Abstract
Corbicula fluminea is an aggressive invasive species that has successfully colonized aquatic habitats worldwide, producing changes in natural environments and impacting endemic populations. As disturbed environments may favor the adaptive success of exotic species over native ones, we studied the fitness response of two freshwater bivalves, invasive C. fluminea and native Diplodon delodontus, to the presence of metals in the Parana de la Palmas River. To assess the health status of both species, we analyzed morphological and histological alterations in the digestive gland and their relation to metal concentrations in this organ and in the water. Water and specimens sampling took place in the first section of the delta of the Parana River in June and September. Most metal concentrations increased in the river, although in the digestive gland only iron in D. delodontus and nickel and zinc in C. fluminea increased seasonally. Digestive gland factor and hepatosomatic index indicated higher values in C. fluminea in both periods. Despite histological analysis showing a decrease in atrophic tubules frequency in both species, which was followed by histomorphometric parameters of the epithelium and lumen of the digestive tubules, C. fluminea exhibited a greater physiological capacity for metal metabolism and recovery. These results suggest that the physiological characteristics of the Asian clam C. fluminea, combined with its reproductive efficiency and broad dispersal capacities, could explain its invasive dispersal competence over the D. delodontus in the disturbed environmental conditions observed in the Parana de las Palmas River.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Species invasions are a major conservation threat and associated with human activities, one of the main drivers of biodiversity loss, particularly in aquatic environments (Thomaz et al. 2015). Among the invaders in freshwater ecosystems, Corbicula fluminea (Müller 1774) is a highly successful invasive bivalve mollusk that has spread rapidly through aquatic ecosystems worldwide (Pernecker et al. 2021). The first records of this Asian clam in the Neotropical region were made in the Río de la Plata estuary in the late 1970s (Ituarte 1981) and subsequently in other regions of South America (Cao et al. 2017). The success of exotic species in adapting to a new environment may be greater if that environment is disturbed compared with a pristine habitat. Such an adaptive process is mainly explained by native species using their energy resources to cope with changes in physicochemical variables, rather than using them to compete with invasive species for the ecological niche (Darrigran and Arcaria 2009).
The Paraná de la Palmas River is the second largest river basin in South America and is the main tributary of the Rio de la Plata basin in Argentina (Cataldo 2001). Along its course, it receives, directly and through its effluents and tributaries, discharges from agricultural, livestock, and forestry areas, as well as the impact generated by the continuous transit of commercial vessels (Cataldo 2001). The presence of industrial, agricultural, and domestic waste in aquatic ecosystems is widely recognized as a potential threat to the natural environment (Sabatini et al. 2011). Although metals occur naturally in the environment, human activities often increase their concentration, disrupt their natural cycles, or even introduce additional metals into aquatic ecosystems (Tulonen et al. 2006).
Aquatic invertebrates accumulate different metals, essential or non-essential, in variable concentrations (Ruiz et al. 2018), depending on their physiological condition, rate of absorption, accumulation, excretion, exposure time, and also environmental variables. However, essential metals become toxic to the organism above a certain concentration threshold, up to those required to support their life cycle (Rizzo et al. 2010). These potentially toxic metals include cobalt, chromium, copper, molybdenum, manganese, nickel, selenium, and zinc. However, metals that are not associated with biological functions and are therefore harmful to life include mercury, silver, cadmium, and lead. In addition, as a consequence of their non-biodegradable nature, which leads to their bioaccumulation, all metals can be highly toxic and freely cross the cell membrane of an organism from its surrounding (Fichet et al. 1999; Rizzo et al. 2010).
In bivalves, absorption, accumulation, and detoxification of metals in the digestive gland have been demonstrated. Not only those ingested through the diet but also those originating from other tissues to be later excreted are strategic from a toxicological point of view (Zaldibar et al. 2007). Therefore, their morphological and histological analysis may indicate metabolic imbalances due to an increased deposition of glycogen or lipids (vacuoles in their hepatocytes) and structural alterations or pathologies resulting from chronic exposure, among other things (Rodríguez de la Rua et al. 2005). Histology provides information on the toxic effects of contamination on organ anatomy and function. It has been observed that epithelial cells can show changes in their composition and morphology in the presence of contaminants (Soto and Mena 1999). Morphological changes and histopathological lesions have become necessary and widely used tools as suitable biomarkers, given the increasing development of monitoring and assessment of the toxic effects of different contaminants in aquatic ecosystems (Oropesa et al. 2017).
Bivalve mollusks, such as clams and mussels, have long been an important food source for humans and have recently been used as bioindicators of environmental pollution. Their sedentary and filter-feeding nature, along with their availability in large numbers in natural habitats, resistance to variable environmental conditions, ability to take up and accumulate various substances present in their ecosystem, and ease of sampling make them potential candidates for environmental biomonitoring programs (Helmholz et al. 2016). Metal contamination of bivalve mollusks provides a time-integrated measure of the bioavailability of the contaminants (Phillips and Segar 1986).
