Abstract
Objectives
To investigate longitudinal associations of smoking and a change in smoking status with leisure-time physical inactivity. In addition, to control whether familial confounding (genetics and shared environment) influences the associations.
Methods
Data were based on the population-based Finnish Adult Twin Cohort of 5254 twin individuals born in 1945–1957 (41% men) and who participated in all four surveys over a 35-year follow-up (1975–2011). Logistic and conditional logistic regression models with multiple covariates were used for analyses.
Results
Compared to never-smokers, long-term daily smokers (1975–1990) had the highest likelihood for both long-term inactivity and to change into inactive by 2011. Recurrent smoking was associated with long-term inactivity. Instead, in comparison to persistent daily smokers, quitting smoking decreased the likelihood of becoming physically inactive at leisure time. The associations remained in the analyses which accounted for multiple covariates and/or familial confounding.
Conclusions
Daily smoking increases the likelihood of remaining or becoming physically inactive over the decades. Our results emphasize not only the importance of preventing smoking initiation, but also to support early smoking cessation in promotion of lifelong physical activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Physical inactivity and tobacco smoking are major preventable risk factors for non-communicable diseases, including cardiovascular diseases, chronic respiratory diseases, diabetes, and many cancers (Lee et al. 2012; Lim et al. 2012). Both contribute to increasing health care costs (Krueger et al. 2014) and shortened life spans (Lim et al. 2012). Globally, around one-third of adults are reported as being physically inactive during their leisure time (World Health Organization 2011), whereas more men (31%) than women (6%) are daily smokers (Ng et al. 2014). Even though age-adjusted prevalence rates of daily smoking (Ng et al. 2014) and leisure-time physical inactivity (Borodulin et al. 2016) have shown decreasing trends over recent decades in many high-income countries, the prevalence of smoking and inactivity is still high or is even increasing in other countries (Dumith et al. 2011; Ng et al. 2014).
Smoking often coexists with other unhealthy lifestyle habits (Auer et al. 2014; Morris et al. 2016). Accumulating evidence from longitudinal studies indicates that adults who are daily smokers are less physically active in their leisure time than non-smokers (Auer et al. 2014; Azagba and Asbridge 2013; Paavola et al. 2004). However, the association between smoking and leisure-time physical inactivity has not been consistent (Bauman et al. 2012; Kaczynski et al. 2008). More longitudinal studies with a follow-up over several decades and several time points to track changes in smoking and leisure-time physical activity (LTPA) are needed to establish whether a change in smoking behavior would affect physical activity behavior.
Both smoking (Li et al. 2003; McGue et al. 2014) and LTPA (de Geus et al. 2014; de Vilhena e Santos et al. 2012) are known to have a moderate-to-strong genetic component. Studies about the familial confounding underlying smoking and physical inactivity are, however, rare. Twin studies provide possibility to a co-twin control design which applies for determining whether the association between the factors is influenced by familial confounding (McGue et al. 2010). All twin pairs raised together have the same family background and thus share many environmental exposures in childhood. Dizygotic (DZ) twin pairs also share, on average, 50% of their segregating genes, whereas monozygotic (MZ) twin pairs are virtually identical on the gene sequence level (Boomsma et al. 2002). Twin pairs discordant for leisure-time physical inactivity (one twin within a pair is active and the co-twin inactive) would provide the possibility to determine whether the association between smoking and inactivity is influenced by familial confounding. If familial factors play a role, the association between smoking status and inactivity should exist in the analyses of the whole cohort (i.e., twins treated as individuals), but not among pairs discordant for inactivity. If genetics plays a role, then the association should be present within DZ twin pairs, but not within MZ twin pairs. Further, if the association were found both within MZ and DZ pairs, the finding would suggest independence from familial factors and support a direct association between smoking and physical inactivity. Due to the lack of longitudinal studies that control for familial confounding, we investigated the longitudinal association between smoking status and leisure-time physical inactivity in the Finnish adult twin cohort with a follow-up from 1975 to 2011. Our specific aims were to analyze the effects of smoking behavior change and familial confounding on these associations.
