Abstract
Studies in alpine environments indicate that nurse plants can facilitate other species mainly through direct mechanisms (i.e., improvements in local abiotic conditions). However, far fewer studies consider indirect facilitation, including the effect on plant–plant interactions of symbiosis with soil fungi. We asked whether the nurse shrub Hypericum laricifolium affected the colonization and activity of fungal symbionts of plants showing contrasting patterns of local spatial association with this nurse in four sites between 4100 and 4400 m in the tropical Andes. We selected three abundant herb species (Senecio wedglacialis, Castilleja fissifolia, and Agrostis tolucensis) which showed positive spatial associations with the shrub, and two herbs (Agrostis breviculmis and the exotic Rumex acetosella), which showed predominantly negative associations. We collected roots of these species from individuals under the shrub’s crown and outside, and measured colonization and activity of arbuscular mycorrhizal fungi (AMF) and colonization of dark septate fungi (DSE), as well as glomalin content in soil samples from both study situations. We found no consistent effect of the nurse across species or elevations on the degree of AMF and DSE colonization, but there was a consistent increase in AMF phosphatase activity in plants positively associated with the shrub, as well as an increase in the content of easily extractable glomalin in soils under its influence across elevations. Thus, our results suggest that an increased AMF metabolic activity and soil stabilization mediated by the increase in extractable glomalin could be linked with an indirect facilitation effect of this nurse shrub on its beneficiary plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Positive plant–plant interactions (i.e., facilitation) have been recognized as key processes for the assembly and organization of communities (Bruno et al. 2003; Lortie et al. 2004; Callaway 2007). Direct positive effects such as shading, moisture retention, and soil nutrient enrichment are important facilitative mechanisms. However, nurse plants may also facilitate other species indirectly by favoring the development of symbiotic interactions with mycorrhizae and other soil microorganisms (Callaway 2007).
Arbuscular mycorrhizal fungi (AMF) can influence plant–plant interactions by providing an advantage to host plants, through the increase in the capacity of incorporation of water and nutrients (mainly P and N), and protection against root pathogens (Ruiz-Lozano and Azcón 1995; Caravaca et al. 2003; Wehner et al. 2010). In turn, this symbiosis may indirectly mediate facilitative processes through plant–soil community feedbacks (Callaway 2007). Plant species that act as nurses can affect the mycorrhizal community, increasing the abundance of mycorrhizal propagules and the size of the mycelial networks under their influence (Requena et al. 1996; Carrillo-García et al. 1999; Casanova-Katny et al. 2011). This could result in increased colonization and activity of symbiont fungi in the rhizosphere shared by nurse and beneficiary plants, and favor the establishment, growth, and reproduction of associated plants (Montesinos-Navarro et al. 2012).
Moreover, there is evidence of several enzymes that influence the P nutrition processes mediated by AMF. Alkaline phosphatase activity has been found within vacuoles of phosphate-accumulating hyphae, and particularly around the fungal tonoplast, while acid phosphatase activity has been found in the cytoplasm of radical cells and at the ends of the external hyphae of the fungus (Tisserant et al. 1993). Their function within root cells is associated with the hydrolysis of polyphosphates to forms of inorganic P, promoting their transfer to the plant through the arbuscules (Tarafdar and Marschner 1994).
AMF can also play an important role in soil structure and stability, due to their extensive network of extraradical mycelium and the exudation of glomalin from their hyphae, which has been shown to promote soil particle cohesion and increase water retention (Augé 2001; Rillig 2004; Barbosa et al. 2019). Therefore, it could be expected that a higher mycorrhizal development under nurse plants should result in higher glomalin contents, favoring the establishment of their beneficiary plants and their water and nutritional balance.
Dark septate endophytes (DSE) belong to another fungal group that can also influence the success of plants in different environments (Della Mónica et al. 2015; Bueno de Mesquita et al. 2018). DSE are relatively less studied than AMF, but it has been shown that they can also have a beneficial effect on plant growth by providing sources of available C, N, and P from decomposing organic matter, due to the presence of extracellular enzymes produced by these fungi (Mandyam and Jumpponen 2005; Della Mónica et al. 2015). However, although the benefits that AMF and DSE can provide to plants are clear, little is known about how these soil fungi modulate plant–plant interactions, and therefore, the ability of plants to cope with stressful environmental conditions.
The available evidence indicates that shrubs can play a key role as nurse plants in arid ecosystems (Pugnaire et al. 1996; Flores and Jurado 2003; Hortal et al. 2013), and, more recently, in alpine regions (Cáceres et al. 2015; Ramírez et al. 2015; Bråthen and Lortie 2016; Llambí et al. 2020). The limited available research suggests that in arid communities, changes generated by nurse shrubs increase the abundance of mycorrhizal mycelia and spores, probably driven by increased nutrient and moisture content in the understory (Rodríguez-Echeverría et al. 2016), in turn promoting plant growth and survival more than adjacent open microsites (Azcón-Aguilar et al. 2003; Lozano et al. 2017).
However, as far as we know, no comparable studies on the effect of nurse shrubs on symbiotic soil fungi are available from alpine environments, with a few studies concentrating on cushion plants, which have been more extensively studied as nurse plants in mountain ecosystems (Filazzola and Lortie 2014). Casanova-Katny et al. (2011) reported that in the Chilean Andes, cushion species can induce a greater AMF colonization in positively associated plants growing within these nurses, with respect to open areas, and that most beneficiary plants also presented colonization by DSE. Likewise, Molina-Montenegro et al. (2015) reported that the presence of endophytic fungi isolated from cushion roots can improve establishment, performance, and survival of both native and exotic plants known to be facilitated by cushion species.
In the case of tropical alpine ecosystems, research has shown that different plant growth forms including cushions, giant rosettes, and shrubs can play an important role as nurse plants and foundation species (Anthelme et al. 2012, 2017; Cáceres et al. 2015; Ramírez et al. 2015; Hupp et al. 2017; Mora et al. 2019; Llambí et al. 2020). In the case of the northern Andes, the widespread and abundant nurse shrub Hypericum laricifolium has been shown to clearly modify local abiotic factors, reducing incident radiation under their compact canopies, reducing soil temperature variability, and increasing local soil moisture and soil organic matter contents (Cáceres et al. 2015; Ramírez et al. 2015; Llambí et al. 2020). In turn, these changes in microhabitat conditions can lead to both positive and negative effects of shrubs and other plant species, although positive associations clearly prevail. Overall, higher plant cover and diversity has been found in microsites associated with the shrub crown, with respect to open areas (Cáceres et al. 2015), suggesting that these shrubs can have important effects on community structuring in the alpine tropics.
Moreover, high mountain ecosystems exhibit abrupt variations in stress along altitudinal gradients, making them ideal systems for analyzing the role of abiotic drivers in modulating plant interactions across environmental gradients (Körner 2007). In many alpine regions, shrubs are important in defining the vegetation physiognomy in the alpine zone and in its transition with the subnival zone, due to the gradual replacement of shrubs by cushions (or giant rosettes) as important structural elements with elevation, which is a common pattern in the high tropical Andes (Ramsay and Oxley 1997; Llambí et al. 2020) and in other high mountain regions (e.g., Himalaya, Chen et al. 2019). In our study area, Llambí et al. (2020) showed that the abundance of the shrub H. laricifolium decreased with elevation, and that its demographic effects on the exotic invader Rumex acetosella changed, with positive effects at lower elevations in the alpine belt and negative effects at higher elevations in the subnival belt. Regarding AMF and DSE colonization along elevation gradients, Lugo et al. (2018) evaluated the roots of 20 species of native grasses of the Andean Puna in Argentina between 3320 and 3870 m, finding no general relationship between AMF/DSE colonization and elevation. Even so, a previous study of AMF in the roots of other grasses of the Puna along a wider altitudinal gradient (between 3320 and 4314 m) did show a decrease in colonization with increasing elevation (Lugo et al. 2012).