C. fluminea, similar to other corbiculids, is a short-lived species with a lifespan of 2–3 years and is characterized by its rapid growth and early maturation with high fecundity; it is a simultaneous, self-fertilizing hermaphrodite that broods its young larvae (glochidia) in their inner demibranch (Hornbach 1992; Darrigran 2002; McMahon 2002; Cao et al. 2017). The reproductive organs of C. fluminea consist of an anterior testis and a posterior ovary, both of which discharge into a common gonoduct (Britton and Morton 1979). This Asian clam also has a broad dispersal capacity, adapting to different types of substrates and habitat conditions, allowing it to colonize new environments (Gomes et al. 2016). Yet, the genus Corbicula is notably characterized by its morphological diversity and wide range of reproductive strategies—with dioecious and hermaphroditic species—from indirect development with free-swimming larvae to gill incubation of larvae until the stage of benthic juveniles, making taxonomic description a challenge (Korniushin 2004). In Argentina, this invasive species is currently widespread throughout the La Plata Basin (provinces of Chaco, Santa Fe, Misiones, Corrientes, Entre Ríos, Buenos Aires, and Córdoba), as well as in the Santa Catalina Stream, the Sauce Grande River (Buenos Aires Province), the San Roque Dam (Córdoba Province), and further south, in the Patagonia region, in the Colorado and Negro rivers in Río Negro Province (Hunicken 2020). In contrast, species within the genus Diplodon have extended life spans, up to 100 years, with slow growth, delayed maturity, and high effective fecundity, being predominantly gonochoristic with only few hermaphroditic species (McMahon 2002; Noya Abad et al. 2022). D. delodontus broods its glochidial larvae in its marsupial chambers within inner demibranchs and is found native to the province of Buenos Aires in the Paraná and La Plata rivers of the La Plata Basin (Cao 2020).
The introduction and following dispersal of the invasive bivalve C. fluminea (Müller 1774) in the Paraná de las Palmas River place the heterogeneity of the aquatic ecosystem at risk since its burial behavior in the sediment and consequential bioturbation of the water column might displace or reduce the habitat and/or limit the availability of food for native bivalves such as D. delodontus (Cao et al. 2017).Therefore, to assess the health status of both the invasive C. fluminea and the native D. delodontus in the Parana de las Palmas River, we analyzed morphological and histological alterations in the structure of the digestive gland tubules and their relation with the presence of metals in the aquatic environment.
Materials and methods
Sampling site and experimental design
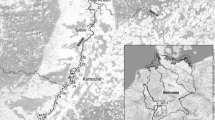
In 2017 and 2018, preliminary sampling evidenced the presence of C. fluminea and D. delodontus in low-density assemblages in the form of patches in the studied area. The sampling site was established at the downstream far zone of the Paraná de las Palmas River and beginning of the first section of the Buenos Aires Delta (34° 16′ 3.6″S, 58° 39′ 9.8′W; Fig. 1). In September 2018, at the same site, 60 adult exemplars of the invasive Asian clam C. fluminea (shell length > 1 cm) were set inside 10 cages (6 individual/cage) along with 10 adults of native D. delodontus (shell length > 7 cm) set in a separate 10 cages (1 individual/cage). The cages were placed on the silty-sandy sediment at the bottom of the river, at a depth of about 5 m, in suitable conditions at the sampling site and tied to the nearest pier. Five cages of each species (10 cages) containing 30 C. fluminea and 5 D. delodontus were collected in June 2019 (austral Winter season), and the remaining cages were collected in September 2019 (austral Spring season). Temperature, pH, dissolved oxygen, and conductivity were measured in situ with a multiparametric electrode (Lutron DO-5510) in both periods. Concurrently, water samples were collected following Alberro et al. (2011) at the same depth using plastic containers previously washed with 5% HNO3 and kept cold until reaching the laboratory, where they were stored at 4 °C for further metal concentration analysis as described in Peluso (2020). Total suspended solids (TSS) were determined according to the APHA 2005 protocol, using the gravimetric method. Finally, morphometric indices, along with metal concentrations and histological determinations in the digestive gland were performed individually.
Morphometric index
Individuals were measured for height, width, and length, after which the digestive gland was dissected and then weighed. Using these data, the digestive gland mass factor (DGF) was calculated considering the relationship between [wet weight of the digestive gland (g)/valve length (cm)] × 100 and the hepatosomatic index (HSI), [wet weight of the digestive gland (g)/wet weight of total soft tissue (g)] × 100, to measure the energy reserves of an animal (Sabatini et al. 2011).
Metal concentration
Metal concentrations were determined in water and over digestive gland homogenates from the two species (in a weight–volume ratio of 1:5), using 0.154 M phosphate buffer (pH 7) with protease inhibitors [benzamidine 10 mM and phenylmethylsulfonyl fluoride (PMSF) 0.5 mM].
Metal presence and concentration was determined using the flame atomic absorption spectrophotometer technique (FAAS; Shimadzu 6700, Kyoto, Japan) regarding iron (Fe) and zinc (Zn). Cadmium (Cd), chromium (Cr), copper (Cu), nickel (Ni), and lead (Pb) were determined using graphite furnace atomic absorption spectrophotometry (GFAAS; at Shimadzu 6800, Kyoto, Japan). Instrumental parameters and graphite furnace programs were provided by the manufacturer (Conti et al. 2011). These techniques quantify the entire concentration of metal, without discriminating the chemical forms (ions, inorganic complexes, and organic compounds) or the different species of the same element (oxidation number). Result traceability was obtained from the analysis of the certified reference material Antarctic Krill MURSTISS-A2 (Italian Research Program in Antarctica). The average recovery percentages (five repetitions) were 101.3 ± 2.3% for iron; 102.1 ± 2.9% for zinc; 93.4 ± 2.7% for cadmium; 98.1 ± 1.0% for chrome; 101.1 ± 1.3% for copper; 98.5 ± 2.5% for nickel; and 96.5 ± 0.6% for lead. Detection limits were defined as limit of detection (LOD) = 3Sb/m, where Sb is the standard deviation of 10 replicates of the blank and m is the slope of the calibration curve (3Sb, n = 10), considering the lowest concentration level that can be determined, statistically different from blank, with 99% confidence. Limits of detection (3 Sb, n = 10) were 0.030 mg/L for iron; 0.010 mg/L for zinc; 0.0001 mg/L for cadmium; 0.0002 mg/L for chromium; 0.020 mg/L for copper; 0.006 mg/L for nickel, and 0.002 mg/L for lead.
