Abstract
Objectives
To explore current evidence of the physiological embedding of stress to discuss whether adverse childhood experiences (ACE) causing chronic or acute stress responses may alter fundamental biological functions.
Methods
A non-systematic review of the literature was carried out using keyword searches in Pubmed and the web of science from May to October 2011. In reference to the literature identified, we examine the potential biological pathways potentially linking exposure to ACE and cancer development and progression in adulthood.
Results
These mechanisms, in interaction with social position, and mediated by subsequent environmental exposures, may ultimately lead to the development of cancer. The experience of acute or chronic stressors during sensitive periods of childhood development which can induce several known biological responses, are likely to have an impact on subsequent biological and behavioural functions depending on the timing of initial exposures, and subsequently mediated by later exposures. For this reason, childhood exposure to adversity is a likely source of both acute and chronic stressors, and can be examined as an important initial exposure on a pathway towards adult ill health.
Conclusions
Such pathways justify a life course approach to understanding cancer aetiology, which may have its origins early in life.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
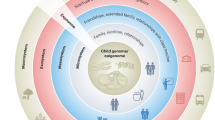
Psychosocial stress is likely to affect individuals differentially depending on where they are positioned in the social structure across their life course (Brunner and Marmot 2001). Exposure to stressors in childhood is of particular relevance in life course epidemiology for understanding the production of health inequalities, and in aiming to reduce them through interventions. This is mainly because developmental processes occurring at different phases throughout childhood, which vary in their biological and behavioural complexity, render children’s physiological and cognitive functions more plastic and capable of adaptation.
The aim of this paper is to explore current evidence of the physiological embedding of stress to discuss whether adverse childhood experiences (ACE) causing chronic or acute stress responses may alter fundamental biological functions. For the purpose of this discussion, we have identified ACE as a set of traumatic and stressful psychosocial conditions not under the child’s control that tend to co-occur (Rosenman and Rodgers 2004) and are persistent over time (Clark et al. 2010; Felitti et al. 1998). We have defined ACE as: intra-familial events or conditions in the child’s immediate environment causing chronic or acute stress responses. These include notions of maltreatment and deviation from societal norms, and need to be distinguished from events or conditions linked to the socioeconomic and material environment. We hypothesise more specifically that such alterations to biological systems may lead to the development of cancer, both through the initiation of harmful physiological modifications and through a propensity towards harmful health behaviours (Delpierre and Kelly-Irving 2011). These mechanisms, in interaction with social position, and mediated by subsequent environmental exposures, may ultimately lead to the development of cancer.
Methods
Our hypothesis is discussed in the light of current literature from biological, medical and public health science. First, within the context of the early origins of adult disease, the developmental sensitivity specific to early life is described and the potential long-term impact of adverse experiences via physiological stress responses is raised. Secondly, the potential physiological mechanisms via which early life experiences may lead to a biological susceptibility to cancer are then highlighted, including neuroendocrine, epigenetic and behavioural responses. Finally, an overview of how these exposures and mechanisms may interact along chains of causality and across the life course is discussed.
This paper is an overall discussion of pertinent literature available stemming from a number of different disciplines. It is based on a non-exhaustive non-systematic search of literature in the aim of highlighting the broad themes emerging and how these might fit together. An exploration of the literature was carried out in two ways.
First, key works of interest such as the edited book by D.B. Bailey, J.T. Bruer, F. Symons & J.W. Lichtman Critical thinking about critical periods (Bailey et al. 2001); the first paper in the series published by Felitti et al. based on the ACEs study were used to identify other relevant sources and references (both citing them and that they cited).
Secondly, keyword searches were carried out between May and October 2011 using Pubmed and the Web of science databases. To explore the literature on childhood exposures, the following sets of keywords were entered ‘adverse childhood experiences & health’; ‘critical periods & child* & health’; ‘sensitive periods & child* & health’. To identify literature on adverse experiences and/ or stress and biological responses these searches were carried out: ‘stress & telomere*’; ‘stress & immun*’; ‘stress & inflam*’; To identify literature on adverse experiences and/ or stress and cancer the following keywords were used: ‘advers* & cancer’; ‘soci* & biology & cancer (limited to humans)’; ‘soci* & biology & cancer (limited to animals)’; ‘soci* & cancer’; ‘stress & cancer’. A keyword search to identify work on epigenetic mechanisms in cancer development was as follows: ‘epigen* & cancer’
Since the topics being searched cover a large array of disciplines the papers were selected for their relevance to the topic being explored: (1) environmental (in the broadest sense) exposures likely to cause physiological stress responses, (2) biological mechanisms leading to the development of cancer, (3) animal and human evidence for the link between (1) and (2). All the papers used to inform this discussion were also used to identify other works of interest in their references.