Hence, although we know that the effect of alpine shrubs on other plants and the colonization by symbiont fungi can change along elevation gradients, how shrubs affect the symbiosis between associated plants and soil fungi, particularly across different elevations, remains unexplored. The objective of this study was to evaluate the effects of the nurse shrub Hypericum laricifolium on the presence and activity of symbiont fungi (AMF and DSE), along an elevation gradient from the alpine to the subnival belt in the high tropical Andes of Venezuela. To explore the possible role of these symbiotic associations in the interactions between nurse shrubs and their associated species, we compared AMF and DSE colonization, and AMF viability in terms of phosphatase activity in species positively or negatively associated with H. laricifolium, growing under and outside their crown, in four study sites between 4100 and 4400 m. In these four sites, we also compared the glomalin content in the soil between areas under the shrub’s crowns and outside.
We addressed the following questions: (1) is the effect of the nurse shrub H. laricifolium on AMF and DSE colonization and AMF activity different for plant species with contrasting patterns of local spatial association with it? How do these effects of shrubs change with elevation? and (2) does the presence of nurse shrubs affect the local glomalin content of soils along the elevation gradient?
Based on the above considerations and background, we hypothesized that: (1) under nurse shrubs, fungal symbiont colonization and AMF metabolic activity (measured through the enzymatic activity of acid and alkaline phosphatases in roots) should be higher than outside and this effect should be more evident in plant species that show a positive association with the shrub. Furthermore, the effects of the shrub should be more evident at sites along the elevation gradient where there is a more positive association between the shrub and these species; and (2) glomalin production from AMF should increase under the shrubs influence, compared to adjacent areas outside across the elevation gradient.
The results of this study should provide useful information to explore whether plant–soil feedback processes are important in explaining the effects of nurse plants on beneficiary species in high mountain ecosystems, and how these effects vary along elevation gradients. This is particularly important given the scarcity of studies addressing AMF and DSE colonization, AMF enzymatic activity, and glomalin production in tropical alpine ecosystems.
Methods
Study area
This work was carried out in the Piedras Blancas páramo, located in the northeastern portion of the “Sierra La Culata” National Park in the Venezuelan Andes (8° 52′ 1″ N; 70° 54′ W). In this páramo, we selected four sampling sites along an elevation gradient (4100, 4200, 4300, and 4400 m; Fig. 1). All the sites chosen have northeast to east aspect and are located on slopes with an inclination between 10° and 25°. In each site, we worked in an area of 100 × 100 m, where total vegetation cover and topography were relatively homogeneous.
This region is the driest in the Venezuelan páramo, with an average annual precipitation of 760 mm (Pico El Águila weather station, the closest to our study sites at 4118 m). Rainfall is distributed in two distinct seasons: a rainy period (April–November) receiving more than 100 mm per month, and a dry period (December-March) with 20 mm per month or less (Rada et al. 2019). The daily temperature oscillations are very marked, with maximum air temperatures above 20 °C in the day, and minima below − 5 °C at night during the dry season (Rada et al. 2019).
There are almost daily freeze–thaw cycles in the soil surface that cause high mobility of superficial particles and fragments. These processes maintain the edaphic habitats of the páramo under daily stress conditions, not only due to the biological consequences of low temperatures but also due to the instability of the soils, which can adversely affect the establishment and development of plant seedlings (Monasterio 1979; Pérez 1993).
There is a high percentage of uncovered substrate, varying between 50 and 90%, depending on elevation and topographic position, with a highest plant cover in valley bottoms (wetlands), and the lowest cover on steep slopes where soils show higher mobility (Monasterio 1979). According to Monasterio (1979), 4000–4200 m is the approximate ecotone elevation between the Andean and High-Andean páramo (corresponding to the alpine and subnival belts, see Cuesta et al. 2017). Thus, our elevation gradient represents the transition between these environments; the lower sites (4100 and 4200 m) correspond to the higher portion of the alpine belt, with rosette-shrub vegetation dominated by our nurse shrub H. laricifolium and the stem rosette Espeletia schultzii. The highest section of our gradient (4300 and 4400 m) corresponds to the subnival belt, characterized by an open rosette vegetation, with large open spaces, dominated by the giant rosette Espeletia timotensis and cushion plants, while H. laricifolium shrubs become a less abundant element (see below, and Fig. 1). The low vegetation stratum across the gradient shows an increasingly sparser cover of native acaulescent rosettes (Hypochaeris, Oenothera, Draba), tussock forming grasses (Agrostis, Festuca, Poa), prostrate herbs (Belloa, Geranium), and forbs (Castilleja, Senecio; Cáceres et al. 2015; Mora et al. 2019).
The soils of the study sites have a sand-loamy texture and a soil pH (5 to 10 cm below the surface) that declines monotonically from 5.19 ± 0.13 at 4100 m to 4.50 ± 0.28 at 4400 m. Measurements in the dry season indicate that the same is true for soil organic matter (SOM) which declines from 15.80 ± 0.99% at 4100 m to 6.70 ± 0.80% at 4400 m (0–10 cm below the surface). In contrast, relative soil water content (SWC) increases progressively with elevation from 2.08 ± 0.77% at 4100 m to 6.68 ± 0.95% at 4400 m (0–10 cm below the surface). Also, at the four sites along our study gradient, both SOM and SWC are significantly higher under H. laricifolium shrubs than in areas away from the nurse (Llambí et al. 2020).
Focal nurse shrub
Hypericum laricifolium Juss. (Hypericaceae) is a widespread sclerophyllous shrub in the Cordillera de Mérida and one of the most abundant species of the Venezuelan páramos, where it generally integrates Andean rosette-shrub communities. Its elevation range in the Venezuelan Andes has been reported between 2200 and 4400 m (Briceño and Morillo 2002), although we have recorded its presence up to 4500 m in our study region. This species presents dense rounded crowns with microphyllous, opposite, decussate, and sessile leaves. In addition, H. laricifolium is recognized as a mycorrhizal species, whose degree of mycorrhizal colonization has been considered high (Barnola and Montilla 1997; Sarmiento and Llambí 2011).
Variations in the abundance of H. laricifolium have been described along the elevation gradient studied here, with higher average cover in the sites at 4100 and 4200 m (19.20 and 12.10%, respectively), decreasing progressively with increasing elevation as vegetation becomes sparser at 4300 and 4400 m (3.62 and 0.28%, respectively) (Llambí et al. 2020).
Analysis of the spatial associations between the shrub H. laricifolium and other plant species of the community along our elevation gradient showed a predominance of positive associations of grasses and forbs, especially in the lower elevation sites. At 4100 and 4200 m, of a total of 32 and 28 species recorded, respectively, 19 and 36% had a significantly higher density under the shrub canopy, while only 3 and 4% had a significantly higher density in the open areas. At 4300 and 4400 m, 37–39 species were recorded, with 15–19% of the species showing positive associations with the shrub, and 10 and 5% were negatively associated (Cáceres-Mago 2020).