Histology of digestive gland
Histological assessment of the digestive glands of C. fluminea and D. delodontus was made by observation under an optical microscope. Organs were fixed in calcium formaldehyde for 24–48 h and then dehydrated through a progressive series of ethanol, up to 96%, intermediately infiltrated by xylene and embedded in histological paraffin. Tissue sections of 6 μm thickness were obtained using a Leica RM2125 microtome, subsequently stained with hematoxylin–eosin solution (H&E). Sections were randomly analyzed in an optical microscope (Zeiss Axiostar) and photographically recorded with a Canon camera (Canon G10).
To evaluate the digestive gland condition, alterations in the epithelium and lumen of the digestive gland tubules were compared, and a histomorphometric analysis was made.
Histomorphometry
Histomorphometric analyses were performed by evaluating changes in the epithelium and tubular lumen of the digestive tubules. Images of the digestive gland H&E-stained sections were randomly taken at 400× magnification (30 images per individual) and examined using Image J software 1.51j8 (Rasband 2012). Each digestive tubule was described individually, and the proportion of each type of digestive tubule per individual was then determined. Digestive tubule status was analyzed in terms of the epithelial layer area (S0), digestive tubule lumen area (Si), tubule perimeter (P0), lumen perimeter (Pi), mean epithelial thickness (h) [h = 2(S0 − Si)/P0 + Pi] (Marigómez et al. 1990), and circularity (c) [c = 4(S0/P02)] (Carella et al. 2015).
Statistical analysis
Analysis of the water physicochemical variables was carried out using Student’s t-test comparison of two means (Student 1908) and a posteriori Tuckey tests, using the InfoStat program. Comparisons between species and both sampling periods for each of the variables analyzed were carried out using two-factor analysis of variance (ANOVA) followed by Tuckey tests (Sokal and Rohlf 1995) using the GraphPad Prism 6 program. Concentrations of chromium, nickel, and lead were compared between species and periods using Student’s means comparison tests (Student 1908) with Tuckey tests a posteriori where results were lower than the measurement blank.
Results were compared using parametric two-way ANOVA test (season and types of digestive tubule as factors) for each species separately after normality (Kolmogorov–Smirnov test) and homogeneity of variances (Levene test) were tested, and transformations were applied when required. Significant differences were compared using Tukey’s test (Sokal and Rohlf 1995), and significant interactions were analyzed by simple effects analysis. When assumptions were not verified, non-parametric Mann–Whitney (M-W) and Kruskal–Wallis (K-W) analyses were performed. All the statistical analyses were carried out with the Statistica 7.1 software. p = 0.05 was taken as the threshold of significance.
Results
Metals in water and digestive gland
Temperature, conductivity, hardness, and total suspended solids increased significantly from June to September (p < 0.05), while oxygen and pH did not (Table 1). For metals measurements, all metals except for lead and copper increased significantly in September (p < 0.05).
In the digestive gland, chromium showed higher concentration values in D. delodontus compared with C. fluminea in both June and September, with significant differences between months (p > 0.05; Table 2). Similarly, D. delodontus presented higher values for nickel in both periods, although only significantly in September (p < 0.05). Nevertheless, nickel increased in both species from June to September, with significantly higher values for C. fluminea in September. However, cadmium concentration in organs showed the opposite tendencies between the species regarding months, a significant decrease from June to September for C. fluminea while an increase for D. delodontus in the same period. However, no significant differences were found for D. delodontus (p > 0.05). Meanwhile, lead decreased in both species from June to September, with statistical variation found in C. fluminea (p < 0.05) and in September between species. Iron increased from June to September in both species, but only significantly in D. delodontus (p < 0.05). Significant values were also observed between species in September. Zinc also showed increasing values between June and September in both species, yet only significantly in C. fluminea, and between species in September (p < 0.05). Copper concentrations decreased from June to September, showing significant differences between species in both periods (p < 0.05). In conclusion, bioaccumulation in D. delodontus reached higher concentrations of cadmium, chromium, iron, lead, and nickel in both periods and zinc in June, while C.fluminea had the highest concentrations of copper in both periods and zinc in September.
The concentration of bioaccumulated metals observed in the digestive gland was not proportional to that in the water for any of the species. Concentrations of copper, iron, and zinc were found to be significantly higher in the animals than in the water. (Table 3).
Morphometric index
The digestive gland factor (DGF) varied in June between species, when C. fluminea was 62.51% higher than D. delodontus (p < 0.05). In September no significant differences between species were observed (p > 0.05); still results indicated higher values in C. fluminea. Also, no significant differences were found upon comparing June and September for each species; nevertheless, there was a small decrease in the invasive species C. fluminea, while a limited increase was observed in the native D. delodontus. The hepatosomatic index (HIS) presented no differences between sampling periods regarding species; however, C. fluminea exhibited higher values (p < 0.05) both in June (53.24%) and September (39.85%) compared with D. delodontus (Fig. 2).