Methods
Sample
Four waves of postal surveys (participation rates 72–89%) from the population-based Finnish Older Twin Cohort of same-sex adult twins were gathered in 1975, 1981, 1990, and 2011 (Kaprio 2013). To maintain continuity, the original questions used in 1975 were used wherever possible over time. Our analysis was restricted to twins born between 1945 and 1957 invited to the 2011 survey (Piirtola et al. 2016). Among the 5575 individuals participating in all four surveys, 321 individuals (6% of the total sample) had missing or incomplete LTPA data in at least one survey and were therefore excluded. In 1975, there were no differences in leisure-time physical inactivity, smoking status, or mean alcohol consumption between the 321 excluded and the 5254 included participants. However, the mean age of the 321 excluded individuals (51% men) was 0.7 (SD 0.22) years higher (P = 0.001), and they had on average 1.18 (SD 0.15) years less education (P < 0.001) and had a 0.32 kg/m2 (SD 0.16) higher BMI (P = 0.042) compared to the 5254 included participants at baseline. Our final sample consisted of 5254 individuals. Analyses and results related to selection bias and dropout during the 35-year follow-up (1975–2011) are presented in the Electronic Supplementary Material [ESM] 1.
Leisure-time physical inactivity
The focus of this study was on a low level of LTPA, that is, leisure-time physical inactivity (outcome variable), and especially in long-term inactivity or change into inactive. The LTPA questions were the same in all surveys except in 1990. The LTPA questions and the metabolic equivalent of task (MET) calculations for this cohort have been described in detail earlier (Piirtola et al. 2014, 2016). Briefly, LTPA (exercise) was elicited by asking, “Is your leisure-time physical exercise on average as intensive as…”, with four response alternatives: walking, walking and jogging, jogging, or running. In addition, daily time for commuting by physically active means (walking, jogging, and cycling) to and from work was gathered as a separate question and included in the total MET index of LTPA. The MET index/day was calculated by multiplying the general intensity (4–13 METs) and average duration and frequency of activities at each time point and converted into MET h/day. A sub-study has shown intraclass correlations of 0.68 for the LTPA (exercise) and 0.93 for commuting activities between the questionnaire-based MET index and a comprehensive structured face-to-face interviewed MET index (Waller et al. 2008). At each survey, the mean daily LTPA energy expenditure ≤1.5 MET hours (≤10.5 MET h/week) was applied to define inactive (Dumith et al. 2011; Lee et al. 2012). Those with a MET index >1.5 were defined as active. Our focus was on the long-term LTPA behavior. Thus, a combined LTPA status in 1975 and 1981 was used for a baseline LTPA status; an inactive group was formed from those inactive both in 1975 and 1981 (n = 1728), and those active in one or both surveys were defined as active (n = 3526) (Fig. 1).

LTPA leisure-time physical activity, MET metabolic equivalent, A active (>1.5 MET h/day), I inactive (≤1.5 MET h/day), II inactive in 1975 and 1981, AA active in 1975 and 1981, AI active in 1975, inactive in 1981, IA inactive in 1975, active in 1981, IIII inactive in all four time points (1975, 1981, 1990 and 2011), AIII active in 1975, inactive in 1981, 1990 and 2011, AAII active in 1975 and 1981, inactive in 1990 and 2011, AAAI active in 1975, 1981 and 1990, inactive in 2011, IIAI inactive in 1975 and 1981, active in 1990, inactive in 2011, IAII inactive in 1975, active in 1981, inactive in 1990 and 2011, AAAA active in all four time points (1975, 1981, 1990 and 2011), IAAA inactive in 1975, active in 1981, 1990 and 2011, IIAA inactive in 1975 and 1981, active in 1990 and 2011, IIIA inactive in 1975, 1981 and 1990, active in 2011, AAIA active in 1975 and 1981, inactive in 1990, active in 2011, AIAA active in 1975, inactive in 1981, active in 1991 and 2011
Categorization of leisure-time physical activity by surveys (1975, 1981, 1990, 2011), at combined baseline (1975/1981), and in analyzing remaining (long term) inactive or becoming inactive both in the whole cohort (n = 5254) and in the leisure-time physical activity discordant twin pairs among the Finnish Older Twin Cohort, Finland, 1975–2012.
Smoking status
In each survey, smoking status (main independent variable) was elicited the same way and categorized into five classes: never-smoker, former smoker, occasional smoker, daily smoker, and other (i.e., illogical reporting/missing information) (Kaprio and Koskenvuo 1988). For the statistical analyses, the baseline smoking status (1975/1981) was categorized into five classes: never-smokers, quitters before 1975, quitters between 1975 and 1981, daily smokers, and others (EMS 2A). Correspondingly, the long-term smoking status (1975/1990) was categorized into seven major classes: consistently never-smokers, quitters before 1975, quitters during 1975/1981, quitters during 1981/1990, recurrent smokers (i.e., those unsuccessful in cessation and restarting smoking), persistently daily smokers, and others (ESM 2B). The smoking category “others” was included in the analyses to maintain all participants in the analyses. No interpretation was made of the results from the “other” group given its heterogeneity.