Results
Early life exposure to stress: sensitive periods
Barker and Osmond (1986, p. 1080) described strong correlations between infant mortality rates between 1921 and 1925 and adult mortality from ischaemic heart disease from 1968 to 1978. They hypothesised that “adverse influences in childhood associated with poor living standards increase susceptibility to other influences, associated with affluence, encountered in later life”. They suggested that the geographic variations in the distribution of ischaemic heart disease were a reflection of past differences in early life nutrition. In the same year, Barker and Osmond showed that high rates of infant mortality were strongly correlated with subsequent rates of coronary heart disease mortality in the same generation. They suggested that exposure to adverse conditions in utero and in infancy were associated with developing coronary heart disease in adulthood.
The mechanism of ‘sensitive periods’ in life course epidemiology is borrowed from notions originally identified in neurobiology and physiology (Daw 1997; Fox et al. 2010). During a phase of rapid development, a biological system is more sensitive to exposures in the environment and especially deviations from ‘normal’ exposures expected during that particular phase of development for that particular system (Bruer 2001). Given the vast array of developmental processes occurring between conception and adolescence, no single sensitive period can be identified, rather, differing levels of sensitivity are constantly shifting for different systems which in turn vary in their complexity. Every developmental window is in fact characterized by a different susceptibility depending on various environmental factors. Developmental processes occurring earlier in a human life course are linked to fundamental biological functions most basic for human existence. Exposures occurring during the development of these functions are therefore likely to affect basic sensory or neurological processes in the long term. The developmental time windows rendering biological systems sensitive to environmental exposures are longer as the complexity of the systems increase. Later, during the development of higher functions, such as socio-emotional behaviours, sensitive periods are likely to be longer and vary greatly based on age and other individual characteristics like gender (Anderson et al. 2009). Research on animals has suggested that while females are not as sensitive to acute stress compared to males, they are less able to adjust to chronic stress situations than males (Bale 2006). Biological programming during sensitive periods of development is likely to be affected by the involvement of sex differences, but is also likely to affect sexual dimorphism. Indeed “elucidation of the mechanisms by which sex-specific susceptibility arises is likely to provide critical insight into disease aetiology” (Bale et al. 2010). Links between biological programming in utero and cancer development in adulthood have been discussed in terms of exposure to androgen hormones, however, exposure to stress-related hormones (Potischman et al. 2004), during gestation is difficult to ascertain and its link with later cancer development unconfirmed in human epidemiology.
Due to this developmental sensitivity that is more pronounced in children the experience of acute or chronic stressors, which can induce several known biological responses, could have an impact on subsequent biological and behavioural functions depending on the timing of initial exposures, and be mediated subsequently by later exposures. For this reason, childhood exposure to adversity is a possible source of both acute and chronic stressors, and can be examined as a potentially important initial exposure on a pathway towards adult ill health.
ACE and cancer: the intra-familial environment
After finding evidence “that family life in childhood and adolescence may, independently of material factors, have important long-term consequences for health in adulthood”. Sweeting and West (1995, p. 171) note that “the role of the family in relation to health inequalities has been largely ignored”. Characterising the intra-familial environment in childhood and adolescence, wherein adversities may be experienced, but equally where positive psychosocial mechanisms favouring better social and health outcomes is likely to be important in understanding the production of health inequalities and hence in carrying out early interventions. Kelly et al. (2011) found that home learning, family routines and parenting characteristics in children aged 3 and 5 were key elements of the early environment that could be targeted in the aim of closing income gaps in early child health and development, impacting thereby on health inequalities along the life course. The younger the child, the more important the influence of the intra-familial environment is likely to be on their development, as s/he may not enter into formal education infrastructures until as late as 6 or 7, at which point the breadth of the child’s experiences and relationships broadens to include other influential individuals.
Early life exposure to ACE, like trauma, abuse or maltreatment in childhood has been linked to alteration of the brain structure and the neurobiological stress–response systems which in turn has consequences for health and emotional well-being (Anda et al. 2006). Several studies have described associations between ACE and health outcomes such as ischaemic heart disease (Dong et al. 2004), obesity (Thomas et al. 2008), perceived health (Dube et al. 2010) and psychopathology (Clark et al. 2010) among others. Several studies identify a dose–response association, where an increasing number of accumulated adversities is associated with a higher risk of morbidity (Clark et al. 2010; Danese et al. 2009; Dube et al. 2003b). Regarding cancer specifically, data are sparse although relevant explanations for mechanisms linking adverse events and initiation or progression of cancer have been provided from animal models (Antoni et al. 2006b; Lutgendorf et al. 2010).
