Abstract
The resilience of aquatic ecosystems hinges on our ability to protect the native species that reside within them. The river redhorse (Moxostoma carinatum) is one such example and populations have become low enough to warrant a threatened status by the State of Michigan. An insufficient understanding of the species’ habitat use outside of its spawning season hinders the ability of fisheries managers to implement appropriate habitat protection and restoration measures. To enhance our understanding of river redhorse habitat use, we implanted 15 individuals with radio transmitters during the spring spawning run and tracked their locations over the course of a summer. River redhorse movement varied greatly with some individuals remaining within the spawning area throughout the summer and others traveling as far as 50 km down river. Once post-spawn movement ceased, river redhorse established themselves in small home ranges between 0.04 and 0.12 km2. We found no obvious selection for depth, sediment type, macrophyte presence, or water velocity. Instead, river redhorse strongly selected for habitat containing freshwater mollusks, the primary food source for the species. This suggests that they were seeking foraging habitat during this time period. These findings provide insight into river redhorse management, indicating that the recovery of the species may depend on our ability to protect these newly discovered feeding areas. Future river redhorse management efforts should therefore focus on the protection of native mussels and snails and the maintenance of migration routes between spawning and summer habitats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fisheries management in the United States is primarily concerned with game species, which provide significant economic value for the country (Reynolds et al. 2008). As a result, non-game species receive far less attention and are often ignored until they become imperiled enough to warrant state or federal listing (Ricciardi & Rasmussen 1999). Once listed, management agencies must develop plans for the recovery of the species; however, with minimal prior knowledge of their life-history species, recovery plans can be difficult to formulate and may not cover the full breath of the species’ needs.
The river redhorse (Moxostoma carinatum) is one example of a non-game species brought into the focus of management agencies by its imperiled status. It once occupied a large area of eastern North America including parts of the Great Lakes, St. Lawrence River, and Mississippi River watersheds (Lee et al. 1980; Becker 1983; Fig. 1). This range was severely restricted following declines in the middle-to-late 1900s, and the species was thought to be extirpated from Michigan, Iowa, and Indiana (Becker 1983). The river redhorse is currently imperiled or critically imperiled in 13 of the 22 states and provinces in which they are present, including in Michigan where the species is currently listed as threatened (Michigan Natural Features Inventory 2007; NatureServe 2018). Threats facing the river redhorse include the presence of flow control structures, river fragmentation, river channelization, siltation, degraded water quality, and loss of native mollusks, which are its main food source (Becker 1983; Lee et al. 1980; Reid 2003). In addition to these physical threats, the river redhorse has not been well studied, which has prevented the effective management of the species (Parker 1988).
Studies on river redhorse are limited, but work has been done to document the species’ spawning behavior and habitats, and physical morphology. The species is known to spawn over gravel and cobble substrates in fast-flowing water in both the main stem of large rivers and their tributaries (Hackney et al. 1968; Jenkins 1970). In the river redhorse’s northern range, spawning begins in May when water temperatures rise above 15 °C and concludes in June (Campbell 2002; Reid 2003; Reid et al. 2006). Eggs hatch in 3–4 days, but very little is known about the species’ behavior or growth patterns post-spawn. River redhorse mature in approximately 4 years, though this is likely variable and dependent on local conditions (Becker 1983; Beckman & Hutson 2012; Jenkins 1970), and have been found to live up to age 28 (Reid et al., 2006). Individuals can grow to over 75 cm in length and reach weights of over 4.5 kg. Mature river redhorse possess large pharyngeal teeth that enable them to feed on both native and invasive mollusks and have even been proposed as a potential biological control for zebra mussels (Dreissena polymorpha; French 1993). However, only limited research has been done to explore this potential and the river redhorse’s populations would likely need to rebound to effectively perform this ecosystem service.