Covariates
Covariates related to leisure-time physical inactivity and/or smoking were assessed primarily from the 1981 questionnaire, except for socioeconomic status, which was elicited only in 1975 and consisted of six main classes: upper and lower non-manual workers, skilled and unskilled manual workers, farmers, and others (including students, conscripts, full-time homemakers, and otherwise not classified). Other covariates were age (years), education (obtained as nine categories of education attainment, then converted into years of education), marital status (dichotomized as single, divorced or widowed, vs married or cohabiting), body mass index (BMI) (kg/m2, computed from self-reported weight and height), alcohol consumption (grams of absolute alcohol per day, based on the average consumption of beer, wines, and spirits), life satisfaction (categorized into three classes: satisfied, intermediate, and dissatisfied), and working status including physical loading (sedentary work, more active work, not at work for any reason).
Statistical analyses
Stata SE version 13.1 (StataCorp, College Station, TX, USA) was used for all analyses. Logistic regression models were used to calculate odds ratios (OR) with 95% confidence intervals (CI) for the whole cohort (twins treated as individuals). To account for the dependency of twin individuals within a pair, a cluster option was used to obtain robust standard errors. All analyses were first adjusted for age and sex, then for multiple covariates. First, the cross-sectional association was analyzed between baseline smoking status (1975/1981) and baseline inactivity (1975/1981) (Fig. 1). Then, the associations were analyzed between baseline smoking status and future inactivity in 1990 or in 2011. Thereafter, we analyzed the associations of smoking status with long-term inactivity (remaining inactive) and a change in LTPA status (becoming inactive) from the baseline to 2011. Because the effect of LTPA in 1990 on the association between the long-term smoking status and inactivity over 35 years was minimal (data not shown), LTPA in 1990 was not included as a covariate in the final analyses. In all analyses, the group of consistent never-smokers was used as a reference group. In analysis to clarify the effect of quitting smoking on inactivity, the persistent daily smokers were used as reference.
We tested the interaction between sex and smoking status (at baseline or long term) on inactivity (at baseline, in 1990, or in 2011) with a log-likelihood test. A statistically significant interaction between sex and smoking status was found for inactivity (P = 0.039) at baseline but not for inactivity in 1990 (P = 0.58). Further, interaction between sex and long-term smoking status (1975/1990) was not found on inactivity in 2011 (P = 0.10). Thus, men and women were combined in all analyses with sex as a covariate. We also tested if the effect of long-term smoking status on inactivity would differ across zygosity in twin individuals. However, no interaction between zygosity and smoking was found (P = 0.69).
Finally, we repeated the longitudinal analyses for associations of baseline and long-term smoking status with inactivity using a co-twin control design (McGue et al. 2010). For the cross-sectional pairwise analyses, each twin individual was classified as either inactive or active, as was done in analyzing the whole cohort (Fig. 1). For the longitudinal discordant analyses, twin individuals were dichotomized either into predominantly inactive (n = 1337) or predominantly active (n = 2955) according to each individual’s 35-year LTPA behavior (Fig. 1) (Piirtola et al. 2016). The dichotomization was done to retain enough power in the analyses. Individuals and twin pairs with mixed LTPA behavior (n = 962) were excluded from these longitudinal discordant analyses. The data of 5254 twin individuals included altogether 1604 full twin pairs: 588 MZ and 944 DZ twin pairs. A further 72 pairs were of uncertain zygosity based on the questionnaire-based classification algorithm, showed a high degree of agreement with multiple polymorphic genetic markers (Sarna et al. 1978). At baseline, we identified 588 twin pairs (374 DZ; 190 MZ pairs, 24 uncertain zygosity) discordant for their LTPA. The number of long-term LTPA discordant twin pairs was 370 (238 DZ; 114 MZ pairs, 18 uncertain zygosity). The discordant analyses were performed using conditional (fixed-effects) logistic regression.