Our definition of ACE has been influenced by previous epidemiological studies of ACE, notably the San Diego study (Felitti et al. 1998) the Australian study (Rosenman and Rodgers 2004), as well as discussions on ACE by a WHO expert committee in 2009. We have sought to disentangle adversity and stress responses caused by the material environment, such as poverty, from adversity caused by dysfunction or disruption amid the child’s important relationships, such as those with family members. Adversity caused by poverty is no doubt a source of psychosocial stress, however, the child is more likely to cope with this type of stress if his/her close family relationships are positive and confident. ACEs are therefore likely to interact with socioeconomic disadvantage; however, the exploration of this is beyond the scope of this paper. We hypothesise that the physiological embedding of stress induced by adversity in childhood is linked with a modification of subsequent stress responses, and alterations of biological mechanisms favouring the development of cancers in adult life.
In 1998, the ACE study described a strong graded relationship between ACE and cause of death, including from cancer (Felitti et al. 1998). The authors explained this as an indirect association via health-related behaviours where exposed individuals coped with adversity-induced stress by obtaining a pharmacological or psychological benefit from tobacco or alcohol use. Since then, the same study reported both indirect (via smoking) and direct associations between ACE and lung cancer (Brown et al. 2010). In a separate study, Fuller-Thomson and Brennenstuhl (2009) found an association between childhood physical abuse and self-reported cancer after adjusting for smoking, alcohol and other confounders, suggesting that a direct association between childhood adversity and cancer may exist. The main methodological flaw in these studies is that ACE was self-reported by adults who were asked questions about trauma and adversity they may have experienced during childhood. Such questions are inevitably vulnerable to recall bias, where adults with poor health may be more likely to report adversity during childhood, but often this is the only method available to researchers exploring the consequences of childhood adversity. This issue is raised by Korpimaki et al. (2010) in their study on childhood adversity and cancer in a working age population. The authors do use a retrospective questionnaire to identify childhood adversity, and limit their analyses to individuals whose cancer was diagnosed subsequent to answering questions on childhood adversities, thereby attempting to address the problem of recall bias (Korpimaki et al. 2010). Their study found no association between working-age cancer and reporting childhood adversities.
Embodiment, how ACE becomes incorporated: biological pathways to cancer development
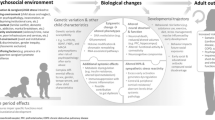
In the following section, we describe the two main biological pathways through which the experience of ACE may get “under the skin”. Stress induced by ACEs could influence the onset of cancer via: (1) a “direct” effect of stress on biological systems including neuroendocrine responses and epigenetic modifications, and (2) an “indirect” biological effect of health behaviours as a response to stress. The most likely scenario is a combination of both mechanisms simultaneously over time. Neuroendocrine, inflammatory responses and epigenetic modifications potentially have an impact on both types of pathway (Fig. 1).
Direct biological embedding of stress: neuroendocrine responses
One of the main biological mechanisms used by organisms to adapt to their environment involves stress response systems, through the hypothalamic–pituitary–adrenal (HPA) axis and sympathetic–adrenal–medulla (SAM) system. These systems control the release of stress hormones such as glucocorticoides (cortisol), catecholamines (adrenaline and noradrenaline) and other hormones (prolactine, oxytocin). The association between stress and cancer development and progression has been shown in biological studies. In animals over the life course, modification of HPA and SAM activities have been shown to alter many biological mechanisms implicated in tumorogenesis, including tumour growth, cell migration and invasion, inflammatory and immune responses (Antoni et al. 2006a). This is well described in vitro and in animal models of chronic restraint stress or of social isolation (Antoni et al. 2006a; Gidron and Ronson 2008; Lutgendorf et al. 2010; Thaker et al. 2006). When exposed to chronic stress, “the body remains in a constant state of overdrive” (Lutgendorf et al. 2010) with adverse consequences on the regulation of systems implicated in cancer progression. Such causal relationships between environmental factors and biological mechanisms involved in cancer have been well demonstrated in animals (Antoni et al. 2006a). In terms of ACE, maternal care defects in animals have been shown to increase HPA responsivity to stress. These effects seem to be derived from changes in forebrain corticosteroid receptor systems, which determine glucocorticoid negative feedback sensitivity (Meaney et al. 1994). As a key step in controlling the stress response, the glucocorticoid pathway has been dissected in models of early life stresses and found to be associated with epigenetic modifications, which we will discuss below.
In humans, evidence is more difficult to obtain, therefore available studies are sparse and mainly based on correlations and not on causal associations. However, an increasing literature suggests links between psychosocial factors, like stress, depression or social isolation and cancer progression through activation of HPA and SAM systems (Antoni et al. 2006a). Accordingly, altered levels of stress hormones have been observed in human cancers (Antoni et al. 2006a) and a number of correlations between psychosocial stress, biological pathways related to tumorigenesis and cancer have been reported in humans. For instance, psychosocially stressed patients have fewer leucocytes, decreased cytotoxic T cell and natural killer cell activities, high levels of serum cortisol (basal), acute phase proteins, increased plasma concentrations of inflammatory cytokines and more inflammatory responses including DNA damage, growth and angiogenic factors and proteases (Gidron and Ronson 2008). Stress-related immunological changes bring about declines in natural killer cell activity by depressing their ability to respond to tumours or virally infected cells, and causing a reduction in the body’s defences linked to the repair of damaged DNA (Kiecolt-Glaser and Glaser 1999).