Successful management of the river redhorse will depend on the ability of scientists and resource managers to fill the gaps in our knowledge of the species. One of the key gaps is the species’ habitat use outside of their spawning season including habitat use by juveniles, observations of which are extremely rare, and by adults, which has been documented but without consensus outside of their spawning season. Yoder and Beaumier (1986) suggested that adult river redhorse avoid slow-flowing waters in favor of riffles and runs. Mongeau et al. (1992) found adult river redhorse in deep channels with slow current near sections of rapids, while Campbell (2002) found them over 10 km away from rapids in a variety of habitats including areas with slow current, soft substrate, and abundant vegetation. The use of slow-flowing water was also suggested by Reid (2003) who found that adult river redhorse were absent from the shallow, fast-flowing water during the fall that they had used during the previous spring as spawning habitat. Reid et al. (2006) further expanded on this noting that river redhorse have been found to use a variety of habitats, including fast and slow current, hard and soft substrate, as well as areas with and without aquatic vegetation. A more recent study documented seasonal habitat use where adults of the species occupied shallow riffles with rapid flow during the spring, boulder filled runs during the summer, runs of moderate velocity and depth during the fall, and deeper runs during the winter (Butler and Wahl 2017). Furthermore, issues with identifying habitat characteristics become compounded when we consider the means by which studies have identified them. Many studies rely on capture location of river redhorse using electrofishing to draw conclusions on the species’ habitat use. While this is easier than a tracking study, it biases our understanding of habitat use toward areas that are easily sampled by electrofishing equipment and limits our ability to characterize the actual habitat used by the species.
The lack of consensus in habitat use outside of the spawning season coupled with the approaches used to determine it may suggest that we have not yet identified the main characteristics that the river redhorse selects for in its environment. To provide further insight into this issue, we sought to identify the key microhabitat characteristic(s), rather than the generalized habitat types, that river redhorse select for during the summer months. The improved detail during an understudied time period and the lack of reliance on electrofishing should provide a better understanding of habitat characteristics used by the river redhorse and lead to more informed management of the species.
Methodology
Study site
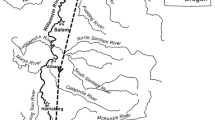
The Grand River watershed covers an area of 14,440 square kilometers in Michigan’s lower peninsula and varies greatly along its course (Hanshue and Harrington 2017). The upper river segments of the Grand River are characterized by steep gradients and relatively stable flows, whereas the lower river segments are predominantly flat with minimal channel slope. Our study was conducted in these lower river segments covering approximately 65 river kilometers bounded by 6th Street Dam, a large run-of-river dam in the City of Grand Rapids, and Lake Michigan (Fig. 2). While most of the river in this area has a nearly flat slope, dropping approximately 0.05 m/km, the stretch within the city of Grand Rapids experiences a much steeper gradient of 1.04 m/km and is also influenced by the 6th Street Dam, which accounts for approximately two and a half meters of drop, and four smaller low head dams. The steep gradient and multiple run-of-river dams in this stretch of the Grand River result in unique habitat characteristics that are rare in the rest of the study area. These characteristics include faster flows, shallower water, and a lower proportion of fine sediment.
Field methods
River redhorse were collected during May of 2018 using a combination of backpack electrofishing and boat electrofishing. Collections targeted the spawning run to increase the likelihood of capture and to allow for an analysis of post-spawn movement and habitat use. Captured individuals were deemed suitable for tagging if their body mass was at least 190 g, at which point telemetry tags would constitute less than 2% of their overall mass and would not adversely affect swimming ability (Brown et al. 1999; Jepsen et al. 2002; Winter 1996). An approximately equal number of males and females were used for this study to identify any differences in habitat use between the two sexes. Seven individuals were determined to be male by the presence of tubercles or tubercle scars on their head and fins, while eight lacked tubercles and were assumed to be female. The sex of the presumed females was confirmed by the presence of eggs during surgery and length at age analyses. Length at age analyses indicated that all tagged individuals were adults and that the possibility of juvenile, non-tuberculate males being misclassified as females was low.
River redhorse individuals were anesthetized in an immersion bath of river water and AQUI-S® at a concentration of 20 mg/L. Once an individual lost equilibrium, it was transferred from the bath to a v-shaped surgical board where a radio tag (ATS model F1580 trailing whip tag) was implanted into their body cavity via the shielded needle technique (Ross and Kleiner 1982). Following surgery, fish were allowed to recover in a flow-through tank and released into the river downstream of their capture points. Collection and surgery took place in accordance with the Grand Valley State University Institutional Animal Care and Use Committee, study number 18-12-a, and with the Michigan Department of Natural Resources Threatened and Endangered Species permit number 2231.