Results
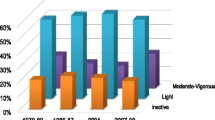
The mean age of the participants (41% men) was 24 years (range 18–31) in 1975 and 60 years (range 53–67) in 2011. At baseline, 33% of the participants were inactive (31% of men; 34% of women), 45% were never-smokers, 12% had quit smoking before 1975 and 13% between 1975 and 1981, whereas 22% were daily smokers. Table 1 describes the smoking status and the covariates by sex and the LTPA level at baseline. In 1990, 34% were inactive (39% of men; 31% of women), and 36% in 2011 (43% of men; 33% of women). The long-term smoking behavior (1975/1990) is shown in the EMS 3.
At baseline, both daily smokers and quitters between 1975 and 1981 had a higher likelihood of being inactive (Table 2), and the association remained after accounting for multiple covariates. In the longitudinal analyses, being a daily smoker at baseline (1975/1981) was associated with inactivity 9 years later in 1990 both in the age- and sex-adjusted and in the multiple-adjusted models (Table 2). Being a daily smoker or quitter between 1975 and 1981 was also associated with being inactive 30 years later in 2011 also in the multiple-adjusted model.
In the age- and sex-adjusted analyses of the long-term smoking behavior (1975/1990), persistent daily smokers showed a higher likelihood for both remaining inactive and becoming inactive in 2011 (Table 3). Additionally, recurrent smokers were more likely to remain inactive. Any history of smoking showed a trend for remaining or becoming inactive in 2011, while accounting for multiple covariates did not change the associations.
An additional analysis was run for the effect of quitting smoking on remaining or becoming inactive between the baseline and 2011. Compared to persistently daily smokers, quitters before 1975 (OR = 0.41, 95% CI 0.25, 0.67), quitters during 1975/1981 (OR = 0.53, 95% CI 0.32, 0.89), and quitters during 1981/1990 (OR = 0.42, 95% CI 0.25, 0.71) were less likely to become inactive in the multiple-adjusted analyses. The association between quitting smoking and remaining inactive was significant only in quitters during 1975/1981 (OR = 0.57, 95% CI 0.38, 0.85).
Furthermore, we tested the effect of familial confounding on the associations (Table 4). Daily smoking was associated with inactivity both in the cross-sectional and in the longitudinal discordant pair analyses. The association was strongest for persistent daily smokers, but evident also for any kind of smoking history in the longitudinal analyses. In the analyses for MZ and DZ twins separately, the association remained significant for the DZ pairs, whereas the estimates for MZ pairs attenuated.
Discussion
Principal findings
Our study within a cohort of 5254 twin individuals with responses to four comprehensive surveys over a 35-year follow-up showed that daily smoking, especially being a long-term daily smoker or a recurrent smoker, was associated with long-term leisure-time physical inactivity. In comparison to persistent daily smokers, quitters had a lower likelihood of inactivity. Accounting for familial confounding indicated that any kind of smoking history was associated with inactivity over 35 years. Our study adds to the previous findings suggesting a direct effect of smoking on physical inactivity.
Comparison with other studies
In particular, our finding of the association of long-term daily smoking with long-term physical inactivity merits attention. Despite some previously reported evidence about a lesser amount of LTPA among persistent daily smokers compared to never-smokers in a long-term continuum (Auer et al. 2014; Azagba and Asbridge 2013; Nagaya et al. 2007; Paavola et al. 2004; Perkins et al. 1993), our study is among the first with a follow-up over three decades (Auer et al. 2014; Borodulin et al. 2012) and more than two time points in the analyses (Auer et al. 2014; Azagba and Asbridge 2013; Nagaya et al. 2007; Paavola et al. 2004). In a 25-year follow-up with eight time points, increasing years of smoking were associated with a decrease in the amount of LTPA, whereas an increase in LTPA was associated with successful smoking cessation (Auer et al. 2014), which is in line with our results. However, indications exist that not all daily smoking is associated with less LTPA (Azagba and Asbridge 2013). For example, a 6-year follow-up study with several survey points showed that only daily smokers with nicotine dependence, that is, heavy smokers, who usually have a longer smoking history and who are on average less successful in smoking cessation, had a higher likelihood for inactivity compared with less dependent smokers (Azagba and Asbridge 2013). We did not have measures of nicotine dependence, a limitation that would call for further studies. It is also relevant that there is no global definition for physical inactivity (Dumith et al. 2011; Lee et al. 2012; Sedentary Behaviour Research Network 2012). Thus, the effect of smoking status on leisure-time physical inactivity might differ across studies depending on the definition of physical inactivity used.