Average ± standard deviation (n = 5) obtained from the digestive gland mass factor (DGF) and hepatosomatic index (HSI), comparing between Corbicula fluminea (Cf), Diplodon delodontus (Dd) and sampling periods [June: (winter) and September:(spring)]. Different letters (A, B and C) indicate significant differences (p < 0.05)
Histological assessment
The digestive gland in both C. fluminea and D. Delodontus comprises a multiple blind-ending alveolo-tubule structure surrounding the stomach and connected to it through main ciliated and secondary non-ciliated ducts. Each duct or digestive tubule (DT) is formed by a single-layer epithelium supported by a well-defined basal membrane (Fig. 3). The digestive tubule epithelium is formed mainly of two different cell types: digestive cells and basophilic cells. The proportion of cell types varied according to the digestive tubule status phase. On the basis of observations, three phases were defined to describe the condition of each digestive tubules: the holding or retention phase (type I), showing high epithelium and a broad-flat lumen; the absorption phase (type II), with high and folded epithelium with angular lumen and presence of space vacuolar cells; and the disintegration or atresic phase (type III), which exhibited reduced thin epithelium, large lumen, and abundant presence of debris. The different heights of the cell cause the epithelium to fold in some sections, producing “Y” and “star” lumen shapes particularly in the absorptive phase of the digestive tubule (Fig. 3C, D). Also, epithelium folds produce chambers in the profile of the lumen. Another important feature was noticed toward the dense accumulation of the luminal material, which also contain leucocytes. Furthermore, plenty of connective tissue lays among the tubules in which hemolymphatic cell types can be distinguished, such as small, rounded hemocytes with conspicuous nucleus, as well as granular and semi-granular hemocytes. These hemocytes were observed more abundantly in the disintegrating or atrophic phase (Fig. 3E, F).
Photomicrographs of the digestive gland of clams C. fluminea (A, C, E) and D. delodondus (B, D, F). A, B Digestive tubule types classified as type I holding, C, D type II absorptive, and E, F type III disintegrating or atrophic. Digestive and basophilic cells in the digestive tubule epithelium (asterisk and black arrow, respectively). Hemocytes are indicated with a white arrow. Sections of the digestive gland are stained with hematoxylin–eosin solution. Scale bars: 20 µm (A, C, E) and 50 µm (B, D, F). vc, vacuolate cells; tl, tubule lumen
Histological assessment of the digestive gland normal phasic activity indicated a predominance of type II (absorptive) in C. fluminea and D. delodontus in both June (70% and 73.33%, respectively) and September 2019 (74.44% and 80%, respectively). In comparison, holding phase type I and disintegrating or atrophic phase type III showed lower values and significant differences (p < 0.05) between species and between months for C. fluminea (Fig. 4).
Histomorphometric analysis
Individuals of C. fluminea and D. delodontus were examined (360 digestive tubules images), in June and September 2019. Digestive gland condition was analyzed in both species independently by comparing morphometric parameters between digestive tubule phases and sampling months (Table 3). The area of the DT epithelium showed significant differences between months for C. fluminea (two-way ANOVA, F(2,46) = 13.624, p = 0.000287), in particular for the absorption phase (type II), which decreased between June (1. 22 ± 0.35 mm2) and September [0.98 ± 0.33mm2; Kruskal–Wallis H test (K-W H)(0.02) = 2.295, p = 0.043], while no differences were verified for D. delodontus (p > 0.05). Regarding tubule circumference (P0), differences between months were observed in C. fluminea [ANOVA F(0.645) = 15.159, p < 0.05], with a reduction in measurements for the three DT phases from June to September and a marked difference in the absorption phase, although not significant (K-W H (2.198) = 2.788, p = 0.055). In contrast, no significant differences were found in D. delodontus [Mann−Whitney−Wilcoxon test (M-W, W) = 2993, p = 0.865].
Regarding the lumen area (Si) of the digestive tubule, a cyclical succession of decreasing area between types I and II was observed, followed by a marked increase from type II to III, and finally a slight decrease between III and I in both species for the 2 months. Furthermore, differences in lumen area values were found in relation to phases (ANOVA F(88.662) = 125.38, p < 0.05); Tukey’s test, p < 0.05), as well as differences between months (M-W, W = 5893.5, p < 0.05) in C. fluminea and in D. delodontus (M-W, W = 2452, p < 0.05). In C. fluminea, the absorption phase showed the greatest difference between months (K-W H (1.129) = 0.302, p < 0.05), where a reduction was observed from June (0.10 ± 0.05 mm) to September (0.06 ± 0.03 mm). The lumen perimeter showed significant differences in C. fluminea in relation to the phases [two-way ANOVA F(2.136) = 18.054, p < 0.05], mostly between types II and III and types III and I (Tukey’s test, p < 0.05).
The mean epithelial thickness (h) of the DT showed differences in C. fluminea for both phases [two-way ANOVA F(3.717) = 74.510, p < 0.05] and months [two-way ANOVA F(0.472) = 9.46, p < 0.05]. The greatest difference between phases was between absorption and disintegration or atresia (Tukey’s test, p < 0.05), while in D. delodontus only differences in epithelial height were observed in relation to phases [ANOVA F(403.28) = 13.184, p < 0.05]. Regarding circularity (c), differences were observed in C. fluminea between sampling months [two-way ANOVA F(0.440) = 10.044, p < 0.05; Tukey’s test, p < 0.05] and between phases and months [two-way ANOVA F(0.135) = 3.096, p < 0.05], where significant differences were observed in the absorption phase between months (Tukey’s test, p < 0.05). However, no differences were found in that species for DT phase [two-way ANOVA F(0.013) = 0.297, p = 0.743]. No significant differences were found for D. delodontus (W = 2288, p = 0.4026).