Tagged individuals were located twice a week for the first 2 weeks via homing telemetry with an ATS model R410 radio receiver and 3-element yagi antenna to ensure that they remained healthy and to maintain knowledge of their position. No habitat data were collected during the 2-week acclimation period. Tracking was done via boat except in the city of Grand Rapids where water levels and low head dams prevented boat access. Fish in this section of the river were tracked while wading. No evidence was found that tracking via boat influenced the position of the fish, but some individuals were seen to swim away from their original position when located via wading. When this occurred habitat samples were taken from the initial position of the fish. Tracking took place on a rotating schedule to completely cover the entire study area including the mouth of the Grand River at Lake Michigan. All transmitter frequencies were scanned for while tracking throughout the river. If the position of a tagged individual did not change during subsequent tracking events or only moved downstream, then the individual would be assumed dead; however, this did not occur. Accurate locations were determined by reducing the gain on the receiver until it was positioned over top of the fish’s location.
After the 2-week acclimation period, fish were tracked two-to-three times per week, and data on their location and habitat use were documented. Fish locations were recorded with a Garmin GPSmap 62 and later downloaded into ArcGIS for spatial analysis. All habitat characteristics were documented immediately after the location of a fish was determined.
We collected data on the sediment, mollusks, macrophytes, water depth, and water velocity at each tracked location. Sediment samples were collected with either a Ponar grab sampler or by hand depending on the depth of the water and the tracking technique being used. Sediment samples were quantified using the Wentworth scale (Wentworth 1922). Presence or absence of mollusks, including gastropods and bivalves, and aquatic macrophytes were also documented at this time via the Ponar sampler and visual detection, respectively. Water depth was measured using an extendable survey pole and was recorded to the nearest centimeter. Water velocity was measured using a Marsh-McBirney model 2000 portable flow meter at six-tenths depth and was recorded to the nearest millimeter per second. Water temperatures were taken from the USGS gauging station number 04119400 near Eastmanville, Michigan, which is located in the center of the tracking area (Fig. 2, USGS 2019).
To make comparisons between habitat use and habitat availability, a grid was overlaid within each individual home range. These grids were scaled to the size of each home range to ensure complete coverage of the available habitat and 150 random points were distributed within the grid (Marcum and Loftsgaarden 1980). The habitat characteristics at these random points were quantified using the same methods used at the tracked locations to evaluate habitat selection.
Data analysis
Spatial data were processed using ArcGIS version 10.4.1 (ESRI, Redlands, California). All tracked locations were downloaded as shapefiles and used to create 95% minimum convex polygons as estimates of each river redhorse’s home range. Heat maps of these points were developed using kernel density estimation and overlaid onto the minimum convex polygons to further understand the area used by each individual. Home ranges were only developed for individuals located at least ten times and land area was excluded from the home range prior to any analyses. The number of tracked locations per fish ranged from 0 to 19 (Table 1). Movement over the course of the summer was calculated by first merging the tracked locations with a line drawn through the center of the river. The position of each tracked location along the line was then compared to the release point of each fish. Distance from the release point was graphed with positive numbers indicating positions upstream of the release point and negative numbers indicating positions downstream of the release point.
We compared the microhabitat characteristics used by each individual with the characteristics available to them within their home range. By comparing the habitat within each individual’s home range, we eliminated the possibility of arbitrarily defining what was available to the fish (Aebischer et al. 1993). The proportional habitat use of each fish was used as the sample unit rather than the individual point locations to alleviate issues with serial correlation. These proportions were subject to the unit sum constraint and so were numerically ranked before statistical comparisons took place (Alldredge and Ratti 1992). Categories were required to rank continuous data, so water depth was divided into seven categories each representing a half-meter increment. Water velocity was similarly divided into five categories each representing quarter-meter per second increments. All habitat ranks had a non-normal distribution, and so, a non-parametric Kruskal–Wallis test was used for comparisons. Habitat use by males (n = 7) and females (n = 8) was compared using a Kruskal–Wallis test and no significant differences were found; data were then pooled between the sexes. Kruskal–Wallis tests were used to examine differences between habitat use and habitat availability. If a difference was found between the proportional use of a habitat characteristic and its proportional availability, that habitat type was said to be selected for or against depending on the values at hand. All statistical tests were performed in R version 3.3.2 (R Core Team 2016).