Another important finding was that in comparison to persistent daily smokers, quitting smoking was related to a lower likelihood of inactivity. Earlier results suggest that each additional year of successful cessation increases the positive net effect of smoking cessation on an increase in LTPA compared to those who have continued smoking (Auer et al. 2014). We were only interested in those with the lowest amount of LTPA, not in an increase in LTPA. However, an increase in habitual exercise after smoking cessation was indicated in a study with annual time points over seven years among healthy men, whereas a smoking relapse also decreased the amount of LTPA (Nagaya et al. 2007).
Interpretation of the discordant analyses
An unique aspect of this study was the twin study design, which permits controlling for familial confounding. In twin pairs discordant for LTPA, the twin who was a daily smoker had a higher likelihood of being inactive in both the cross-sectional and longitudinal analyses compared to his/her non-smoking twin brother or sister. The longitudinal associations between smoking status over 1975–1990 and inactivity pointed to the same direction regardless of quitting, in other words, any kind of smoking history increased the likelihood for inactivity. Hence, our results indicate the importance of not only support for quitting smoking, but also the prevention of smoking initiation in the first place. Notably, in the long-term discordant analyses, the point estimates attenuated to statistical non-significance in the MZ pairs for all smoking categories, suggesting limited power in the separate analyses of MZ and DZ twins.
Both LTPA and smoking have a strong genetic component (de Geus et al. 2014; de Vilhena e Santos et al. 2012; Li et al. 2003; McGue et al. 2014), whereas the heritability of physical inactivity has been reported to be only modest (de Geus et al. 2014). Since smoking usually starts already during the teenage years (Auer et al. 2014), the influence of parental practices and living conditions in childhood is obvious. To the best of our knowledge, prospective twin studies analyzing the association of smoking with LTPA while controlling for familial confounding are rare if any. However, we acknowledge an earlier study conducted among the younger Finnish twin cohort, where the longitudinal association in the reverse direction, that is, between physical inactivity in adolescence and smoking in adulthood, was not confounded by familial factors (Kujala et al. 2007).
Pathways between smoking and inactivity
The mechanisms underlying the association between smoking and physical inactivity are not fully known. One potential explanation is the association between respiratory capacity and LTPA, since smoking reduces pulmonary function and therefore compromises LTPA performance (Higgins et al. 1991). However, smoking is related to inactivity already in adolescence (Aarnio et al. 2002) when the negative effects of smoking on pulmonary functions are evident but still modest (Gold et al. 1996). Long-term smoking is, however, associated with many chronic diseases (Lim et al. 2012), which might reduce physical activity. Other factors (social behavior, life circumstances, or early experiences with smoking experimentation) may also explain the association of smoking with inactivity. Notably, smoking has also been used as a weight control mechanism (Wee et al. 2001). Another pathway might be related to psychosocial factors such as mood, stress, and depression, which may affect smoking and consequently inactivity (Kaczynski et al. 2008). Long-term smoking has been shown to be associated with depression (Korhonen et al. 2011), which might modify LTPA behavior (Kaczynski et al. 2008). Instead, smoking cessation can reduce depression, anxiety, and stress and improve positive mood (Taylor et al. 2014b), whereas LTPA is known to reduce depressive symptoms (De Moor et al. 2008). However, in our study life dissatisfaction, used as a proxy for depression (Koivumaa-Honkanen et al. 2004), did not weaken the association between smoking and physical inactivity. Despite the fact that successful smoking cessation has been reported to improve mental health in a meta-analysis (Taylor et al. 2014b), a clear causal association between smoking and depression was not supported by another Mendelian randomization meta-analysis (Taylor et al. 2014a).
The pathway between smoking and inactivity may also be affected by the complex associations between several other lifestyle choices, because health-related lifestyle choices usually occur in combinations (Auer et al. 2014; Morris et al. 2016). For example, changing one lifestyle behavior could act as a catalyst to change another (Kaczynski et al. 2008), and in influential lifestyle changes, smoking seems to play a central role (Auer et al. 2014; Morris et al. 2016; Paavola et al. 2004). In our study, quitters still had a higher likelihood for inactivity, even when controlling for alcohol consumption and BMI as reflectors of other lifestyle factors. Hence, interventions aimed at influencing lifestyles should include simultaneous support for many behavioral changes, such as smoking cessation.