In epidemiological studies, evidence of a direct association between exposure to stress and cancer incidence is mixed and inconclusive (Schraub et al. 2009). These studies combine a number of definitions or forms of stress reported in adulthood that may be relevant to the chain of risk leading to cancer development, but do not relate to ACEs specifically. A Danish cohort study on 8,736 men and women found no direct association between cumulative stressful life events collected retrospectively and cancer incidence, though they did identify a relationship between stress and unhealthy lifestyles (Bergelt et al. 2006). Ollonen et al. (2005) found support for an overall association between stressful life events and breast cancer risk in their Finnish case–control study (Ollonen et al. 2005). Evidence from the West of Scotland collaborative study, a prospective cohort study of 5,743 men and 991 women, found an association between medium levels of reported daily stress and breast/prostate cancer development after adjusting for prior confounders and mediating risk factors (Metcalfe et al. 2007). Conversely, Nielsen et al (2005) found a significant reduction in the hazard ratio of women exposed to perceived stress after adjusting for confounders. The authors explain that chronic stress impairs oestrogen synthesis, which is a known risk factor for breast cancer (Nielsen et al. 2005). A meta-analysis of studies on the association between stress and breast cancer did not support an association between stressful life events and breast cancer risk (Duijts et al. 2003).
Biological embedding of stress: epigenetic modifications
Recent articles linking environment, DNA hypomethylation and cancer development suggest that the environment may modify DNA and gene expression (Nise et al. 2010). Epigenetic modification as a molecular-level mechanism involved in the developmental origins of disease has thus been used to explain a number of common pathologies such as cardiovascular diseases, psychopathologies and cancer (Hochberg et al. 2011; Szyf 2009). Furthermore, epigenetic modifications have been put forward as a plausible link between environmental factors, alterations in gene expression and diseases susceptibility (Jirtle and Skinner 2007). Here, we discuss the current literature suggesting that environmental exposures and psychosocial stress (via exposure to ACE) in particular, may contribute to cancer development through epigenetic events.
The social environment and methylation
Epigenetics refers to any information heritable during cell division other than the DNA sequence itself (Feinberg 2007). McGowan and Szyf (2010) have used the following definition “the combination of mechanisms that confer long-term programming to genes and could bring about a change in gene function without changing gene sequence” (McGowan and Szyf 2010). The most well-established epigenetic methylation modification has been observed whereby a methyl group is added to DNA. Methylation of critical regulatory regions affects gene expression, hypermethylation is usually associated with the silencing of genes whereas hypomethylation with gene activation. This alteration can be stable, and long-term but also reversible due to the existence of DNA demethylases. Epigenetic processes are therefore essential for understanding gene function and expression (Hochberg et al. 2011). It is the normal regulatory process of gene expression and as such, is essential for the normal growth, development and aging of higher organisms. However, it also underlies genomic modifications that can impact susceptibility to disease (Feinberg 2007).
In recent years, groundbreaking research has been carried out establishing the link between psychosocial and socioeconomic exposures in early life and epigenetic modifications potentially leading to adverse health outcomes later in life (Borghol et al. 2011). In animals, this has been described through the modification of the glucocorticoid receptor (GR). Methylation was observed in the hippocampus of rat pups in response to maternal care whereby the levels of methylation at the 5′-end of the GR gene promoter in the hippocampus were inversely proportional to the extent to which rat pups were licked, groomed and nursed by their mothers. Furthermore, the increased level of methylation at the GR promoter was correlated with reduced GR transcription confirming that levels of gene expression were indeed affected by methylation (Weaver et al. 2004). Accordingly in humans, hypermethylation and reduced expression of the glucocorticoid receptor gene was found in the post-mortem hippocampus of suicide victims with a history of abuse in childhood but not among suicide victims without a history of childhood abuse or controls who died suddenly from causes other than suicide (McGowan et al. 2009).
Given that the glucocorticoid pathway is strongly involved in life-stress modulation, methylation of the GR supports the idea that psychosocial stress, and ACE in particular, may have an impact, on tumorigenesis and that this may be triggered and sustained by epigenetic changes. This idea is further supported by the fact that aberrant DNA methylation is common in various human tumours [stomach, kidney, colon, pancreas, liver, uterus, lung, cervix (Feinberg 2004)]. These aberrant epigenetic changes could stem from the activation of biological systems induced by early social exposures, such as the GR pathway downstream. However, direct evidence is lacking from studies on humans.