Discussion
Concentrations of chromium, copper, nickel, zinc, iron, cadmium, and lead in the Paraná de las Palmas River were found to be two times or more above the levels reported by Cataldo et al. (2001) in 1996. These significant increments reached the highest values in September 2019. Páez-Osuna (2005) suggests that, although chromium, nickel, zinc, and copper are naturally present in aquatic environment, their over-limited concentrations might be directly linked to waste produced by the intensification of industrial, agricultural, livestock, and urban activities previously developed in the area (Cataldo et al. 2001). In addition, research has shown that most of industrial effluents and domestic waste are discharged into the river with little or no prior treatment (Peluso 2020). And although the high flow rate of the Paraná de las Palmas River (about 18,000 cubic meters per second) would probably have a very important “cleansing” effect, (Cataldo 2001), the tidal regime and the sudestadas phenomenon (southeast storms), depending on the water level and the direction of the current, directly influence the hydrological behavior of the river (Pizarro et al. 2007). Therefore, the hydrological regime of the Paraná de las Palmas River is the main cause of the variation in metal concentrations observed in June and September (winter and spring seasons), together with the shift in the intensity of anthropic activities throughout the year in the surrounding area.
The increase in the river water levels due to flooding could cause dilution and thus a decrease in the concentration of pollutants, while periods of low water levels could cause an increase in the concentration of suspended solids, re-suspending in the water column the chemical elements that are bioavailable in the sediment, as previously reported by Cataldo (2001). However, during high hydrometric level events associated with ocean tides, the upper section of the Paraná de las Palmas River contributes pollutants to the lower section, thereby reaching higher concentrations of pollutants than those observed during low hydrometric events, as occurred in our study, since the high levels events were recorded in September, while the low ones occurred in June.
Several species of bivalve mollusks accumulate metals in concentrations proportional to those present in their environment, which may also fluctuate over time (Sabatini et al. 2011). In bivalves the digestive gland is the main site for metal bioaccumulation and detoxification, concentrating more metals than other organs (Sabatini et al. 2011). Variation between sampling periods indicated the highest metal concentrations in C. fluminea digestive gland occurred in September, which is associated with all the metals measured in water in the Paraná de las Palmas River, except for lead and copper, which increased significantly in that period (p < 0.05). Nevertheless, the divergent results of metal concentrations found in water and in the digestive gland could be related to the presence of metals in the diet of both species or even in the sediment that they inhabit since bioaccumulation depends on the metal concentration, its environmental bioavailability (water, diet, and particles), and its mechanisms of absorption, accumulation, and excretion (Wang et al. 1995). Such mechanisms differ according to the metal nature and whether they are essential for the metabolism and growth of the organism (Wang et al. 1995; Chapman et al. 1996). Furthermore, metals persistence in the environment is directly associated with its chemical speciation, incorporation, and sediment accumulation, thus playing a key role in its bioavailability in and toxicity to aquatic organisms (Van Ginneken et al. 1999; Rizzo et al. 2010). Once incorporated to the sediment, metals can be mobilized and released back into the water column through changes in solubility and transfer through the trophic web as a result of physical and chemical changes (Altindag and Yigit 2005; Harikumar et al. 2009).
Regarding the morphometric index of C. fluminea and D. delodontus in the Paraná de las Palmas River, differences were observed between sampling periods. The limited increase of the digestive gland factor in the native species could be related to the fact that D. delodontus is in the period of preparation of the marsupium and accumulation of energy reserves for the gamete maturation, a period that is completed by the end of summer in different species of the same genus (Semenas and Brugni 2002). Díaz and García (2001) indicated that copper and lead inhibit the activity of enzymes responsible for glycogen synthesis and mobilization, which could induce a negative effect in the reproductive process. For its part, the invasive species displays several reproductive events throughout the year, the most relevant being during springtime, and apparently does not exhibit periods of reproductive inactivity; therefore, glycogen reserves remain constant all year round (Cao et al. 2017). The constancy of the hepatosomatic index between sampling periods for each of the species, although with higher concentration in C. fluminea, would indicate that food availability did not change during the period of these samplings in the Paraná River.
The digestive gland tubules of C. fluminea and D. delodontus were morphologically analyzed to assess the environmental quality of the water in the Paraná de las Palmas River and its effects on both species. In general, the structure of the digestive gland in both species corresponds to the basic model of microphagous feeders in mollusks and previous descriptions in other bivalve anatomy (Diniz et al. 2008; Costa et al. 2013). The characterization of the rhythms of the intracellular digestion based on morphological differences of the digestive tubules is the result of the variation within the digestive cells that constitute it, depending on food ingestion and its subsequent intracellular digestion. In this way, an arbitrary division into phases that represent the sequence of events in this dynamic process can be made. The description of types of digestive tubules model used in the present study coincides with the one applied to describe the mussels Mytilus galloprovincialis (Rocha et al. 2016). The proportion pattern of digestive tubules found in both species with prevalence of absorbing type but most importantly the seasonal decrease in atrophic tubule may be strongly linked to reproductive cycle in C. fluminea, as mentioned above. It is also important to highlight that all the individuals observed presented two or more types of tubule phases in the digestive gland. Despite widely used in bivalve species as a biomarker of general stress induced by various contaminant, including metals, the frequency of digestive tubule atrophy observed in C. fluminea and D. delodontus in Paraná de las Palmas River could be associated with degenerative processes within their life cycle (Sabatini et al. 2011; Carella et al. 2015).
