynamics emerged when considering naïve and experienced individuals’ foraging effort separately (Fig. 4). When the feeding stations were present, a marginal and provisional increase in the foraging traffic of naïve individuals was recorded. The increase in traffic rate at the colony level was primarily due to an increased and stable response of reactivated, experienced individuals. In honey bees, when a forager flies back to the hive after a successful foraging trip, it transfers information about nectar odour and taste of the visited flowers without dancing, via simple trophallaxis (von Frish 1967). In both honey bees’ and stingless bees’ colonies, successful foragers can stimulate a greater number of hive-mates to forage by sharing nectar with higher frequency (Farina 1996; Sánchez et al. 2004). If experienced bees resume their visits to known, previously exhausted nectar sources, a “foraging reactivation” takes place (Gil and Farina 2002; Sánchez et al. 2004). The experienced individuals can navigate back to the previously rewarding foraging sites through a “memory recall” mechanism (Reinhard et al. 2004). Similarly, successful bumble bee foragers bring home the odour of newly discovered food sources and actively alert nestmates, increasing colony foraging activity and conditioning resource choices of nestmates (Dornhaus and Chittka 1999, 2001, 2004).
While experienced wasps maintained high foraging activity, naïve foragers did not (Fig. 4, interval III). Indeed, these last individuals significantly reduced their activity. This result could be a homeostatic dynamic, resulting from task reorganization at the colony level. The strong resource influx due to the newly successful activity of experienced individuals would meet colony requirements and, with a necessary temporal delay, less successful naïve foragers may switch to other tasks (Johnson 2010).
Wind strength had an effect both on the trained nest foraging activity and on the number of naïve foragers arriving at the training site during experiment 3. The role of this environmental factor on the foraging activity of wasps and other flying hymenopterans was highlighted in the past (e.g., Blackith 1958; Comba 1999). Wind can indeed affect thermoregulation and flight costs, potentially impacting foraging ability and flight directionality (Comba 1999).
Our results, demonstrating learned associative preferences in food choice by “naïve” foragers, prove the existence of nest-based information transfer in V. vulgaris. The increase in foraging activity of the trained colony, in the presence of the feeding stations, provides evidence of foraging activation in the common wasp. These phenomena are likely cue-mediated, but active signals on different sensory channels are possible. Our observations on the arrival of newcomers at the feeding stations are indeed consistent with the possibility of communication and location-specific recruitment to food resources in social wasps, and suggest piloting as a possible foraging mechanism in the common wasp. Our findings, together with other studies on Vespula (e.g., Parrish and Fowler 1983; Harris et al. 1994; Kim et al. 2007; Wilson-Rankin 2014), show interesting inter-specific differences within the genus, making generalizations difficult. We hence encourage comparative studies within the genus Vespula and further experiments to investigate the possibility of recruitment in social wasps.
References
Blackith R (1958) Visual sensitivity and foraging in social wasps. Insectes Soc 2:159–169
Comba L (1999) Patch use by bumblebees (Hymenoptera: Apidae): temperature, wind, flower density and traplining. Ethol Ecol Evol. 11:243–264
D’ Adamo P, Corley JC, Lozada M (2001) Attraction of Vespula germanica (Hymenoptera: Vespidae) foragers by conspecific heads. J Econ Entomol 94:850–852
D’ Adamo P, Lozada M (2009) Flexible foraging behavior in the invasive social wasp Vespula germanica (Hymenoptera: Vespidae). Cons Biol Biodiv 102:1109–1115
Aguilar I, Fonseca A, Biesmeijer JC (2005) Recruitment and communication of food source location in three species of stingless bees (Hymenoptera, Apidae, Meliponini). Apidologie 36:313–324
Biesmeijer JC, Slaa EJ (2004) Information flow and organization of stingless bee foraging. Apidologie 35:143–157
Dall SRX, Giraldeau L-A, Olsson O, McNamara JM, Stephens DW (2005) Information and its use by animals in evolutionary ecology. Trends Ecol Evol 20:187–193
Dechaume-Moncharmont F-X, Dornhaus A, Houston AI, McNamara JM, Collins EJ, Franks NR (2005) The hidden cost of information in collective foraging. Proc R Soc B Biol Sci 272:1689–1695
Dornhaus A, Chittka L (1999) Evolutionary origins of bee dances. Nature 401:38–38