Local spatial associations with the nurse shrub
Vegetation sampling was carried out during the dry season (March 2016) in the four study sites to analyze the type of local spatial associations between our focal nurse shrub (H. laricifolium) and the plant species present in the communities found at different elevations.
In each site, we established two study situations: (1) under the influence of H. laricifolium, defined as areas under the shrub crown projection, and (2) outside shrubs, corresponding to randomly located microsites that were not under the crown projection of H. laricifolium. Then, we haphazardly selected 40 H. laricifolium shrubs and 40 areas outside them, and placed a flexible wire ring of 60 cm in diameter under the shrubs (positioning the central stem at the center of the ring) and in a paired random location outside, 2–3 m away from the nurse. This distance was chosen to avoid being under the direct influence of the shrub’s crowns and immediate rhizosphere, but not so far as to include a strong effect of environmental heterogeneity into the paired sampling design (based on Cáceres et al. 2015 detailed analysis of the spatial scale of environmental patchiness in our study region). Within each sampling ring, we counted the number of individuals of all plant species present in the delimited area, considering each ramet as an individual (i.e., each stem visible on the ground). We used this information to calculate the average density of each species in the two sampling situations (under shrubs and outside them) for each study site.
Based on this information, we selected species for the assessments on the symbiosis with soil fungi according to the following criteria: (a) relatively abundant species distributed throughout the entire elevation gradient; (b) existence of consistent positive or negative spatial association with the shrub along the gradient; (c) we included both native species and the most common exotic invader in the region for comparison, Rumex acetosella (see Llambí et al. 2020).
Effects of nurse shrub on symbioses between soil fungi and associated plants
For the assessment of AMF and DSE colonization and enzymatic activity of acid and alkaline phosphatases, root samples were taken from at least five individuals of each species selected according to the above criteria, under five different shrubs (at least one individual per shrub) and in paired sampling sites outside the shrub crowns (random locations 2–3 m away from each focal shrub). This was done in the four study sites along the elevation gradient and the collection of this plant material was carried out during the dry season (March 2016).
Effects of nurse shrubs on the fungal colonization
Roots were stained following the method of Phillips and Hayman (1970). They were first rinsed in water to remove soil remains and then boiled in 10% KOH solution for 1 h. KOH solution was washed. After clearing, the roots were then soaked in 0.5% HCl for 15 min. The acid was drained (unwashed), and trypan blue (0.05% in lacto-glycerin) was added and then boiled for 10 min. The roots were then rinsed in distilled water and mounted lengthwise in lacto-glycerin on microscope slides.
Mycorrhizal colonization was quantified following McGonigle et al. (1990), which evaluates the presence or absence of fungal structures (arbuscules, vesicles, hyphal coils, and hyphae alone), regardless of their abundance. Three replicas per situation (under shrubs and paired areas outside the shrub crown 2–3 m away) were evaluated in each of the four study sites. Each replica represented the observation of 100 intersections of the root sample using the 20X objective of the microscope. Mycorrhizal structures are stained blue, while DSE are identified as septate hyphae and microsclerotia without staining (brown in color). To calculate the colonization percentage (% C), the following expression was used:
Effects of nurse shrubs on the activity of acid/alkaline phosphatases in roots
Due to the influence of phosphatase enzymes on P nutrition, the determination of phosphatase activity provides information on the functionality or effectiveness of AMF, in terms of P uptake and transfer to the plant. Acid phosphatase (APA) and alkaline phosphatase activities (ALPA) of the root were determined following the procedures of Gianinazzi-Pearson and Gianinazzi (1976), which consists of the colorimetric determination of the p-nitrophenol (pNP) released during the incubation of the root extract with a buffered solution (pH 4.0 and 8.5) of p-nitrophenylphosphate (pNPP). The estimation of p-nitrophenol released is based on the fact that in alkaline solutions, phenol shows an intense yellow color.
Roots were washed with water to remove soil remains. 50 mg of roots were weighed and cold macerated with 1 ml of 0.1 M borate buffer at pH 8.8. The macerate obtained was transferred to micro-centrifuge tubes. The supernatant was centrifuged at 5600 rpm for 20 min, and this procedure was repeated until a transparent supernatant was obtained.
To determine the enzymatic activity, a solution of 50 μL of the root extract with 10 μL of MgCl2 (20 mM), 200 μL of the reaction buffer (0.1 M acetate buffer at pH 4.0 for AP, and 0.05 M tris-citric acid buffer at pH 8.5 for ALP), and 80 μL of the pNPP (50 mM) substrate up to a final volume 400 μL filled with distilled water was incubated at 37 °C in a water bath for 30 min. The entire procedure was carried out in cold. The enzymatic hydrolysis was stopped after 30 min by adding NaOH (0.1 N), and the yellow color was read in a spectrophotometer at 410 nm. Five replications were made per situation (under shrubs and outside) for the four study sites.
Because the abundance of A. tolucensis decreased with elevation, the amount of plant material collected for the assessment of mycorrhizal parameters was not sufficient at 4300 and 4400 m. Likewise, A. breviculmis was absent in the first two altitudes. For this reason, the percentage of AMF and DSE colonization and phosphatase activity in A. tolucensis was determined at 4100 and 4200 m, and in A. breviculmis at 4300 and 4400 m.
Effects of nurse shrubs on soil glomalin
Measurements of the content of glomalin in soil were performed by taking soil samples from 5 to 10 cm below the soil surface during the dry season (March 2016). We collected one sample from each of five different haphazardly chosen Hypericum shrubs and five sites outside of the nurse, located 2–3 m away. This was done in each study site.
The concentration of glomalin in the soil was evaluated by the method of Wright and Upadhyaya (1998), and quantified according to the method of Bradford (1976). For the assessment of total glomalin (TG), 1 g of sieved (2 mm) soil was weighed and transferred to resistant polyethylene centrifuge tubes, and 8 ml of sodium citrate (pH 8.0) were added. The tubes were shaken and autoclaved for 60 min at 121 °C. The tubes were allowed to cool and were then centrifuged at 3000 rpm for 15 min. The liquid was then transferred by a funnel to a 50 ml volumetric flask. 8 ml of sodium citrate (pH 8.0) were added again to the residue contained in the centrifuge tube, and it was autoclaved for a second time for another 60 min, followed by centrifugation for 15 min; this centrifuged liquid was added to the previous one. The extractions with citrate were repeated until the reddish color of the solution disappeared and were replaced by a soft milky brown color. Finally, the centrifuged liquid was filled up to 50 ml with citrate solution (pH 8.0). From this sample extract, a small fraction was taken into Eppendorf tubes and centrifuged at 10,000 rpm for 15 min.
Quantification of TG was performed by mixing 50 µL of the sample, 750 µL of distilled water, and 200 µL of Bradford reagent, and the absorbance was measured at 595 nm. The Bradford reagent quantifies proteins through the coupling of Coomasie blue dye with the protein, establishing a direct relationship between color development and protein concentration, which is determined colorimetrically (Bradford 1976).
The easily extractable glomalin (EEG) was quantified from the same soil samples taken for the quantification of TG. 1 g of sieved (2 mm) soil was weighed and transferred to resistant polyethylene centrifuge tubes, and 8 ml of sodium citrate (pH 7.0) were added, and then by once it was autoclaved (121 °C) for 30 min. Then, the procedure was the same as for the quantification of TG. In both cases (TG and EEG), five replications per situation (under shrubs and outside) were made for each of the four study sites.