Results
Fifteen river redhorse were collected, tagged, and given identification numbers based on the last three digits of their radio transmitters (Table 1). Individuals ranged from 1.21 to 3.02 kg and from 49 to 63 cm total length which is consistent with sizes of adult river redhorse reported in the literature (Beckman and Hutson 2012; Jenkins 1970; Trautman 1981), and with the sizes of river redhorse caught during surveys in the Grand River earlier in the year (Fig. 3). Based on these sizes, individuals likely ranged from 7.5 to 12 years of age (Beckman and Hutson 2012). Of the 15 fish that were tagged, 14 were located at least once following tagging; fish 291 was never relocated. Twelve fish were located throughout the summer and were used in movement analyses; fish 431 and 473 were not located following the conclusion of spawning. Nine fish (152, 171, 201, 231, 271, 349, 372, 393, and 453) were tracked at least ten times and were used in home range analyses.
Following release, eight individuals moved upstream into the spawning grounds where they had been captured, while four individuals left the spawning area and moved downstream (Fig. 4a, b). Five fish remained within the spawning area over the course of the summer, while the others established home ranges farther down river. Fish that transitioned downstream began to leave the spawning grounds 3–10 days after tagging following a week long warming trend in which water temperatures rose from 16 to 26 °C (USGS 2019). Fish were seen to travel as much as 47 km in an 8-day period following this warming trend, but then remained in a relatively small area over the remainder of the summer. One individual moved 30 km downriver before returning upriver to its original home range, covering a distance of 68 km in late June to early July (Fig. 4b).
a Distance traveled by six river redhorse in the Grand River from release during their spawning season until early September. Positive numbers indicate tracked locations upstream of the release point. Individuals included in this graph traveled less than 10 km from the release site and were found at least once during the summer of 2018. b Distance traveled by six river redhorse in the Grand River from release during their spawning season until early September. Positive numbers indicate tracked locations upstream of the release point. Individuals included in this graph traveled more than 10 km from the release site and were found at least once during the summer of 2018
River redhorse home ranges were small, between 0.04 and 0.12 km2, and spread throughout the study area with the greatest concentration occurring within the city of Grand Rapids (Fig. 5). Available habitat in this area was primarily less than 1 m in depth, composed of gravel/cobble substrates, and contained fast-moving water. However, river redhorse were found to use a variety of habitat characteristics including depths ranging from 0.2 to 3.87 m, velocities ranging from 0.08 to 1.5 m/s, substrates made up of sand, gravel, and cobble, and sections of the river both with and without aquatic vegetation.
Home ranges of nine river redhorse tracked in the Grand River through the summer of 2018. Home ranges varied between 0.04 and 0.12 km2, and were widely distributed. Five individuals established home ranges within the city of Grand Rapids (a), one approximately two kilometers downriver (b), two approximately fifteen kilometers downriver (c), and one nearly 46 km downriver (d)
Kruskal–Wallis tests detected no significant differences between habitat use and habitat availability for water depth, sediment type, macrophyte presence, and water velocity. However, a subsequent power analysis determined that the likelihood of finding a difference between these habitat categories was low due to the small sample size and limited variability between used and available habitat. Kruskal–Wallis tests did return significant results for the presence of mollusks (p < 0.001). Mollusks were found in just 12% of all the available habitat throughout the sampled area, but were present in nearly 80% of tracked locations indicating selection favoring the presence of mollusks.
Discussion
Successful management of the river redhorse will depend on our ability to fill the gaps in our knowledge of the species. One of the key gaps has been the species’ habitat use outside of their spawning season. Studies have reported river redhorse using deep, slow-flowing waters during the summer and fall (Reid 2003; Reid et al. 2006), fast-flowing gravel-filled riffles and runs (Becker 1983; Hackney et al. 1968; Scott and Crossman 1973), and differential habitat use during different seasons (Butler and Wahl 2017). In the Grand River, river redhorse used the same variety of habitat characteristics found in the previous studies including both fast- and slow-flowing waters and hard and soft substrates. However, microhabitat analyses within the river redhorses’ home ranges indicated that the species will use many of the previously documented habitat characteristics (depth, sediment, macrophyte presence, and water velocity) in proportion to their availability.