Dornhaus A, Chittka L (2001) Food alert in bumblebees (Bombus terrestris): possible mechanisms and evolutionary implications. Behav Ecol Sociobiol 50:570–576
Dornhaus A, Chittka L (2004) Information flow and regulation of foraging activity in bumble bees (Bombus spp.). Apidologie 35:183–192
Farina WM (1996) Food-exchange by foragers in the hive—a means of communication among honey bees? Behav Ecol Sociobiol 38:59–64
Farina WM, Grüter C, Díaz PC (2005) Social learning of floral odours inside the honeybee hive. Proc R Soc B Biol Sci 272:1923–1928
Von Frisch K (1967) The Dance Language and Orientation of Bees. Belknap Press of Harvard University Press, Cambridge
Gil M, Farina WM (2002) Foraging reactivation in the honeybee Apis mellifera L.: factors affecting the return to known nectar sources. Naturwissenschaften 89:322–325
Gonzaga MO, Araújo MS (2007) Individual specialization in the hunting wasp Trypoxylon (Trypargilum) albonigrum (Hymenoptera, Crabronidae). Behav Ecol Sociobiol 61:1855–1863
Grangier J, Lester PJ (2011) A novel interference behaviour: invasive wasps remove ants from resources and drop them from a height. Biol Lett 7:664–667
Harris RJ, Moller H, Winterbourn MJ (1994) Competition for honeydew between two social wasps in South Island beech forests, New Zealand. Insectes Soc. 41:379–394
Hrncir M, Mateus S, Nascimento FS (2007) Exploitation of carbohydrate food sources in Polybia occidentalis: social cues influence foraging decisions in swarm-founding wasps. Behav Ecol Sociobiol 61:975–983
Jaffe K, Issa S, Sainz-Borgo C (2012) Chemical recruitment for foraging in ants (Formicidae) and termites (Isoptera): a revealing comparison. Psyche 2012:1–11
Jandt JM, Jeanne RL (2005) German yellowjacket (Vespula germanica) foragers use odors inside the nest to find carbohydrate food sources. Ethology 111:641–651
Jandt JM, Riel L, Crain B, Jeanne RL (2005) Vespula germanica foragers do not scent-mark carbohydrate food sites. J Insect Behav 18:19–31
Jarau S, Hrncir M, Zucchi R, Barth FG (2000) Recruitment behavior in stingless bees, Melipona scutellaris and M. quadrifasciata. I. Foraging at food sources differing in direction and distance. Apidologie 31:81–91
Jeanne RL, Hunt JH, Keeping MG (1995) Foraging in social wasps: Agelaia lacks recruitment to food (Hymenoptera: Vespidae). J Kansas Entomol Soc 68:279–289
Jeanne RL, Taylor BJ (2009) Individual and social foraging in social wasps. In: Jarau S, Hrncir M (eds) Food exploitation by Social Insects: Ecological, Behavioral and Theoretical Approches. CRC Press, Boca Raton, pp 53–79
Johnson BR (2010) Task partitioning in honey bees: the roles of signals and cues in group-level coordination of action. Behav Ecol 21:1373–1378
Kajobe R, Echazarreta CM (2005) Temporal resource partitioning and climatological influences on colony flight and foraging of stingless bees (Apidae; Meliponini) in Ugandan tropical forests. Afr J Ecol 43:267–275
Kim KW, Noh S, Choe JC (2007) Lack of field-based recruitment to carbohydrate food in the Korean yellowjacket, Vespula koreensis. Ecol Res 22:825–830
Lester PJ, Gruber MAM, Brenton-Rule EC, Archer M, Corley JC, Dvorak L, Masciocchi M, Van Oystaeyen A (2014) Determining the origin of invasions and demonstrating a lack of enemy release from microsporidian pathogens in common wasps (Vespula vulgaris). Diversity Distrib 20:964–974
Lozada M, D’Adamo P (2011) Cognitive plasticity in foraging Vespula germanica wasps. J Insect Science 11:1–11
Malham JP, Rees JS, Alspach PA, Beggs JR, Moller H (1991) Traffic rate as an index of colony size in Vespula wasps. NZ J Zool 18:105–109
Maschwitz U, Beier W, Dietrich I, Keidel W (1974) Futterverständigung bei wespen der gattung Paravespula. Naturwissenschaften 11:506–506
Moreyra S, D’ Adamo P, Lozada M (2006) Odour and visual cues utilised by german yellowjackets (Vespula germanica) while relocating protein or carbohydrate resources. Aust J Zool 54:393–397
Naumann MG (1970) The Nesting Behavior of Protopolybia pumila in Panama (Hymenoptera: Vespidae). Ph.D. diss, University of Kansas
Naumann MG (1975) Swarming behavior: evidence for communication in social wasps. Science 189:642–644
Nieh JC (2004) Recruitment communication in stingless bees (Hymenoptera, Apidae, Meliponini). Apidologie 35:159–182
Nieh JC (2009) Convergent evolution of food recruitment mechanisms in bees and wasps. In: Gadau J, Fewell J (eds) Organization of Insects Societies: From Genome to Sociocomplexity. Harvard University Press, Cambridge, pp 264–286
Oldroyd BP, Fewell JH (2007) Genetic diversity promotes homeostasis in insect colonies. Trends Ecol Evol 22:408–413