DNA methylation and cancer
Since methylation is a potentially reversible biological signal, DNA methylation patterns can be used as a plastic biological framework that might play a role in the adaptive responses to changing environments early in life and possibly throughout life (Szyf 2009). Patterns of methylation can be acquired during life, or be inherited from the mother’s behaviour affecting the DNA methylation patterns of the offspring (Weaver et al. 2004). Consequently, an individual’s health status is the result of a remodelled epigenome, which itself is the outcome of complex cumulative interactions between the genotype and the environment over time (Hochberg et al. 2011), wherein early life exposures are so critical (Gluckman et al. 2011).
Cancer involves both global and gene-specific hypomethylation and hypermethylation as well as chromatin modifications. Many growth-promoting genes are activated through hypomethylation (Feinberg and Tycko 2004); in contrast, tumour suppressor gene silencing has been linked to promoter hypermethylation (Jones and Baylin 2002). Specific global DNA methylation patterns can be considered as signatures of specific cancers and are being assessed by whole genome techniques (Feinberg and Tycko 2004). Experimental data from research on mice support a causal role for epigenetic changes in cancer (Eden et al. 2003) and further suggest that hypomethylation is more important in the earliest stages of carcinogenesis whereas hypermethylation has a greater role during tumour progression (Laird et al. 1995; Yamada et al. 2005).
“Indirect” biological pathways
The indirect biological mechanism potentially linking ACE to cancer is via risky health behaviours that are, among other factors, stress-reducing in the short term, such as smoking and alcohol consumption. Previous studies on ACE have established links between adversity in childhood and an increased risk of smoking, alcoholism, early sexual activity and having multiple sexual partners (Anda et al. 1999, 2002, 2006; Chung et al. 2010; Dube et al. 2003a, 2010), all of which are risk factors for cancer. Epigenetic mechanisms, which can be instigated by exposure to stressors such as ACE, have been identified as underlying addiction and neurobiological responses to addiction (Wong et al. 2011) as well as being linked to disruptive behaviour problems in children (Tremblay 2010).
Chains of causality: how it all fits together
We have described the possible biological pathways and mechanisms linking ACE to cancer in adulthood, acting directly on physiological processes, or via behaviours. It is important to simultaneously grasp these biological responses to stress in childhood and their consequences, however, not to overstate them as being deterministic of future negative outcomes. Thankfully, human development and capacity for adaptation is complex, and ACEs are not a death knoll for exposed individuals. A subtle interplay between biological functions and the environment continues throughout life. The importance of sensitive periods in life course epidemiology cannot easily be disentangled from the other mechanism of accumulation. Those individuals most at risk of developing cancer after initial exposure to ACEs are more likely to have accumulated negative exposures subsequently. The accumulation of environmental exposures over time leads an individual along a trajectory towards their ultimate health outcome. Socioeconomic status is a predictor of psychosocial stress (Matthews et al. 2010; Wilkinson 1999), but also, an individual’s position on the social gradient is likely to affect how their biological and behavioural functions embody stress when they become exposed. When considering the “biology of disadvantage” (Adler and Stewart 2010) socioeconomic status is positioned as a distal predictor of ill health mediated by psychosocial factors. However, stress and adversity can occur across the social gradient, and the way in which neurobiological and behavioural processes deal with these psychosocial exposures is likely to be different based on social position (Seeman et al. 2010).
The importance of the early childhood environment is pivotal, however, a nuanced understanding of its significance is required. Though findings suggest a sensitive period effect of exposure to stressors and the impact of this on biological responses which could lead to the development of cancers, it is too over-simplified to think that the organism’s plasticity ‘stops’, becomes fixed, and ends in inevitable cancer development. On the contrary, adaptations are possible across the life course, and the discussion on how to intervene in subjects with an increased susceptibility during specific phases of development and in specific spheres (educational, nutritional, etc.) is essential. Children who have experienced adversity must not be deemed ‘damaged goods’ and destined to develop psychopathologies and now cancer. Instead, the incredible facility for adaptation that is a characteristic of all animals at a biological level is even greater in humans, due to their added cognitive and socio-emotional complexity, potentially allows for later adjustment. At the earliest life stage, when parenting is most intense, interventions to improve parental investment in the child may prevent maladaptive affective patterns and the deregulation of biological systems thus potentially improving the child’s response to stressful events, and preventing poor health or harmful health behaviours from developing (Mayes et al. 2005). It is clear that the ‘sensitive periods’ mechanism cannot easily be dissociated from the cumulative effect of exposures. The combination of these two mechanisms leads an individual along a trajectory where the probability of a poor health outcome increases.
Discussion
This discussion is based on literature stemming from epidemiology, neurobiology and biological sciences, but is a non-exhaustive selection of the evidence and how it might fit together across the disciplines. The main limitation is likely to be publication bias, whereby papers showing a lack of evidence for the links between (1) ACEs and physiological stress responses, (2) physiological stress responses and the biological mechanisms of cancer development and (3) the combination of (1) and (2) are unlikely to be either pursued by researchers or to be published in journals. Therefore, evidence to the contrary is probably difficult to identify. Of course, the authors may have also missed relevant papers or themes which may have biased the discussion. However, the overall objective of this type of paper is to move a discussion forward and link different areas or disciplines of research with may ultimately affect how public health issues are addressed.