While the river redhorse tracked in the Grand River did not exhibit any obvious selection for depth, sediment, macrophyte presence, or water velocity, they strongly selected for habitat containing freshwater mollusks, the primary food source for the species, suggesting that they were seeking foraging habitat during this time period. Previous studies have noted this possibility when attempting to explain the summer habitat use of the river redhorse (Butler and Wahl 2017; Campbell 2002; Yoder and Beaumier 1986), but none have identified the presence of suitable food in their study areas. Considering the commonality of post-spawn dispersal toward feeding habitat in other fish species such as shortnose sturgeon (Acipenser brevirostrum) and razorback sucker (Xyrauchen texanus; Schlosser 1991; Hall et al. 1991; Mueller et al. 2000), it seems possible that the presence of suitable food is driving the river redhorses’ summer habitat use, though a more detailed manipulative study is needed to test this.
The foraging habitat hypothesis is supported by the relatively small size of the home ranges calculated in our study and by the habitat use within these home ranges. River redhorse home ranges were small and use within the home range was often unequally distributed and positively associated with the presence of mollusks. As a result, home ranges appeared to follow the distribution patterns commonly seen in mollusk populations (Mulcrone and Rathbun 2018). Mollusk beds are typically small and their positions are difficult to predict. Mollusk presence has been correlated with environmental parameters ranging from watershed-wide characteristics like slope and land use, to microhabitat characteristics like water chemistry, water depth, and water velocity (Arbuckle and Downing 2002; Hardison and Layzer 2001; Hastie et al. 2000). In addition, due to issues arising from siltation, mollusk beds are often thought to be associated with areas where high-velocity water flushes fine sediment (Allen and Vaughn 2010; Williams et al. 1993). The general stochasticity displayed by the mollusk community may explain the variability seen in reports of the river redhorse’s habitat use in the previous studies. By establishing themselves over mollusk beds, river redhorse maintain easy access to their principal food source, but as a result, they are seen to occupy habitat with a wide range of characteristics that may have little-to-no impact on their selection of that habitat.
Mollusks are highly influential on their surrounding ecosystem. They provide bio-deposition of nutrients, physical habitat, increased food availability, and habitat stabilization for a number ofbenthic invertebrates (Aldridge et al. 2007; Strayer 2014; Vaughn et al. 2008). However, without a detailed understanding of habitat requirements, it can prove difficult to locate and manage for native mollusks (Haag and Williams 2014). For this reason, the link described here between the presence of mollusks and the presence of river redhorse may prove beneficial for malacologists. The apparent selection for the presence of mollusks seen in river redhorse could be used to locate patchily distributed mollusk beds that traditional surveys may miss. As an example, fish 201 in our study was commonly found in mollusk containing habitat that was in over 3 m of fast-flowing water. This area proved almost impossible to survey via traditional mollusk sampling techniques during a follow-up examination by Ottawa County Parks, but is now noted as known mollusk habitat thanks to the analyses in this paper.
The selection for mollusk containing habitat highlights the importance of biotic interactions in species distribution, research, and recovery. Biotic interactions, like the co-occurrence seen between river redhorse and mollusks, can indicate that an important relationship exists between species and that the management of one requires consideration of the other (Lamothe et al. 2019). At the very least, co-occurrence suggests that the management of one co-occurring species could benefit the other and that some direct interaction between the two species, important or not, may exist (Halpern et al. 2007). In either event, biotic interactions need to be considered when attempting to manage for a species yet research and restoration efforts are often more focused on abiotic factors (Angermeier and Winston 1999; Wenger et al. 2011). This holds true for the river redhorse where most studies focus on abiotic factors as an explanation for the distribution and occurrence of the species (Becker 1983; Hackney et al., 1968; Reid 2003; Reid et al. 2006; Scott and Crossman 1973). While abiotic factors are important, the exclusion of biotic factors can reduce the effectiveness of management decisions.
The river redhorse’s selection for mollusk containing habitat is a biotic factor that needs to be considered by resource managers. It suggests that protecting and enhancing mollusk communities are particularly important for the management of the river redhorse. This can include maintenance of adequate water quality, prevention of excess sedimentation, and ensuring the health of host fish species that have coevolved to carry mussel glochidia. With this complex web of interactions all potentially influencing river redhorse populations, the best policy is to maintain the natural structure and function of a river-floodplain ecosystem (Stanford and Ward 1993; Ward 1989). Ecosystem-based management is one way to do so as it avoids ecosystem degradation and specifically accounts for the requirements of non-target ecosystem components to promote ecosystem structure and function and to maintain necessary biotic interactions (Lamothe et al. 2019;Pikitch et al. 2004). This has a twofold benefit of both maintaining necessary habitat and providing corridors between habitat types. Our results documented different feeding and spawning areas used by river redhorse in the lower Grand River, and the preservation of the connection between these two habitats will be key to protecting the species.