Data analysis
Average plant density of the evaluated species, fungal colonization, enzymatic activity of phosphatases, and content of glomalin (total and easily extractable) under H. laricifolium vs. outside and between elevations were compared using a two-way Permutational Analysis of Variance (using the PERMANOVA + for Primer 6.0 software, Anderson et al. 2008). Each response variable was analyzed separately. Given that we had a low number of replicates and our data were not normally distributed, we decided to use PERMANOVA, a robust technique that removes the assumption of normality required in fully parametric ANOVA procedures (especially problematic in designs with more than one factor). We defined the nurse plant treatment as a fixed factor with two levels (under shrubs, outside) and the elevation as a fixed factor with four levels (4100, 4200, 4300, and 4400 m). In all PERMANOVA tests, the number of permutations was set to 9999 and the probability of type I error was established at 95% (a = 0.05). Post hoc tests were performed using permutational t tests.
For the average plant density of the evaluated species, we also used the mean relative interaction index (RII; Armas et al. 2004) as a complementary method to compare the density of each species under shrubs with that outside from them, which was calculated based on paired plots located in patches with similar environmental conditions. RII was computed as
where DUS corresponds to the density of the species under the influence of the shrub and DOS corresponds to the density of the species in open areas. We then calculated means and 95% confidence intervals as a statistical parameter. RII values range from 1 to − 1, where significant positive values (i.e., the 95% confidence interval does not include zero) suggest a positive effect of the shrub and significant negative values suggest inhibitory effects. Therefore, this index allows us to measure the intensity of local spatial interactions, positive or negative, between the shrubs and other species (based on a paired sampling design).
Results
Local spatial associations with the nurse shrub
Although the identity of the species in the plant community that was positively or negatively associated with the shrub changed across the study sites, we found consistent patterns for many dominant species. Based on this, the species selected were the forbs Senecio wedglacialis and Castilleja fissifolia, the grasses Agrostis tolucesis and Agrostis breviculmis, and the exotic forb Rumex acetosella.
S. wedglacialis, C. fissifolia, and A. tolucensis were among the most abundant species under the shrub’s crowns (Fig. 2), with a positive effect of the nurse on the density of these species (p˂0.05 in all cases; see Online Resource 1). Meanwhile, the grass Agrostis breviculmis showed a significant negative association with the shrub at the highest elevation sites (at 4300 and 4400 m), with a significant effect of the nurse plant treatment (p = 0.0289). The exotic R. acetosella was consistently among the dominant species outside in the four study sites and its density was significantly higher in the open areas (p = 0.0007).
Average density of individuals of plant species with different patterns of association with Hypericum laricifolium, inside and outside the crown of shrubs along an elevation gradient between 4100 and 4400 m, in the Piedras Blancas Páramo, Venezuela. (a) Senecio wedglacialis, (b) Castilleja fissifolia, (c) Agrostis tolucensis, (d) Agrostis breviculmis, and (e) Rumex acetosella. Error bars: standard error. Capital letters indicate differences between elevations, while lower case letters indicate significant differences between local sampling situations (two-way PERMANOVA and permutational t tests, α ≤ 0.05)
Regarding the effect of elevation, we found no significant change in density for S. wedglacialis (p = 0.6920), while it showed a significant effect for C. fissifolia (p = 0.0001), with a humpback shape (with a peak at 4300 m). A. tolucensis’s density decreased at higher elevations (p = 0.001), while A. breviculmis was not recorded in the lower areas of the gradient, and its abundance was higher at 4400 m compared to 4300 m (p = 0.0002). Finally, R. acetosella showed its highest density at 4100 m (p = 0.0001). There was no significant interaction between the two study factors (i.e., elevation and the presence of the shrub) for any of the species evaluated (p˃0.05 in all cases).
The patterns of association described above were also reflected in the RII based on density changes of the selected species inside and outside the crown of shrubs along the elevation gradient (Online Resource 2), with three of the species showing positive RIIs (S. wedglacialis, C. fissifolia, and A. tolucesis) and our two other species showing predominantly negative RIIs (A. breviculmis and R. acetosella).
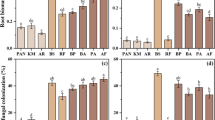
Effects of nurse shrubs on the fungal colonization of associated species
All plant species evaluated were colonized by both AMF and DSE (Fig. 3). The effect of the nurse shrub on AMF colonization of S. wedglacialis changed with elevation (significant interaction between both factors, p = 0.0186). AMF colonization was significantly higher under shrubs in the two highest sites (4300 and 4400 m), compared to the open areas (p = 0.0144 and p = 0.0012, in the t tests respectively). Regarding S. wedglacialis’s DSE colonization, there was a significant effect of the nurse plant treatment (p = 0.0006), being consistently higher outside the shrubs. There was also a significant effect of elevation (p = 0.0101), with higher values at both extremes of the gradient (4100 and 4400 m), and no significant interaction (p = 0.3304).
Colonization of arbuscular mycorrhizal fungi (AMF) and dark septate endophytes (DSE) on roots of plant species with different patterns of association with Hypericum laricifolium, inside and outside the crown of shrubs along an elevation gradient between 4100 and 4400 m, in the Piedras Blancas Páramo, Venezuela. (a) Senecio wedglacialis, (b) Castilleja fissifolia, (c) Agrostis tolucensis, and Agrostis breviculmis, (d) Rumex acetosella. Error bars: standard error. Different letters indicate significant differences between local sampling situations (two-way PERMANOVA and permutational t tests, α ≤ 0.05)
AMF colonization in C. fissifolia roots did not show significant differences due to the presence of the shrub (p = 0.0876), nor due to elevation (p = 0.0649), and there was no significant interaction between both factors (p = 0.1424). The percentage of DSE colonization showed a significant interaction between both factors (p = 0.0182); DSE colonization only showed significant effect of the nurse treatment at 4300 m (p = 0.0045), being higher under the shrubs. Also, an increase in the presence of DSE was recorded in the highest sites (4300 and 4400 m).
The percentage of AMF colonization of A. tolucensis did not show significant differences linked with the presence of the shrub (p = 0.4882), but AMF colonization was significantly higher at 4200 m than at 4100 m (p = 0.0086), with no significant interaction between factors (p = 0.3118). For this plant species, DSE colonization did not show significant effects of the nurse plant treatment (p = 0.7415), nor of the elevation (p = 0.1546), and there was no significant interaction (p = 0.6638). In A. breviculmis, AMF colonization showed a significant effect of the nurse (p = 0.0019), being higher in the roots of individuals growing under the crown. There was neither a significant effect of elevation (p = 0.5408), nor a significant interaction (p = 0.1166). For the DSE colonization, there was a significant interaction between the two study factors (p = 0.0217), being higher under shrubs at 4300 m.
AMF colonization of R. acetosella showed a significant effect of the nurse treatment (p = 0.0002), being consistently higher under the shrub along the entire elevation gradient. There was no effect of elevation (p = 0.2604), nor an interaction between factors (p = 0.4068). DSE colonization showed a significant interaction between the two study factors (p = 0.0009), with an increase in open areas in the lower zones (4100 and 4200 m), and a greater DSE colonization under the shrubs in the highest sites (4300 and 4400 m).
Typical mycorrhizal and DSE structures observed in our root samples are shown in Fig. 4. Microscopic observation of roots showed AMF fungal structures, such as arbuscules, vesicles, hyphal coils, and hyphae, which are observed stained blue. Typical DSE structures such as microsclerotia and septate hyphae were also found, which are observed as unstained structures (i.e., brown).