Conceptualising childhood adversity as an early distal cause of cancer alters the current aetiological understanding of the very early stage of the disease. An organism is thus susceptible to developing a cancer via biological and social/psychosocial pathways. This means that experiencing adversity in childhood may set up the organism’s susceptibility to the future development of cancer, however, the individual’s subsequent life course trajectory is likely to mediate the effect. Though understanding the mechanisms operating behind the relationship between adversity and cancer is paramount, the works discussed here highlight once more the importance of early life exposures and their potential consequences on health. Hence, public health and social interventions to ameliorate intra-familial conditions have a twofold benefit: they are essential to improve the well-being of the children, and are crucial for the future adult’s health. An important message regarding sensitive periods in life course epidemiology is that, for higher functions, “varied stimulation over a prolonged period of time is likely to produce corresponding results—that is, more is better” (McCall and Plemons 2001, p. 276). This means that policies to improve childhood environments cannot be limited to a “one shot” of quality education, or improved health care, but rather a continued nurturing is essential. It also means that there is a good chance to counter the ill effects of adversity in childhood by providing a positive and stable overall environment in the subsequent long term (McCall and Plemons 2001).
References
Adler NE, Stewart J (2010) Preface to the biology of disadvantage: socioeconomic status and health. Ann NY Acad Sci 1186:1–4
Anda RF et al (1999) Adverse childhood experiences and smoking during adolescence and adulthood. JAMA 282(17):1652–1658
Anda RF et al (2006) The enduring effects of abuse and related adverse experiences in childhood. A convergence of evidence from neurobiology and epidemiology. Eur Arch Psychiatry Clin Neurosci 256(3):174–186
Anda RF et al (2002) Adverse childhood experiences, alcoholic parents, and later risk of alcoholism and depression. Psychiatr Serv 53(8):1001–1009
Anderson V et al (2009) Childhood brain insult: can age at insult help us predict outcome? Brain 132(Pt 1):45–56. doi:10.1093/brain/awn293
Antoni MH et al (2006a) The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nat Rev Cancer 6(3):240–248. doi:10.1038/nrc1820
Antoni MH et al (2006b) Opinion—the influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nat Rev Cancer 6(3):240–248. doi:10.1038/nrc1820
Bailey DBJ, Bruer JT, Symons FJ, Lichtman JW (eds) (2001) Critical thinking about critical periods. Paul H. Brookes Publishing Co., Baltimore
Bale TL (2006) Stress sensitivity and the development of affective disorders. Horm Behav 50(4):529–533. doi:10.1016/j.yhbeh.2006.06.033
Bale TL et al (2010) Early life programming and neurodevelopmental disorders. Biol Psychiatry 68(4):314–319. doi:10.1016/j.biopsych.2010.05.028
Barker DJP, Osmond C (1986) Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet 1:1077–1081
Bergelt C, Prescott E, Gronbaek M, Koch U, Johansen C (2006) Stressful life events and cancer risk. Br J Cancer 95(11):1579–1581. doi:10.1038/sj.bjc.6603471
Borghol N et al (2011) Associations with early-life socio-economic position in adult DNA methylation. Int J Epidemiol. doi:10.1093/ije/dyr147
Brown DW et al (2010) Adverse childhood experiences are associated with the risk of lung cancer: a prospective cohort study. BMC Public Health 10:20. doi:10.1186/1471-2458-10-20
Bruer JT (2001) A critical and sensitive period primer. In: Bailey DB Jr, Bruer JT, Symons FJ, Lichtman JW (eds) Critical thinking about critical periods. Paul H. Brookes, Baltimore, p 299
Brunner E, Marmot M (2001) Social organization, stress, and health. In: Marmot M, Wilkinson RG (eds) Social determinants of health. Oxford University Press, Oxford, pp 17–43
Chung EK, Nurmohamed L, Mathew L, Elo IT, Coyne JC, Culhane JF (2010) risky health behaviors among mothers-to-be: the impact of adverse childhood experiences. Acad Pediatr 10(4):245–251
Clark C, Caldwell T, Power C, Stansfeld SA (2010) Does the influence of childhood adversity on psychopathology persist across the lifecourse? A 45-year prospective epidemiologic study. Ann Epidemiol 20(5):385–394. doi:10.1016/j.annepidem.2010.02.008