Histomorphometric studies have long been applied to establish a correlation response between the presence of contaminants and tissue alteration caused by the organism’s stress response (Marigómez et al. 1990). Considering that the epithelial cells lining the tubules of the digestive gland respond negatively to the effects of various contaminants, causing tubule lumen enlargement and/or epithelial thinning, morphometric variations in epithelial layer and lumen constitute a reliable index of digestive gland activity (Rocha et al. 2016). In such way, the morphometric parameters used to differentiate digestive tubule (DT) conditions demonstrated that epithelial layer area (S0), digestive tubule lumen area (Si), mean epithelial thickness (h), and circularity (c) offered a reliable criterion to characterize DT phases in both species, particularly in C. fluminea, and therefore evaluate their health status in the Paraná de las Palmas River (Marigómez et al. 1990; Carella et al. 2015; Rocha et al. 2016). Foremost, it is necessary to take in consideration that the digestive gland of bivalves is an organ that undergoes a series of daily and annual cyclical changes related to digestive and reproductive cycles, also shaped by tidal rhythms and hydrological regimes, that may directly affect its morphological condition and its assessment (Usheva et al. 2006). Although alterations in the morphology of the digestive tubules represent a non-specific response to stressful environmental conditions, atrophy condition and hence the thinning of the tubule epithelium combined with lumen enlargement is broadly used and well documented as a morphological measurement of the cellular and tissue level alteration (Garmendia et al. 2011). In this way, quantitative morphological analysis in terms of mean epithelial thickness and lumen area represents a reliable tool in analyzing the health status of the digestive gland in bivalves (Marigómez et al. 2006).
In summary, the present work evaluated the health condition of two bivalve species inhabiting the Paraná de las Palmas River through in situ experiment in which morphological and histological alterations in the digestive gland were analyzed seasonally in relation to the presence of metal in the aquatic environment. The two competitor clams, a native species and an invasive species, showed different responses to the presence of metals both in water and digestive gland accumulate, suggesting that, despite its higher susceptibility to environmental disturbance, particularly observed in the greater tissue alteration in the digestive tubule, C. fluminea displays an efficiency in metabolizing metals that is superior to D. delodontus. These results combined with C. fluminea biological traits, such as its ability to inhabit different substrate types and its reproductive efficiency, might explain its invasive dispersal competence when it comes to colonizing a great diversity of freshwater ecosystems and ecologically overcome the presence and endurance of the native D. delodontus in the Paraná de las Palmas River.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. The authors confirm that the data supporting the findings of this study are available within the article.
Code availability
Not applicable.
References
Altindað A, Yiðit S (2005) Assessment of heavy metal concentrations in the food web of lake Beyşehir Turkey. Chemosphere 60(4):552–556. https://doi.org/10.1016/j.chemosphere.2005.01.009
Argentina. Variabilidad morfológica, molecular y distribución de Diplodon delodontus. Tesis Doctoral en
Britton JC, Morton B (1979) Corbicula in North America: the evidence reviewed and evaluated. Britton JD (Eds) Proceedings of the First International Corbicula Symposium. Texas Christian University Research Foundation Fort Worth. 249–287
Cao LM, Damborenea C, Penchaszadeh PE, Darrigran G (2017) Gonadal cycle of Corbicula fluminea (Bivalvia: Corbiculidae) in Pampean streams (Southern Neotropical Region). PLoS ONE. https://doi.org/10.1371/journal.pone.0186850
Cao LM (2020) Conservación de las poblaciones de bivalvos nativos (Mollusca: Bivalvia: Unionoida) en
Carella F, Feist SW, Bignell JP, De Vico G (2015) Comparative pathology in bivalves: aetiological agents and disease processes. J Invertebr Pathol. https://doi.org/10.1016/j.jip.2015.07.012
Cataldo DH, Boltovskoy D, Stripeikis J, Pose M (2001) Condition index and growth rates of field caged Corbicula fluminea (Bivalvia) as biomarkers of pollution gradients in the Paraná river delta (Argentina). Aquat Ecosyst Health Manage. https://doi.org/10.1080/14634980127712
Cataldo DH (2001) Dinámica poblacional y potencial bioindicador de contaminación acuática del molusco bivalvo Corbicula fluminea en el delta del Río Paraná. Tesis Doctoral. Facultad de Ciencias Exactas y Naturales. Universidad de Buenos Aires. http://digital.bl.fcen.uba.ar/Download/Tesis/Tesis_3351_Cataldo.pdf.
Chapman PM, Allen HE, Godtfredsen K, ZGraggen MN (1996) Evaluation of bioaccumulation factors in regulating metals. Environ Sci Technol. https://doi.org/10.0013/936X/96/0930-448AS12.00/0
Ciencias Naturales. Universidad Nacional de La Plata, Facultad de Ciencias Naturales y Museo. 161 págs
Conti ME, Stripeikis J, Finoia MG, Tudino MB (2011) Baseline trace metals in bivalve molluscs from the Beagle Channel Patagonia (Argentina). Ecotoxicology. https://doi.org/10.1007/s10646-011-0690-5