Fungal structures observed inside the roots of plant species with different patterns of association with Hypericum laricifolium shrub. (a) Arbuscules (arb) (400X), (b) Vesicles (v), hyphae (h) (200X), and (c) hyphal coils (hc) (400X) of arbuscular mycorrhizal fungi (AMF). (d) Microsclerotia (m) and septate hyphae (sh) (400X) of dark septate endophytes (DSE)
Effects of shrubs on the activity of acid/alkaline phosphatases in roots of associated species
Enzymatic assays showed that there was acid (APA) and alkaline phosphatase activity (ALPA) in the roots of all species evaluated along the elevation gradient (Fig. 5). APA in roots of S. wedglacialis was greater under the nurse’s crown (p = 0.0001), and changed with elevation (p = 0.0022, being lowest at 4100 m), with no significant interaction (p = 0.2357). There was a significant interaction between both factors for ALPA (p = 0.0243), being higher under the influence of the shrub in the sites at 4200, 4300, and 4400 m. Activity values were lower at 4100 m under shrubs.
Enzymatic activity of acid and alkaline phosphatases on roots of plant species with different patterns of association with Hypericum laricifolium, inside and outside the crown of shrubs along an elevation gradient between 4100 and 4400 m, in the Piedras Blancas Páramo, Venezuela (PNP = p-nitrophenol). (a) Senecio wedglacialis, (b) Castilleja fissifolia, (c) Agrostis tolucensis, and Agrostis breviculmis, (d) Rumex acetosella. Error bars: standard error. Different letters indicate significant differences between local sampling situations (two-way PERMANOVA and permutational t tests, α ≤ 0.05)
In C. fissifolia, we found that for APA, there was significant interaction between both study factors (p = 0.0004), being significantly higher under the influence of the shrub only at 4100 and 4400 m. Meanwhile, ALPA was significantly higher under the shrubs (p = 0.0001). There was also a significant effect of elevation (p = 0.0011), with no significant interaction (p = 0.5673). The activity of these enzymes decreases significantly at 4400 m, compared to the other sites.
APA of A. tolucensis did not show significant differences between the study situations (under shrubs and outside; p = 0.1324), but there was an effect of elevation (p = 0.0001), being greater at 4200 m. ALPA was significantly higher under the shrub (p = 0.0002) and increased significantly at 4200 m. There was no significant effect of the interaction in any of these cases (p = 0.2574 for APA, and p = 0.8053 for ALPA).
For A. breviculmis, APA showed a significant interaction between the nurse plant treatment and elevation (p = 0.0049), with a higher activity in the roots of individuals growing in open areas at 4300 m, while there were no differences at 4400 m. ALPA showed a significant interaction between factors (p = 0.0004), being significantly higher in the open areas at 4300 m, while it increased under shrubs at 4400 m.
For R. acetosella, there were no significant differences under shrubs vs. outside on APA (p = 0.9416) nor ALPA (p = 0.4977), while there was an effect of elevation (p = 0.0022 for APA and p = 0.0026 ALPA), with no significant interaction between factors (p = 0.052 and p = 0.0548, respectively). In both cases, the enzymatic activity was significantly higher at 4200 m, compared to the other sites.
Effects of nurse shrubs on soil glomalin
There was no a significant effect of the presence of the shrub (p = 0.5754) on the content of total glomalin (TG) in soil, but there was a significant effect of elevation (p = 0.0002; Table 1). The highest TG content was recorded at 4200 and 4400 m. There was no interaction between the study factors (p = 0.3931). However, in the case of easily extractable glomalin (EEG), there was a significant effect of both study factors (elevation, p = 0.0001; nurse shrub, p = 0.0373), with no interaction between them (p = 0.1409; Table 1). EEG content was significantly higher in the areas under the crown of the nurse shrub, compared to the open areas. Furthermore, EEG was higher at higher elevations (i.e., at 4300 and 4400 m; p < 0.05).
Discussion
In this study, we evaluated the effects of an abundant nurse shrub on the symbiosis between soil fungi and associated plants along an elevation gradient in the high tropical Andes. This allowed us to explore if indirect facilitation mechanisms could influence nurse-beneficiary interactions, a topic that has received little attention in alpine ecosystems.
Effects of nurse shrubs on fungal colonization and phosphatase activity
The evaluation of fungal colonization in roots of several common forbs and grasses in superpáramo ecosystems revealed the simultaneous occurrence of arbuscular mycorrhizae and dark septate endophytes in all the evaluated species. For R. acetosella, it is noteworthy that this exotic plant has generally been described as non-mycorrhizal in the páramos (Barnola and Montilla 1997; Sarmiento and Llambí 2011), while our evaluation allowed us to observe the presence of all mycorrhizal structures (see Online Resource 3). We also found acid and alkaline phosphatase activity in the roots of all species evaluated, indicating that fungal colonization is functional in these ecosystems, and that these symbiont fungi actively participate in the phosphorus nutrition of AMF-colonized plants in the highland tropics.
Although there was no generalized effect of the shrub H. laricifolium on the colonization by symbiont fungi in the plant species evaluated, we consistently found an increased phosphatase activity under the crown of H. laricifolium in species that showed positive associations with the focal shrub in terms of their changes in local density and in the relative interaction index (with positive RIIs for S. wedglacialis, C. fissifolia and A. tolucensis). This was not the case for species with a negative spatial association with the nurse (predominant negative RIIs, A. breviculmis and R. acetosella), where the shrub’s effects were more variable, generally negative to neutral. These results partially support our first hypothesis, since changes in phosphatase activity were consistent with the patterns of spatial association between the studied herb species and the nurse shrub. However, the effect of the shrub on AMF and DSE colonization varied depending on the plant species, with positive and negative effects unrelated to their patterns of spatial association with the nurse.
The observed increase in phosphatase activity could be associated with positive effects of the nurse on the growth of the beneficiary species. For example, Cáceres (2002) showed that increases in acid and alkaline phosphatase activity in tree species were associated with the onset of mycorrhizal responses by plants. Therefore, it would be important to analyze whether the increase in phosphatase activity under shrubs found by us translates into higher foliar P concentrations of beneficiary species, as has been reported in other ecosystems (e.g., Sulbarán 2015; Cáceres et al. 2016).
In addition, differences in phosphatase activity in the roots of the plants with different patterns of association with the nurse could be associated with differences in the AMF communities present, as has been reported in other ecosystems (Joner and Johansen 2000). Not all AMFs are equally effective with different plant species, and they may also have different colonization strategies (Klironomos and Hart 2002), which can influence their presence and abundance under contrasting microhabitat conditions (Cuenca and Lovera 1992; Cuenca et al. 1998). Hence, it would be important to identify the AMF present and evaluate the number of infective propagules in our study sites, which would allow an explicit exploration of the capacity of different AMF communities to develop effective symbiosis with beneficiary plants.
Colonization of plants establishing outside these nurses could be restricted by limited dispersal of fungal propagules and less dense mycelia in the upper layer of more exposed soils, where hyphal proliferation may be affected by a higher frequency of daily freeze–thaw cycles (typical of these high tropical alpine sites), which could promote higher soil instability outside the shrub’s crowns (Casanova-Katny et al. 2011; Ramírez et al. 2015). Although, in these extreme environments, the symbiosis with fungi could be more critical for plant survival under the harshest conditions outside the shrub’s crown which could result in a higher colonization/activity of fungal symbionts in plants growing in open areas. However, we found no evidence of this increase in plants growing outside the nurse, except for mycorrhizal activity of A. breviculmis at 4300 m, nor have we found these types of effects reported in the available literature. Hence, the possibility of a net negative effect of our focal nurse shrub on mycorrhizal colonization/activity (which could be species/specific for the soil fungi involved) remains to be explored in more detail.