Study strengths and limitations
This longitudinal cohort study with twin pairs has several strengths. The comprehensive surveys repeated four times provided a unique opportunity not only to follow the same individuals over 35 years, but also to investigate the long-term development of both smoking and LTPA behaviors. The Finnish Twin Cohort is representative of the Finnish adult population (Kaprio and Koskenvuo 1988). The proportion of daily smokers decreased during the 35-year follow-up as a result of increasing cessation with age and the drop-out rate of smokers due to death and non-participation. Overall, this decrease is also in line with the decrease in smoking in adults globally (Midlöv et al. 2014; Morris et al. 2016; Ng et al. 2014). The dataset also provided a powerful case–control setting to control for familial confounding on the associations of interest. In addition to familial confounding, the study also controlled for several covariates with a known influence on smoking and LTPA.
Regarding study limitations, more current daily smokers and occasional smokers in 1975 had died by 2011, and fewer smokers participated in the last survey in 2011. Thus, this dropout most likely dilutes the associations in our analyses. Since we had time points situated 6, 9, and 21 years apart, additional changes regarding both smoking and physical inactivity might have occurred during the 35-year follow-up. Any unrecorded changes in these factors of interest could have had an impact on the observed associations and would dilute the effect. However, we believe that four time points over 35-year of follow-up has rarely been done before. Another limitation in this study is that the measure of LTPA was not based on objective but on self-reported assessments without information about domestic (everyday) activities. However, objective measurements were not available for large cohort studies in the 1970s. Our analyzing methods did not allow taking into account time-dependent changes in covariates, for example in marital status. This may have affected our results. However, the main focus of this study was on the association between smoking status and long-term leisure-time physical inactivity or change into inactive during the follow-up. The analyzing methods were selected to capture whether stability or change in smoking status would affect stability or change in leisure-time physical inactivity over time. However, further studies using alternate methods, such as bivariate cross-lagged path model, are needed to investigate causal relationship between varying smoking status and full spectrum of leisure-time physical activity as well as the influence of covariates on the associations.
Conclusions
Being a long-term persistent daily smoker increases the likelihood of remaining or becoming physically inactive, whereas quitting smoking decreases the likelihood of inactivity. However, since any kind of smoking history associates with physical inactivity in the long-term discordant analyses, efforts to prevent smoking initiation and to support smoking cessation during early adulthood along with the promotion of a healthy lifestyle might be important for promoting physical activity. The association between smoking and inactivity seems to be independent of other factors. Extensive health behavior counselling and effective tobacco control policies are needed in promoting lifelong physical activity.
References
Aarnio M, Winter T, Peltonen J, Kujala UM, Kaprio J (2002) Stability of leisure-time physical activity during adolescence—a longitudinal study among 16-, 17- and 18-year-old Finnish youth. Scand J Med Sci Sports 12(3):179–185. doi:10.1034/j.1600-0838.2002.00250.x
Auer R et al (2014) Change in physical activity after smoking cessation: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Addiction 109(7):1172–1183. doi:10.1111/add.12561
Azagba S, Asbridge M (2013) Nicotine dependence matters: examining longitudinal association between smoking and physical activity among Canadian adults. Prev Med 57(5):652–657. doi:10.1016/j.ypmed.2013.08.020
Bauman AE, Reis RS, Sallis JF, Wells JC, Loos RJ, Martin BW (2012) Correlates of physical activity: why are some people physically active and others not? Lancet 380(9838):258–271. doi:10.1016/S0140-6736(12)60735-1