Ono M, Igarashi T, Ohno E, Sasaki M (1995) Unusual thermal defence by a honeybee against mass attack by hornets. Nature 377:334–336
Overmyer SL, Jeanne RL (1998) Recruitment to food by the German yellowjacket, Vespula germanica. Behav Ecol Sociobiol 42:17–21
Parrish M, Fowler H (1983) Contrasting foraging related behaviours in two sympatric wasps (Vespula maculifrons and V. germanica). Ecol Entomol 8:185–190
Polidori C, Gobbi M, Chatenaud L, Santoro D, Montani O, Andrietti F (2010) Taxon-biased diet preference in the ‘generalist’ beetle-hunting wasp Cerceris rubida provides insights on the evolution of prey specialization in apoid wasps. Biol J Linn Soc 99:544–558
Polidori C, Ballesteros Y, Santoro D, Tormos J, Asís JD (2012) Morphological distance and inter-nest distance account for intra-specific prey overlap in digger wasps (Hymenoptera: Crabronidae). Popul Ecol 54:443–454
Polidori C, Santoro D, Blüthgen N (2013) Does prey mobility affect niche width and individual specialization in hunting wasps? A network-based analysis. Oikos 122:385–394
Provecho Y, Josens R (2009) Olfactory memory established during trophallaxis affects food search behaviour in ants. J Exp Biol 212:3221–3227
R Development Core Team (2012) R: a language and environment for statistical computing. ISBN: 3-900051-07-0
Raveret-Richter M, Tisch VL (1999) Resource choice of social wasps: influence of presence, size and species of resident wasps. Insectes Soc 46:131–136
Raveret-Richter M (2000) Social wasp (Hymenoptera: Vespidae) foraging behavior. Ann Rev Entomol 45:121–150
Reinhard J, Srinivasan MV, Zang S (2004) Scent-triggered navigation in honeybees. Nature 427:411–411
Sánchez D, Nieh JC, Hénaut Y, Cruz L, Vandame R (2004) High precision during food recruitment of experienced (reactivated) foragers in the stingless bee Scaptotrigona mexicana (Apidae, Meliponini). Naturwissenschaften 91:346–349
Santoro D, Polidori C, Asís JD, Tormos J (2011) Complex interactions between components of individual prey specialization affect mechanisms of niche variation in a grasshopper-hunting wasp. J Anim Ecol 80:1123–1133
Scheiner R, Abramson CI, Brodschneider R et al (2013) Standard methods for behavioural studies of Apis mellifera. J Apic Res 52:1–58
Schueller TI, Nordheim EV, Taylor BJ, Jeanne RL (2010) The cues have it; nest-based, cue-mediated recruitment to carbohydrate resources in a swarm-founding social wasp. Naturwissenschaften 97:1017–1022
Steinmetz I, Schmolz E (2003) Use of terrestrial chemical trails for nest orientation in an open nesting wasp. Dolichovespula saxonica F Insectes Soc 50:292–294
Steinmetz I, Sieben S, Schmolz E (2002) Chemical trails used for orientation in nest cavities by two vespine wasps, Vespa crabro and Vespula vulgaris. Insectes Soc 49:354–356
Taylor BJ, Schalk DR, Jeanne RL (2010) Yellowjackets use nest-based cues to differentially exploit higher-quality resources. Naturwissenschaften 97:1041–1046
Taylor BJ, Nordheim EV, Schueller TI, Jeanne RL (2011) Recruitment in swarm-founding wasps: Polybia occidentalis does not actively scent-mark carbohydrate food sources. Psyche 2011:1–7
Taylor BJ (2012) An Investigation of the Role of Signal and Cues in the Coordination of Foraging in Social Wasps. Ph.D. diss, University of Wisconsin-Madison
Taylor BJ, Nordheim EV, Jeanne RL (2012a) Allocation of colony-level foraging effort in Vespula germanica in response to food resource quantity, quality, and associated olfactory cues. Ethology 118:594–605
Taylor BJ, Nordheim EV, Jeanne RL (2012b) Introduction of a scented carbohydrate resource into the nest increses departure rate in Polybia occidentalis. Insectes Soc 59:151–157
Wilson EO (1971) The Insect Societies. Belknap Press of Harvard University Press, Cambridge
Weiss MR, Wilson EE, Castellanos I (2004) Predatory wasps learn to overcome the shelter defenses of their larval prey. Anim Behav 68:45–54
Wilson-Rankin EE (2014) Social context influences cue-mediated recruitment in an invasive social wasp. Behav Ecol Sociobiol 68:1151–1161
Acknowledgments
We thank Lloyd Stringer and Bob Brown for their help during nests’ excavation and data collection, the anonymous reviewers and Insectes Sociaux Associate Editor Miriam Richards for their constructive criticism and valuable comments on the previous versions of the manuscript. This work was funded by Victoria University of Wellington.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Santoro, D., Hartley, S., Suckling, D.M. et al. Nest-based information transfer and foraging activation in the common wasp (Vespula vulgaris). Insect. Soc. 62, 207–217 (2015). https://doi.org/10.1007/s00040-015-0395-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00040-015-0395-5