The effect of the nurse shrub on the presence of soil symbiotic fungi was highly variable along the elevation gradient, as the response to elevation varied depending on the species, regardless of their spatial associations with the shrub. This contradicts our first hypothesis, as we expected that shrub effects on mycotrophy of beneficiary species would be more evident at sites where there is a more positive association with the shrubs. It should be noted that the response to mycorrhization depends both on the AMF and its compatibility with the plant species, as well as on various abiotic factors (e.g., soil nutrient content; Cuenca 2015). Given that this is a complex stress gradient, where soil water availability increases with elevation, while organic matter decreases (Llambí et al. 2020), the effects of these variables along the gradient on the colonization and activity of symbiotic fungi could also be complex. This agrees with what was previously reported by Lugo et al. (2018) in the Argentinian puna, where they found changes in mycorrhizal response (colonization in that case) for several species between elevation, but no general relationship between AMF and DSE colonization and altitude.
Effects of nurse shrubs on soil glomalin
Our second hypothesis was partially confirmed, as easily extractable glomalin (EEG) showed a consistent increase in areas under the shrub in all study sites. However, this was not the case for total glomalin (TG), which showed no significant differences between areas outside and inside the nurse’s influence. TG is considered as the residual protein that has remained in the soil after the death and degradation of the hyphae (the half-life of glomalin can be between 7 and 42 years, Rillig et al. 2001), while the EEG is considered a newly produced protein and is associated with biologically active hyphae (Lovelock et al. 2004). This could help to explain why no effects of the shrub were observed for TG, but they were evident in the case of EEG.
These results indicate that our focal nurse shrubs could generate a greater stability of the soils, not only by decreasing the frequency of nocturnal freezing events (as shown in our study area by Ramírez et al. 2015), but also by increasing EEG content in soils under their local influence. A greater stability of the substrate could then favor the establishment of the beneficiary species, since superficial soil movement can generate a high mortality in seedlings and juveniles of páramo plants, especially in the subnival belt (Pérez 1993).
In addition, better soil aggregation favors its structure by providing a better distribution of pores of different sizes and shapes, favoring air circulation, better infiltration and water storage, root growth, and biological activity (Barbosa et al. 2019). Hence, the symbiosis with AMF could be contributing to the tolerance of the shrub’s beneficiary species to seasonal water stress observed in these environments (Ramírez et al. 2015). In fact, it has been consistently shown that these symbiont fungi have an effect on plant and soil water relations under stress conditions, modifying stomatal conductance, plant transpiration, and photosynthetic rate. In turn, the extraradical mycelial network and glomalin exudation promote soil particle cohesion and increase soil water retention (Augé 2001; Rillig 2004; Barbosa et al. 2019). Therefore, a higher glomalin content in soils under the shrub could also contribute to the increase in soil moisture content induced by the nurse which has been reported in our study region, along with increases in organic matter and nutrients (e.g., nitrogen). This could in turn be linked with observed positive effects of the shrub on the water and nutritional balance of beneficiary plants (Ramírez et al. 2015; Llambí et al. 2020). In fact, the results of Cáceres-Mago (2020) indicate positive effects of our focal shrub on the water status (minimum leaf water potentials) of all the beneficiary plants studied here.
Moreover, EEG showed a consistent response to elevation, being lower in the 4100–4200 m sites, while the positive effect of the shrub on EEG was stronger in these lower sites. Given that soil water is also more limiting in these low elevation sites (Llambí et al. 2020), a more marked effect of shrubs on EEG could be key for beneficiary species in these drier areas. Finally, the differences found in EEG outside vs. under the shrub’s canopy and along elevations could be associated with different production rates due to changes in AMF community composition and/or the physiology of the AMF present (responses to the environment, including their interaction with the host plant and their physiological state; Rillig 2004). It has been shown that different AMF species are capable of producing different amounts of glomalin. Barbosa et al. (2019) reported that inoculation with five mycorrhizal fungi favored soil aggregation, but that each fungus had different effects on glomalin production and the length of extracellular hyphae, and thus on aggregate formation. Therefore, it has been suggested that the effect of AMF should be included in the interpretation of soil aggregation from a “multifunctional” perspective (Barbosa et al. 2019).
Conclusions
This is a pioneering study in tropical high mountain environments that explores the possible role of soil symbiont fungi as indirect facilitation mechanisms, and shows the presence of AMF and DSE colonization and AMF activity in native and exotic species, suggesting fungal symbiotic associations as an important adaptive strategy in these extreme environments. Our results suggests that the facilitating effect of H. laricifolium on the abundance and physiological performance of other beneficiary species in these tropical alpine ecosystems (Ramírez et al. 2015; Llambí et al. 2020; Cáceres-Mago 2020) could be generated not only through the improvement of abiotic conditions (Ramírez et al. 2015; Llambí et al. 2020), but also by promoting the activity of beneficial symbiotic fungi via indirect facilitation effects.
In this context, further research should explore the effect of these nurse shrub in the composition of AMF communities present in these superpáramo environments, to determine if the presence of the shrub alters these fungal communities at the local level, which could be related to the differences found in AMF activity and glomalin production. Moreover, mechanistic links between changes in symbiont activity and their effects on the performance of beneficiary species need to be further explored, including the effects of glomalin on soil stability and of an increased fungal activity on leaf nutrient concentrations and ecophysiological performance (e.g., Cáceres-Mago 2020 analysis of the nurse effects on the water status of our beneficiary plants). It would also be important to extend this approach to study other nurse species alternating in importance with shrubs along the elevation gradient (e.g., cushions, giant rosettes). More generally, the results of this study emphasize to the need of considering both direct and indirect facilitation by nurse plants and the role of soil microorganisms in mediating these interactions for understanding plant community assembly and ecosystem functioning in tropical alpine ecosystems.
References
Anderson M, Gorley RN, Clarke K (2008) Permanova+ for primer. Guide to software and statistical methods. PRIMER-E Ltd.