Boomsma D, Busjahn A, Peltonen L (2002) Classical twin studies and beyond. Nat Rev Genet 3(11):872–882. doi:10.1038/nrg932
Borodulin K et al (2012) Leisure time physical activity in a 22-year follow-up among Finnish adults. Int J Behav Nutr Phys Act 9:121. doi:10.1186/1479-5868-9-121
Borodulin K, Harald K, Jousilahti P, Laatikainen T, Männistö S, Vartiainen E (2016) Time trends in physical activity from 1982 to 2012 in Finland. Scand J Med Sci Sports 26(1):93–100. doi:10.1111/sms.12401
de Geus EJ, Bartels M, Kaprio J, Lightfoot JT, Thomis M (2014) Genetics of regular exercise and sedentary behaviors. Twin Res Hum Genet 17(4):262–271. doi:10.1017/thg.2014.42
De Moor MH, Boomsma DI, Stubbe JH, Willemsen G, de Geus EJ (2008) Testing causality in the association between regular exercise and symptoms of anxiety and depression. Arch Gen Psychiatry 65(8):897–905. doi:10.1001/archpsyc.65.8.897
de Vilhena e Santos DM, Katzmarzyk PT, Seabra AF, Maia JA (2012) Genetics of physical activity and physical inactivity in humans. Behav Genet 42(4):559–578. doi:10.1007/s10519-012-9534-1
Dumith SC, Hallal PC, Reis RS, Kohl HW 3rd (2011) Worldwide prevalence of physical inactivity and its association with human development index in 76 countries. Prev Med 53(1–2):24–28. doi:10.1016/j.ypmed.2011.02.017
Gold DR, Wang X, Wypij D, Speizer FE, Ware JH, Dockery DW (1996) Effects of cigarette smoking on lung function in adolescent boys and girls. N Engl J Med 335(13):931–937. doi:10.1056/nejm199609263351304
Higgins M et al (1991) Pulmonary function and cardiovascular risk factor relationships in black and in white young men and women. The CARDIA Study. Chest 99(2):315–322. doi:10.1378/chest.99.2.315
Kaczynski AT, Manske SR, Mannell RC, Grewal K (2008) Smoking and physical activity: a systematic review. Am J Health Behav 32(1):93–110. doi:10.5555/ajhb.2008.32.1.93
Kaprio J (2013) The Finnish Twin Cohort Study: an update. Twin Res Hum Genet 16(1):157–162. doi:10.1017/thg.2012.142
Kaprio J, Koskenvuo M (1988) A prospective study of psychological and socioeconomic characteristics, health behavior and morbidity in cigarette smokers prior to quitting compared to persistent smokers and non-smokers. J Clin Epidemiol 41(2):139–150. doi:10.1016/0895-4356(88)90088-1
Koivumaa-Honkanen H, Kaprio J, Honkanen R, Viinamaki H, Koskenvuo M (2004) Life satisfaction and depression in a 15-year follow-up of healthy adults. Soc Psychiatry Psychiatr Epidemiol 39(12):994–999. doi:10.1007/s00127-004-0833-6
Korhonen T, Koivumaa-Honkanen H, Varjonen J, Broms U, Koskenvuo M, Kaprio J (2011) Cigarette smoking and dimensions of depressive symptoms: longitudinal analysis among Finnish male and female twins. Nicotine Tob Res 13(4):261–272. doi:10.1093/ntr/ntq251
Krueger H, Turner D, Krueger J, Ready AE (2014) The economic benefits of risk factor reduction in Canada: tobacco smoking, excess weight and physical inactivity. Can J Public Health 105(1):e69–e78
Kujala UM, Kaprio J, Rose RJ (2007) Physical activity in adolescence and smoking in young adulthood: a prospective twin cohort study. Addiction 102(7):1151–1157. doi:10.1111/j.1360-0443.2007.01858.x
Lee IM, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT (2012) Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet 380(9838):219–229. doi:10.1016/S0140-6736(12)61031-9
Li MD, Cheng R, Ma JZ, Swan GE (2003) A meta-analysis of estimated genetic and environmental effects on smoking behavior in male and female adult twins. Addiction 98(1):23–31. doi:10.1046/j.1360-0443.2003.00295.x
Lim SS et al (2012) A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380(9859):2224–2260. doi:10.1016/S0140-6736(12)61766-8
McGue M, Osler M, Christensen K (2010) Causal inference and observational research: the utility of twins. Perspect Psychol Sci 5(5):546–556. doi:10.1177/1745691610383511
McGue M, Skytthe A, Christensen K (2014) The nature of behavioural correlates of healthy ageing: a twin study of lifestyle in mid to late life. Int J Epidemiol 43(3):775–782. doi:10.1093/ije/dyt210
Midlöv P, Calling S, Sundquist J, Sundquist K, Johansson SE (2014) The longitudinal age and birth cohort trends of smoking in Sweden: a 24-year follow-up study. Int J Public Health 59(2):243–250. doi:10.1007/s00038-013-0535-5
Morris LJ, D’Este C, Sargent-Cox K, Anstey KJ (2016) Concurrent lifestyle risk factors: clusters and determinants in an Australian sample. Prev Med 84:1–5. doi:10.1016/j.ypmed.2015.12.009