Costa PM, Carreira S, Costa MH, Caeiro S (2013) Development of histopathological indices in a commercial marine bivalve (Ruditapes decussatus) to determine environmental quality. Aquat Toxicol. https://doi.org/10.1016/j.aquatox.2012.08.013
Darrigran G (2002) Potential impact of filter-feeding invaders on temperate inland freshwater environments. Biol Invasions 4:145–156
Darrigran G, Arcaría N (2009) Las invasiones biológicas en la costa Argentina y en la cuenca del Plata. 179–192. In: López RA Marcomini SC (coord.) 2009. Problemática de los ambientes costeros Sur de Brasil, Uruguay y Argentina. Editorial Croquis S.R.L. ISBN 978–987–1527–24–3
Díaz A, García J (2001) Concentration of the heavy metal Cu and Pb and their relationship with the synthetase glycogen and phosphorylase glycogen enzymatic activity in the mussel (Perna viridis). Zootec Trop 19:115–129
Diniz MS, Santos HM, Costa PM, Peres I, Costa MH, Alves S, Capelo-Martinez JL (2008) Efectos de la exposición al arsénico en Corbicula fluminea: evaluación de las respuestas histológicas, histoquímicas y bioquímicas. Cienc Mar. https://doi.org/10.7773/cm.v34i3.1396
Fichet D, Boucher G, Radenac G, Miramand P (1999) Concentration and mobilisation of Cd, Cu, Pb and Zn by meiofauna populations living in harbour sediment: their role in the heavy metal flux from sediment to food web. Sci Total Environ. https://doi.org/10.1016/S0048-9697(99)00401-5
Garmendia L, Izagirre U, Cajaraville MP, Marigómez I (2011) Application of a battery of biomarkers in mussel digestive gland to assess long-term effects of the Prestige oil spill in Galicia and the Bay of Biscay: lysosomal responses. J Environ Monit. https://doi.org/10.1039/c0em00409j
Gomes C, Sousa R, Mendes T, Borges R, Vilares P, Vasconcelos V et al (2016) Low genetic diversity and high invasion success of Corbicula fluminea (Bivalvia, Corbiculidae) (Müller, 1774) in Portugal. PLoS ONE 11(7):e0158108. https://doi.org/10.1371/journal.pone.0158108
Harikumar PS, Nasir UP, Mujeebu Rahman MP (2009) Distribution of heavy metals in the core sediments of a tropical wetland system. Int J Environ Sci Technol 6:225–232. https://doi.org/10.1007/BF03327626
Helmholz H, Ruhnau C, Pröfrock D, Erbslöh HB, Prange A (2016) Seasonal and annual variations in physiological and biochemical responses from transplanted marine bioindicator species Mytilus spp. during a long term field exposure experiment. Sci Total Environ 565:626–636
Hornbach DJ (1992) Life history traits of a riverine population of the Asian Clam Corbicula fluminea. Am Midl Nat 127:248–257
Hünicken LA (2020) Invasión de Corbicula spp. en Argentina: distribución, variaciones morfológicas y metabolismo de un bivalvo invasor global. Tesis Doctoral. Universidad de Buenos Aires, FCEyN, Departamento EGE. 151
Ituarte CF (1981) Primera noticia acerca de la introducción de pelecípodos asiáticos en el área rioplatense
Korniushin AV (2004) A revision of some Asian and African freshwater clams assigned to Corbicula fluminalis (Müller, 1774) (Mollusca: Bivalvia: Corbiculidae), with a review of anatomical characters and reproductive features based on museum collections. Hydrobiologia 529:251–270. https://doi.org/10.1007/s10750-004-9322-x
Lombardo RJ, O’Farrell I, dos Santos AM (2010) Spatial and temporal ion dynamics on a complex hydrological system: the lower Luján River Buenos Aires Argentina. Aquat Geochem. https://doi.org/10.1007/s10498-009-9064-5
Marigómez JA, Cajaraville MP, Angulo E (1990) Histopathology of the digestive gland-gonad complex of the marine prosobranch Littorina litforea exposed to cadmium. Dis Aquat Org 9:229–238
Marigómez I, Soto M, Cancio I, Orbea A, Garmendia L, Cajaraville MP (2006) Cell and tissue biomarkers in mussel, and histopathology in hake and anchovy from Bay of Biscay after the Prestige oil spill (Monitoring Campaign 2003). Mar Pollut Bull 53:287–304
McMahon RF (2002) Evolutionary and physiological adaptations of aquatic invasive animals: r selection versus resistance. Can J Fish Aquat Sci 59:1235–1244
(Moll. Corbiculidae). Neotropica. 27: 79–83
Noya Abad T, Peluso J, Minaberry YS, Rojas DE, Cristos D, Knack De Almeida H, Yusseppone MS, Aronzon CM, Calcagno JÁ, Sabatini SE (2022) Efectos de las actividades antrópicas sobre parámetros metabólicos en un bivalvo invasor y otro nativo en el tramo inferior del río Paraná. Ecol Austral 32(3):1089–1105. https://doi.org/10.25260/EA.22.32.3.0.1989
Oropesa AL, Moreno JJ, Gómez LJ (2017) Lesiones histopatológicas en peces originadas por la exposición a contaminantes emergentes: recopilando y analizando datos. Rev Toxicol 34:99–108 (ISSN: 0212-7113)
Páez-Osuna F (2005) Efectos de los metales. Golfo de México, Contaminación e Impacto Ambiental: diagnóstico y tendencias. 329–360
Peluso J (2020) Evaluación de la calidad de cuerpos de agua pertenecientes a la cuenca baja del río Paraná mediante indicadores fisicoquímicos y toxicológicos. Tesis doctoral. Instituto de Investigación e Ingeniería Ambiental, IIIA, UNSAM, CONICET
Pernecker B, Czirok A, Mauchart P (2021) No experimental evidence for vector-free, long-range, upstream dispersal of adult Asian clams [Corbicula fluminea (Müller, 1774)]. Biol Invasions. https://doi.org/10.1007/s10530-020-02446-8
Phillips DJH, Segar DA (1986) Use of bio-indicators in monitoring conservative contaminants: programme design imperatives. Mar Pollut Bull 17(1):10–17
Pizarro H, Rodríguez P, Bonaventura SM, O’Farrell I, Izaguirre I (2007) The sudestadas: a hydro-meteorological phenomenon that affects river pollution (River Luján, South America). Hydrol Sci J. https://doi.org/10.1623/hysj.52.4.702