Anthelme F, Buendia B, Mazoyer C, Dangles O (2012) Unexpected mechanisms sustain the stress gradient hypothesis in a tropical alpine environment. J Veg Sci 23:62–72. https://doi.org/10.1111/j.1654-1103.2011.01333.x
Anthelme F, Meneses RI, Valero NNH, Pozo P, Dangles O (2017) Fine nurse variations explain discrepancies in the stress-interaction relationship in alpine regions. Oikos 126:1173–1183. https://doi.org/10.1111/oik.04248
Armas C, Ordiales R, Pugnaire FI (2004) Measuring plant interactions: a new comparative index. Ecology 85:2682–2686. https://doi.org/10.1890/03-0650
Augé RM (2001) Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhiza 11:3–42. https://doi.org/10.1007/s005720100097
Azcón-Aguilar C, Palenzuela J, Roldán A, Bautista S, Vallejo R, Barea JM (2003) Analysis of the mycorrhizal potential in the rhizosphere of representative plant species from desertification-threatened Mediterranean shrublands. Appl Soil Ecol 22:29–37. https://doi.org/10.1016/S0929-1393(02)00107-5
Barbosa MV, Pedroso DF, Curi N, Carneiro MA (2019) Do different arbuscular mycorrhizal fungi affect the formation and stability of soil aggregates? Cien Agrotecnol 43:e003519. https://doi.org/10.1590/1413-7054201943003519
Barnola LG, Montilla MG (1997) Vertical distribution of mycorrhizal colonization, root hairs, and belowground biomass in three contrasting sites from the tropical high mountains, Mérida, Venezuela. Arct Antarct Alp Res 29:206–212. https://doi.org/10.1080/00040851.1997.12003233
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Bråthen KA, Lortie C (2016) A portfolio effect of shrub canopy height on species richness in both stressful and competitive environments. Funct Ecol 30:60–69. https://doi.org/10.1111/1365-2435.12458
Briceño B, Morillo G (2002) Catálogo abreviado de las plantas con flores de los páramos de Venezuela. Parte I. Dicotiledóneas (Magnoliopsida). Acta Bot Venez 25:1–46
Bruno JF, Stachowicz JJ, Bertness MD (2003) Inclusion of facilitation into ecological theory. Trends Ecol Evol 18:119–125. https://doi.org/10.1016/S0169-5347(02)00045-9
Bueno de Mesquita CP, Sartwell SA, Ordemann EV, Porazinska DL, Farrer EC, King AJ, Schmidt SK (2018) Patterns of root colonization by arbuscular mycorrhizal fungi and dark septate endophytes across a mostly-unvegetated, high-elevation landscape. Fungal Ecol 36:63–74. https://doi.org/10.1016/j.funeco.2018.07.009
Cáceres Y, Llambí LD, Rada F (2015) Shrubs as foundation species in a high tropical alpine ecosystem: a multi-scale analysis of plant spatial interactions. Plant Ecol Divers 8:147–161. https://doi.org/10.1080/17550874.2014.960173
Cáceres A, Cáceres-Mago K, Sulbarán A, Márquez M (2016) Crecimiento y actividad enzimática en plantas micorrizadas del bosque seco tropical. Memorias Del Instit Biol Exp 8:141–144
Cáceres A (2002) Algunos aspectos funcionales de la simbiosis con micorrizas arbusculares (MA) en dos especies arbóreas del género Clusia, en el bosque nublado de Altos de Pipe. Doctoral thesis, Universidad Central de Venezuela
Cáceres-Mago K (2020) El arbusto Hypericum laricifolium Juss como ingeniero ecosistémico en un gradiente altitudinal en el páramo desértico: interacciones con la microbiota edáfica. Doctoral thesis, Universidad Central de Venezuela
Callaway RM (2007) Interaction between competition and facilitation. In: Callaway RM (ed) Positive interactions and interdependence in plant communities. Springer, pp 179–254
Caravaca F, del Mar AM, Díaz G, Roldán A (2003) Use of nitrate reductase activity for assessing effectiveness of mycorrhizal symbiosis in Dorycnium pentaphyllum under induced water deficit. Commun Soil Sci Plan 34:2291–2302. https://doi.org/10.1081/CSS-120024064
Carrillo-García Á, La Luz D, León JL, Bashan Y, Bethlenfalvay GJ (1999) Nurse plants, mycorrhizae, and plant establishment in a disturbed area of the Sonoran Desert. Restor Ecol 7:321–335. https://doi.org/10.1046/j.1526-100X.1999.72027.x
Casanova-Katny MA, Torres-Mellado GA, Palfner G, Cavieres LA (2011) The best for the guest: high Andean nurse cushions of Azorella madreporica enhance arbuscular mycorrhizal status in associated plant species. Mycorrhiza 21:613–622. https://doi.org/10.1007/s00572-011-0367-1
Chen JG, He XF, Wang SW, Yang Y, Sun H (2019) Cushion and shrub ecosystem engineers contribute differently to diversity and functions in alpine ecosystems. J Veg Sci 30:362–374. https://doi.org/10.1111/jvs.12725
Cuenca G (2015) Las micorrizas arbusculares: aspectos teóricos y aplicados. Ediciones IVIC
Cuenca G, Lovera M (1992) Vesicular-arbuscular mycorrhizae in disturbed and revegetated sites from La Gran Sabana, Venezuela. Can J Bot 70:73–79. https://doi.org/10.1139/b92-009
Cuenca G, De Andrade Z, Escalante G (1998) Arbuscular mycorrhizae in the rehabilitation of fragile degraded tropical lands. Biol Fertil Soils 26:107–111. https://doi.org/10.1007/s003740050351
Cuesta F, Muriel P, Llambí LD et al (2017) Latitudinal and altitudinal patterns of plant community diversity on mountain summits across the tropical Andes. Ecography 40:1381–1394. https://doi.org/10.1111/ecog.02567
Della Mónica IF, Saparrat MC, Godeas AM, Scervino JM (2015) The co-existence between DSE and AMF symbionts affects plant P pools through P mineralization and solubilization processes. Fungal Ecol 17:10–17. https://doi.org/10.1016/j.funeco.2015.04.004
Filazzola A, Lortie CJ (2014) A systematic review and conceptual framework for the mechanistic pathways of nurse plants. Glob Ecol Biogeogr 23:1335–1345. https://doi.org/10.1111/geb.12202
Flores J, Jurado E (2003) Are nurse-protégé interactions more common among plants from arid environments? J Veg Sci 14:911–916. https://doi.org/10.1111/j.1654-1103.2003.tb02225.x
Gianinazzi-Pearson V, Gianinazzi S (1976) Enzymatic studies on the metabolism of vesicular-arbuscular mycorrhiza. I. Effect of mycorrhiza formation and phosphorus nutrition on soluble phosphatase activities in onion roots. Physiol Veg 14:833–841
Hortal S, Bastida F, Armas C, Lozano YM, Moreno JL, García C, Pugnaire FI (2013) Soil microbial community under a nurse-plant species changes in composition, biomass and activity as the nurse grows. Soil Biol Biochem 64:139–146. https://doi.org/10.1016/j.soilbio.2013.04.018
Hupp N, Llambí LD, Ramírez L, Callaway RM (2017) Alpine cushion plants have species-specific effects on microhabitat and community structure in the tropical Andes. J Veg Sci 28:928–938. https://doi.org/10.1111/jvs.12553
Joner EJ, Johansen A (2000) Phosphatase activity of external hyphae of two arbuscular mycorrhizal fungi. Mycol Res 104:81–86. https://doi.org/10.1017/S0953756299001240
Klironomos JN, Hart MM (2002) Colonization of roots by arbuscular mycorrhizal fungi using different sources of inoculum. Mycorrhiza 12:181–184. https://doi.org/10.1007/s00572-002-0169-6
Körner C (2007) The use of ‘altitude’ in ecological research. Trends Ecol Evol 22:569–574. https://doi.org/10.1016/j.tree.2007.09.006