Nagaya T, Yoshida H, Takahashi H, Kawai M (2007) Cigarette smoking weakens exercise habits in healthy men. Nicotine Tob Res 9(10):1027–1032. doi:10.1080/14622200701591575
Ng M et al (2014) Smoking prevalence and cigarette consumption in 187 countries, 1980–2012. JAMA 311(2):183–192. doi:10.1001/jama.2013.284692
Paavola M, Vartiainen E, Haukkala A (2004) Smoking, alcohol use, and physical activity: a 13-year longitudinal study ranging from adolescence into adulthood. J Adolesc Health 35(3):238–244. doi:10.1016/j.jadohealth.2003.12.004
Perkins KA, Rohay J, Meilahn EN, Wing RR, Matthews KA, Kuller LH (1993) Diet, alcohol, and physical activity as a function of smoking status in middle-aged women. Health Psychol 12(5):410–415. doi:10.1037/0278-6133.12.5.410
Piirtola M, Kaprio J, Ropponen A (2014) A study of sedentary behaviour in the older Finnish twin cohort: a cross sectional analysis. Biomed Res Int 2014:209140. doi:10.1155/2014/209140
Piirtola M et al (2016) Leisure-time physical inactivity and association with body mass index: a Finnish Twin Study with a 35-year follow-up. Int J Epidemiol. doi:10.1093/ije/dyw007 (Published online: March 15, 2016)
Sarna S, Kaprio J, Sistonen P, Koskenvuo M (1978) Diagnosis of twin zygosity by mailed questionnaire. Hum Hered 28(4):241–254. doi:10.1159/000152964
Sedentary Behaviour Research Network (2012) Letter to the editor: standardized use of the terms “sedentary” and “sedentary behaviours”. Appl Physiol Nutr Metab 37(3):540–542. doi:10.1139/h2012-024
Taylor AE et al (2014a) Investigating the possible causal association of smoking with depression and anxiety using Mendelian randomisation meta-analysis: the CARTA consortium. BMJ Open 4(10):e006141. doi:10.1136/bmjopen-2014-006141
Taylor G, McNeill A, Girling A, Farley A, Lindson-Hawley N, Aveyard P (2014b) Change in mental health after smoking cessation: systematic review and meta-analysis. BMJ 348:g1151. doi:10.1136/bmj.g1151
Waller K, Kaprio J, Kujala UM (2008) Associations between long-term physical activity, waist circumference and weight gain: a 30-year longitudinal twin study. Int J Obes (Lond) 32(2):353–361. doi:10.1038/sj.ijo.0803692
Wee CC, Rigotti NA., Davis RB, Phillips RS (2001) Relationship between smoking and weight control efforts among adults in the united states. Arch Intern Med 161(4):546–550
World Health Organization (2011) Global status report on noncommunicable diseases 2010. WHO Press, Geneva
Author information
Authors and Affiliations
Contributions
All authors meet the ICMJE authorship requirements as follows: (1) substantial contributions to conception and design (MP, JK, KS, PS, TK, AR) OR the acquisition of data (JK), AND the analysis and interpretation of data (all authors: MP, JK, KS, PS, TK, AR); AND (2) the drafting of the article or its critical revision for important intellectual content (all authors: MP, JK, KS, PS, TK, AR); AND (3) final approval of the version to be published (all authors: MP, JK, KS, PS, TK, AR). All authors (MP, JK, KS, PS, TK, AR) also agreed be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study protocol was approved by the Ethical Committee of the University of Helsinki, Department of Public Health. This present analysis did not involve any contact with the study participants and only used existing epidemiological data.
Funding
This work was supported by the Academy of Finland Centre of Excellence in Complex Disease Genetics (Grants 213506 and 129680 to JK), the Academy of Finland (Grants 265240 and 263278 to JK) for data collection, and the Ministry for Education and Culture of Finland (to AR) for analyzing and reporting the results. KS was supported by the Academy of Finland (Grant 266592).
Conflict of interest
JK reports personal fees from Pfizer unrelated to the submitted work. TK reports personal fees from Pfizer for consulting on nicotine dependence but unrelated to the submitted work. None of the other authors have anything to declare.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Piirtola, M., Kaprio, J., Silventoinen, K. et al. Association between long-term smoking and leisure-time physical inactivity: a cohort study among Finnish twins with a 35-year follow-up. Int J Public Health 62, 819–829 (2017). https://doi.org/10.1007/s00038-017-0975-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00038-017-0975-4