Rasband WS (2012) ImageJ software
Rizzo A, Daga R, Arcagni M, Perez Catán S, Bubach D, Sánchez R, Ribeiro Guevara S, Arribére MA (2010) Concentraciones de metales pesados en distintos compartimentos de lagos andinos de Patagonia Norte. Ecol Austral 20(2):155–171
Rocha TL, Morais SMTS, Bebianno MJ (2016) Histopathological assessment and inflammatory response in the digestive gland of marine mussel Mytilus galloprovincialis exposed to cadmium-based quantum dots. Aquat Toxicol 177:306–315
Rodríguez de la Rua A, Arellano JM, Gonzalez de Canales ML, Blasco J, Sarasquete C (2005) Acumulación de cobre y alteraciones histopatológicas en el Ostión Crassostrea angulata. Cienc Mar. https://doi.org/10.7773/cm.v31i3.39
Ruíz MD, Iriel A, Yusseppone MS, Ortiz N, Di Salvatore P, Fernández Cirelli A, Ríos de Molina MC, Calcagno JA, Sabatini SE (2018) Trace metals and oxidative status in soft tissues of caged mussels (Aulacomya atra) on the North Patagonian coastline. Ecotoxicol Environ Saf. https://doi.org/10.1016/j.ecoenv.2018.02.064
Sabatini SE, Rocchetta I, Nahabedian DE, Luquet CM, Eppis MR, Bianchi L, Ríos de Molina MC (2011) Estrés oxidativo y alteraciones histológicas producidas por el cobre dietético en el bivalvo de agua dulce Diplodon chilensis. Bioquím Fisiol Comp Part C: Toxicol Farmacol. https://doi.org/10.1016/j.cbpc.2011.07.009
Semenas L, Brugni N (2002) Características poblacionales y ciclo de vida de Diplodon chilensis (d’Orbigny, 1835) (Hyriidae, Bivalvia) en el lago Gutiérrez (Patagonia, Argentina). Ecol Austral 12(1):29–40
Sokal RR, Rohlf FJ (1995) Introducción a la Bioestadística. Reverté, Barcelona, Spain
Soto D, Mena G (1999) Filter feeding by the freshwater mussel, Diplodon chilensis, as a biocontrol of salmon farming eutrophication. Aquaculture. https://doi.org/10.1016/S0044-8486(98)00420-7
Student (1908) The Probable Error of a Mean. Biometrika. Oxford J. https://doi.org/10.2307/2331554
Thomaz SM, Kovalenko KE, Havel JE, Kats LB (2015) Aquatic invasive species: general trends in the literature and introduction to the special issue. Hydrobiologia. https://doi.org/10.1007/s10750-014-2150-8
Tulonen T, Pihlström M, Arvola L, Rask M (2006) Concentrations of heavy metals in food web components of small, boreal lakes. Boreal Environ Res 11:185–194
Usheva LN, Vaschenk MA, Durkina VB (2006) Histopathology of the digestive gland of the bivalve mollusk Crenomytilus grayanus (Dunker, 1853) from Southwestern Peter the Great Bay, Sea of Japan. Histology 32:166–172
Van Ginneken L, Mohammed JC, Blust R (1999) Bioavailability of cadmium and zinc to the common carp, Cyprinus carpio, in complexing environments: a test for the validity of the free ion activity model. Environ Toxicol Chem 18(10):2295–2304
Wang WX, Fisher NS, Luoma SM (1995) Assimilation of trace elements ingested by the mussel Mytilus edulis: effects of algal food abundance. Marine Ecol Prog Ser 129:165e176
Zaldidar B, Cancio I, Soto M, Marigómez I (2007) Digestive cell turnover in digestive gland epithelium of slugs experimentally exposed to a mixture of cadmium and kerosene. Chemosphere. https://doi.org/10.1016/j.chemosphere.2007.06.071
Acknowledgements
We would like to acknowledge Walter Santillán for his participation in taking samples and collecting animals and to Martín Fea for his technical support in the laboratory. This project was funded by grants from the Universidad de Buenos Aires—Secretaría de Ciencia y Técnica UBACYT 20020190200150BA and the Agencia Nacional de Promoción Científica y Tecnológica (ANPCYT)—Proyectos de Investigación Científica y Tecnológica (PICT) 2018-1895 to Sebastián E. Sabatini.
Funding
This project was funded by grants from the Universidad de Buenos Aires—Secretaría de Ciencia y Técnica UBACYT 20020190200150BA and the Agencia Nacional de Promoción Científica y Tecnológica (ANPCYT)—Proyectos de Investigación Científica y Tecnológica (PICT) 2018–1895 to Sebastián E. Sabatini.
Author information
Authors and Affiliations
Contributions
Tatiana Noya Abad, Sebastián E. Sabatini, and Javier A. Calcagno conceived, planned, and carried out the experiments. Tatiana Noya Abad and Sebastián E. Sabatini contributed to sample preparation. Morphometric index analysis was performed by María S. Yusseppone. Yanina S. Minaberry performed metal measurements. Metal concentration assessment in water and digestive glands was performed by Tatiana Noya Abad. Histological preparation and examination were carried out by Henrique Knack de Almeida. All authors discussed the results. Tatiana Noya Abad and Henrique Knack de Almeida took the lead in writing the manuscript, and all authors commented on previous versions. Last version was carefully reviewed by Sebastián E. Sabatini. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this paper.
Ethical approval
Not applicable.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
The authors affirm that human research participants provided informed consent for publication of the images in Fig. 1.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Noya Abad, T., Knack de Almeida, H., Minaberry, Y.S. et al. Effects of metals exposure on morphological and histological structure of the digestive gland in native and invasive clams in the Paraná de las Palmas River (Buenos Aires, Argentina). Aquat Sci 86, 75 (2024). https://doi.org/10.1007/s00027-024-01087-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00027-024-01087-6