Llambí LD, Durbecq A, Cáceres-Mago K, Cáceres A, Ramírez L, Torres E, Méndez Z (2020) Interactions between nurse-plants and an exotic invader along a tropical alpine elevation gradient: growth-form matters. Alp Bot 130:59–73. https://doi.org/10.1007/s00035-020-00235-6
Lortie CJ, Brooker RW, Choler P, Kikvidze Z, Michalet R, Pugnaire FI, Callaway RM (2004) Rethinking plant community theory. Oikos 107:433–438. https://doi.org/10.1111/j.0030-1299.2004.13250.x
Lovelock CE, Wright SF, Clark DA, Ruess RW (2004) Soil stocks of glomalin produced by arbuscular mycorrhizal fungi across a tropical rain forest landscape. J Ecol 92:278–287. https://doi.org/10.1111/j.0022-0477.2004.00855.x
Lozano YM, Armas C, Hortal S, Casanoves F, Pugnaire FI (2017) Disentangling above-and below-ground facilitation drivers in arid environments: the role of soil microorganisms, soil properties and microhabitat. New Phytol 216:1236–1246. https://doi.org/10.1111/nph.14499
Lugo MA, Negritto MA, Jofré M, Anton A, Galetto L (2012) Colonization of native Andean grasses by arbuscular mycorrhizal fungi in Puna: a matter of altitude, host photosynthetic pathway and host life cycles. FEMS Microbiol Ecol 81:455–466. https://doi.org/10.1111/j.1574-6941.2012.01373.x
Lugo MA, Menoyo E, Allione LR, Negritto MA, Henning JA, Anton AM (2018) Arbuscular mycorrhizas and dark septate endophytes associated with grasses from the Argentine Puna. Mycologia 110:654–665. https://doi.org/10.1080/00275514.2018.1492846
Mandyam K, Jumpponen A (2005) Seeking the elusive function of the root-colonizing dark septate endophytic fungi. Stud Mycol 53:173–189. https://doi.org/10.3114/sim.53.1.173
McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA (1990) A new method which gives an objective measure of colonization of roots by vesicular—arbuscular mycorrhizal fungi. New Phytol 115:495–501. https://doi.org/10.1111/j.1469-8137.1990.tb00476.x
Molina-Montenegro MA, Oses R, Torres-Díaz C, Atala C, Núñez MA, Armas C (2015) Fungal endophytes associated with roots of nurse cushion species have positive effects on native and invasive beneficiary plants in an alpine ecosystem. Perspect Plant Ecol Evol Syst 17:218–226. https://doi.org/10.1016/j.ppees.2015.02.003
Monasterio M (1979) El páramo desértico en el altiandino de venezuela. In: Salgado-Laboiriau ML (ed) El medio ambiente páramo. Ediciones del CIET-IVIC/MAB-UNESCO, pp 117–176
Montesinos-Navarro A, Segarra-Moragues JG, Valiente-Banuet A, Verdú M (2012) Plant facilitation occurs between species differing in their associated arbuscular mycorrhizal fungi. New Phytol 196:835–844. https://doi.org/10.1111/j.1469-8137.2012.04290.x
Mora MA, Llambí LD, Ramírez L (2019) Giant stem rosettes have strong facilitation effects on alpine plant communities in the tropical Andes. Plant Ecol Divers 12:593–606. https://doi.org/10.1080/17550874.2018.1507055
Pérez FL (1993) Talus movement in the high equatorial Andes: a synthesis of ten years of data. Permafr Periglac Process 4:199–215. https://doi.org/10.1002/ppp.3430040303
Phillips JM, Hayman DS (1970) Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55:158–161
Pugnaire FI, Haase P, Puigdefabregas J (1996) Facilitation between higher plant species in a semiarid environment. Ecology 77:1420–1426. https://doi.org/10.2307/2265539
Rada F, Azócar A, García-Núñez C (2019) Plant functional diversity in tropical Andean páramos. Plant Ecol Divers 12:539–553. https://doi.org/10.1080/17550874.2019.1674396
Ramírez LA, Rada F, Llambí LD (2015) Linking patterns and processes through ecosystem engineering: effects of shrubs on microhabitat and water status of associated plants in the high tropical Andes. Plant Ecol 216:213–225. https://doi.org/10.1007/s11258-014-0429-5
Ramsay P, Oxley E (1997) The growth form composition of plant communities in the Ecuadorian páramos. Plant Ecol 131:173–192. https://doi.org/10.1023/A:1009796224479
Requena N, Jeffries P, Barea JM (1996) Assessment of natural mycorrhizal potential in a desertified semiarid ecosystem. Appl Environ Microbiol 62:842–847. https://doi.org/10.1128/aem.62.3.842-847.1996
Rillig MC (2004) Arbuscular mycorrhizae, glomalin, and soil aggregation. Can J Soil Sci 84:355–363. https://doi.org/10.4141/S04-003
Rillig MC, Wright SF, Nichols KA, Schmidt WF, Torn MS (2001) Large contribution of arbuscular mycorrhizal fungi to soil carbon pools in tropical forest soils. Plant Soil 233:167–177. https://doi.org/10.1023/A:1010364221169
Rodríguez-Echeverría S, Lozano YM, Bardgett RD (2016) Influence of soil microbiota in nurse plant systems. Funct Ecol 30:30–40. https://doi.org/10.1111/1365-2435.12594
Ruiz-Lozano JM, Azcón R (1995) Hyphal contribution to water uptake in mycorrhizal plants as affected by the fungal species and water status. Physiol Plant 95:472–478. https://doi.org/10.1111/j.1399-3054.1995.tb00865.x
Sarmiento L, Llambí L (2011) Regeneración del páramo después de un disturbio agrícola: síntesis de dos décadas de investigación en sistemas con descansos largos de la cordillera de Mérida. In: Herrera F, Herrera I (eds) Algunas experiencias de restauración ecológica en el país en las últimas décadas. Instituto Venezolano de Investigaciones Científicas, pp 123–145
Sulbarán A (2015) Enzimas relacionadas con los procesos metabólicos del fósforo en plantas micorrizadas de Platymiscium pinnatum en el bosque seco insular de la península de Macanao, estado Nueva Esparta. Universidad Central de Venezuela
Tarafdar JC, Marschner H (1994) Phosphatase activity in the rhizosphere and hyphosphere of VA mycorrhizal wheat supplied with inorganic and organic phosphorus. Soil Biol Biochem 26:387–395. https://doi.org/10.1016/0038-0717(94)90288-7
Tisserant B, Gianinazzi-Pearson V, Gianinazzi S, Gollotte A (1993) In planta histochemical staining of fungal alkaline phosphatase activity for analysis of efficient arbuscular mycorrhizal infections. Mycol Res 97:245–250. https://doi.org/10.1016/S0953-7562(09)80248-7
Wehner J, Antunes PM, Powell JR, Mazukatow J, Rillig MC (2010) Plant pathogen protection by arbuscular mycorrhizas: a role for fungal diversity? Pedobiologia 53:197–201. https://doi.org/10.1016/j.pedobi.2009.10.002
Wright SF, Upadhyaya A (1998) A survey of soils for aggregate stability and glomalin, a glycoprotein produced by hyphae of arbuscular mycorrhizal fungi. Plant Soil 198:97–107. https://doi.org/10.1023/A:1004347701584
Acknowledgements
We thank Gisela Cuenca, Nora Malaver, Ismael Hernández-Valencia, Nathalie Suárez, two anonymous referees, and editor for their insightful comments and suggestions which greatly improved the manuscript. We also wish to thank the team at ICAE, Universidad de Los Andes, in particular Eloy Torres and Nelson J. Márquez, for their support during fieldwork and with the identification of the plant material.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cáceres-Mago, K., Cáceres, A. & Llambí, L.D. Effects of nurse shrubs on symbioses between soil fungi and associated plants along a tropical alpine elevation gradient. Alp Botany 132, 285–300 (2022). https://doi.org/10.1007/s00035-021-00275-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00035-021-00275-6