Danese A et al (2009) Adverse childhood experiences and adult risk factors for age-related disease depression, inflammation, and clustering of metabolic risk markers. Arch Pediatr Adolesc Med 163(12):1135–1143
Daw NW (1997) Critical periods and strabismus: what questions remain? Optom Vis Sci 74(9):690–694
Delpierre C, Kelly-Irving M (2011) To what extent are biological pathways useful when aiming to reduce social inequalities in cancer? Eur J Public Health 21(4):398–399. doi:10.1093/eurpub/ckr076
Dong M et al (2004) Insights into causal pathways for ischemic heart disease: adverse childhood experiences study. Circulation 110(13):1761–1766
Dube SR, Cook ML, Edwards VJ (2010) Health-related outcomes of adverse childhood experiences in Texas, 2002. Prev Chronic Dis 7(3):A52
Dube SR, Felitti VJ, Dong M, Chapman DP, Giles WH, Anda RF (2003a) Childhood abuse, neglect, and household dysfunction and the risk of illicit drug use: the adverse childhood experiences study. Pediatrics 111(3):564–572
Dube SR, Felitti VJ, Dong M, Giles WH, Anda EF (2003b) The impact of adverse childhood experiences on health problems: evidence from four birth cohorts dating back to. Prev Med 37(3):268–277
Duijts SFA, Zeegers MPA, Van der Borne B (2003) The association between stressful life events and breast cancer risk: a meta-analysis. Int J Cancer 107(6):1023–1029. doi:10.1002/ijc.11504
Eden A, Gaudet F, Waghmare A, Jaenisch R (2010) Chromosomal instability and tumors promoted by DNA hypomethylation. Science 300(5618):455. doi:10.1126/science.1083557
Feinberg AP (2004) The epigenetics of cancer etiology. Semin Cancer Biol 14(6):427–432. doi:10.1016/j.semcancer.2004.06.005
Feinberg AP (2007) Phenotypic plasticity and the epigenetics of human disease. Nature 447(7143):433–440. doi:10.1038/nature05919
Feinberg AP, Tycko B (2004) The history of cancer epigenetics. Nat Rev Cancer 4(2):143–153. doi:10.1038/nrc1279nrc1279
Felitti VJ et al (1998) Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med 14(4):245–258
Fox SE, Levitt P, Nelson CA (2010) How the timing and quality of early experiences influence the development of brain architecture. Child Dev 81(1):28–40
Fuller-Thomson E, Brennenstuhl S (2009) Making a link between childhood physical abuse and cancer results from a regional representative survey. Cancer 115(14):3341–3350. doi:10.1002/cncr.24372
Gidron Y, Ronson A (2008) Psychosocial factors, biological mediators, and cancer prognosis: a new look at an old story. Curr Opin Oncol 20(4):386–392
Gluckman PD, Low FM, Buklijas T, Hanson MA, Beedle AS (2011) How evolutionary principles improve the understanding of human health and disease. Evol Appl 4(2):249–263. doi:10.1111/j.1752-4571.2010.00164.x
Hochberg Z et al (2011) Child health, developmental plasticity, and epigenetic programming. Endocr Rev 32(2):159–224. doi:10.1210/er.2009-0039
Jirtle RL, Skinner MK (2007) Environmental epigenomics and disease susceptibility. Nat Rev Genet 8(4):253–262. doi:10.1038/nrg2045
Jones PA, Baylin SB (2002) The fundamental role of epigenetic events in cancer. Nat Rev Genet 3(6):415–428
Kelly Y, Sacker A, Del Bono E, Francesconi M, Marmot M (2011) What role for the home learning environment and parenting in reducing the socioeconomic gradient in child development? Findings from the Millennium Cohort Study. Arch Dis Child 96(9):832–877. doi:10.1136/adc.2010.195917
Kiecolt-Glaser JK, Glaser R (1999) Psychoneuroimmunology and cancer: fact or fiction? Eur J Cancer 35(11):1603–1607
Korpimaki SK, Sumanen MPT, Sillanmaki LH, Mattila KJ (2010) Cancer in working-age is not associated with childhood adversities. Acta Oncol 49(4):436–440. doi:10.3109/02841860903521103
Laird PW et al (1995) Suppression of intestinal neoplasia by DNA hypomethylation. Cell 81(2):197–205. doi:10.1016/0092-8674(95)90329-1
Lutgendorf SK, Sood AK, Antoni MH (2010) Host factors and cancer progression: biobehavioral signaling pathways and interventions. J Clin Oncol 28(26):4094–4099. doi:10.1200/jco.2009.26.9357
Matthews KA, Gallo LC, Taylor SE (2010) Are psychosocial factors mediators of socioeconomic status and health connections? A progress report and blueprint for the future biology. Ann NY Acad Sci 1186:146–173
Mayes LC, Swain JE, Leckman JF (2005) Parental attachment systems: neural circuits, genes, and experiential contributions to parental engagement. Clin Neurosci Res 4(5–6):301–313. doi:10.1016/j.cnr.2005.03.009
McCall RB, Plemons BW (2001) The concept of critical periods and their implications for early childhood services. In: Bailey DB Jr, Bruer JT, Symons FJ, Lichtman JW (eds) Critical thinking about critical periods. Paul H. Brookes, Baltimore, p 299
McGowan PO et al (2009) Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci 12(3):342–348. doi:10.1038/nn.2270
McGowan PO, Szyf M (2010) The epigenetics of social adversity in early life: implications for mental health outcomes. Neurobiol Dis 39(1):66–72. doi:10.1016/j.nbd.2009.12.026
Meaney MJ et al (1994) Early environmental programming hypothalamic–pituitary–adrenal responses to stress. Semin Neurosci 6(4):247–259. doi:10.1006/smns.1994.1032
Metcalfe C, Smith GD, Macleod J, Hart C (2007) The role of self-reported stress in the development of breast cancer and prostate cancer: a prospective cohort study of employed males and females with 30 years of follow-up. Eur J Cancer 43(6):1060–1065. doi:10.1016/j.ejca.2007.01.027
Nielsen NR, Zhang ZF, Kristensen TS, Netterstrom B, Schnohr P, Gronbaek M (2005) Self reported stress and risk of breast cancer: prospective cohort study. BMJ 331(7516):548–550. doi:10.1136/bmj.38547.638183.06
Nise MS, Falaturi P, Erren TC (2010) Epigenetics: origins and implications for cancer epidemiology. Med Hypotheses 74(2):377–382. doi:10.1016/j.mehy.2009.09.008
Ollonen P, Lehtonen J, Eskelinen M (2005) Stressful and adverse life experiences in patients with breast symptoms; a prospective case–control study in Kuopio, Finland. Anticancer Res 25(1B):531–536
Potischman N, Troisi R, Vatten LJ (2004) A life course approach to cancer epidemiology. In: Kuh D, Ben-Shlomo Y (eds) A life course approach to chronic disease epidemiology, 2nd edn. Oxford University Press, Oxford
Rosenman S, Rodgers B (2004) Childhood adversity in an Australian population. Soc Psychiatry Psychiatr Epidemiol 39(9):695–702. doi:10.1007/s00127-004-0802-0
Schraub S, Sancho-Garnier H, Velten M (2009) Should psychological events be considered cancer risk factors? Rev Epidemiol Sante Publique 57(2):113–123. doi:10.1016/respe.2008.12.012
Seeman T, Epel E, Gruenewald T, Karlamangla A, McEwen BS (2010) Socio-economic differentials in peripheral biology: cumulative allostatic load. Ann NY Acad Sci 1186:223–239
Sweeting H, West P (1995) Family life and health in adolescence: a role for culture in the health inequalities debate. Soc Sci Med 40(2):163–175. doi:10.1016/0277-9536(94)e0051-s
Szyf M (2009) The early life environment and the epigenome. Biochim Biophys Acta 1790(9):878–885. doi:10.1016/j.bbagen.2009.01.009
Thaker PH et al (2006) Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med 12(8):939–944. doi:10.1038/nm1447
Thomas C, Hypponen E, Power C (2008) Obesity and type 2 diabetes risk in midadult life: the role of childhood adversity. Pediatrics 121(5):E1240–E1249. doi:10.1542/peds.2007-2403
Tremblay RE (2010) Developmental origins of disruptive behaviour problems: the ‘original sin’ hypothesis, epigenetics and their consequences for prevention. J Child Psychol Psychiatry 51(4):341–367. doi:10.1111/j.1469-7610.2010.02211.x
Weaver ICG et al (2004) Epigenetic programming by maternal behavior. Nat Neurosci 7(8):847–854. doi:10.1038/nn1276
Wilkinson RG (1999) Health, hierarchy, and social anxiety. In: Adler NE, Marmot M, McEwen B, Stewart J (eds) Socioeconomic status and health in industrial nations—social, psychological, and biological pathways. Ann NY Acad Sci 896:48–63
Wong CC, Mill J, Fernandes C (2011) Drugs and addiction: an introduction to epigenetics. Addiction 106(3):480–489. doi:10.1111/j.1360-0443.2010.03321.x
Yamada Y et al (2005) Opposing effects of DNA hypomethylation on intestinal and liver carcinogenesis. Proc Natl Acad Sci USA 102(38):13580–13585. doi:10.1073/pnas.0506612102
Acknowledgments
This work was funded by a grant from the Institut National du Cancer and MKI is funded by the Ligue contre le cancer.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the special issue "Life course influences on health and health inequalities: moving towards a Public Health perspective”.
Rights and permissions
About this article
Cite this article
Kelly-Irving, M., Mabile, L., Grosclaude, P. et al. The embodiment of adverse childhood experiences and cancer development: potential biological mechanisms and pathways across the life course. Int J Public Health 58, 3–11 (2013). https://doi.org/10.1007/s00038-012-0370-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00038-012-0370-0