Our findings have helped fill one of the key gaps in our knowledge of river redhorse. The species’ use of mollusk-rich habitat indicates that while they may occupy many different macrohabitats and be associated with many microhabitat characteristics, these factors likely do not influence patterns of summer habitat use. Furthermore, this suggests that protecting mollusk communities will be critical for the management of the river redhorse going forward and that any degradations to the mollusk community could reduce the potential survival of the river redhorse. Questions still remain regarding habitat use during other seasons and during different stages of the river redhorse’s life history. Future studies should therefore focus on understanding juvenile habitat use and should document the presence of suitable food sources when examining adult habitat use.
Availability of data and materials
The data that support the findings are available in GVSU’s Scholar Works at https://scholarworks.gvsu.edu/theses/942/
References
Aebischer NJ, Robertson PA, Kenward RE (1993) Compositional analysis of habitat use from animal radio-tracking data. Ecology 74(5):1313
Aldridge DC, Fayle TM, Jackson N (2007) Freshwater mussel abundance predicts biodiversity in UK lowland rivers. Aquat Conserv Mar Freshw Ecosyst 17(6):554–564. https://doi.org/10.1002/aqc.815
Alldredge JR, Ratti JT (1992) Further comparison of some statistical techniques for analysis of resource selection. J Wildl Manag 56(1):1–9. https://about.jstor.org/terms
Allen DC, Vaughn CC (2010) Complex hydraulic and substrate variables limit freshwater mussel species richness and abundance. J N Am Benthol Soc 29(2):383–394. https://doi.org/10.1899/09-024.1
Angermeier PL, Winston MR (1999) Characterizing fish community diversity across Virginia landscapes: prerequisite for conservation. Ecol Appl 9(1):335–349
Arbuckle KE, Downing JA (2002) Freshwater mussel abundance and species richness: GIS relationships with watershed land use and geology. Can J Fish Aquat Sci 59(2):310–316. https://doi.org/10.1139/f02-006
Becker GC (1983) River redhorse. Fishes of Wisconsin. University of Wisconsin Press, Madison, pp 674–677
Beckman DW, Hutson CA (2012) Validation of aging techniques and growth of the river redhorse, Moxostoma carinatum, in the James River, Missouri. Southwest Nat 57:240–247. https://doi.org/10.2307/23258010
Brown RS, Cooke SJ, Anderson WG, McKinley RS (1999) Evidence to challenge the “2% Rule” for biotelemetry. N Am J Fish Manag 19(3):867–871. https://doi.org/10.1577/1548-8675(1999)019%3c0867:ETCTRF%3e2.0.CO;2
Butler SE, Wahl DH (2017) Movements and habitat use of river redhorse (Moxostoma carinatum) in the Kankakee River, Illinois. Copeia 105(4):734–742. https://doi.org/10.1643/CE-17-626
Campbell BG (2002) A study of the river redhorse, Moxostoma carinatum (Pisces; Catostomidae), in the tributaries of the Ottawa River, near Canada’s National Capital and in a tributary of Lake Ontario, the Grand River, near Cayuga, Ontario. University of Ottawa Theses. https://doi.org/10.20381/RUOR-11104
Core Team R (2016) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.r-project.org/
French JRP (1993) How well can fishes prey on zebra mussels in eastern North America? Fisheries 18(6):13–19. https://doi.org/10.1577/1548-8446(1993)018%3c0013:HWCFPO%3e2.0.CO;2
Haag WR, Williams JD (2014) Biodiversity on the brink: an assessment of conservation strategies for North American freshwater mussels. Hydrobiologia 735(1):45–60. https://doi.org/10.1007/s10750-013-1524-7
Hackney PA, Tatum WM, Spencer SL (1968) Life history study of the river redhorse, M. carinatum (Cope) in the Cahaba River, AL, with notes on the management of the species as a sport fish. Proc Southeast Assoc Game Fish Commis 21:324–332
Hall JW, Smith TIJ, Lamprecht SD (1991) Movements and habitats of shortnose sturgeon, Acipenser brevirostrum in the Savannah River. Copeia 1991(3):695. https://doi.org/10.2307/1446395
Halpern BS, Silliman BR, Olden JD, Bruno JP, Bertness MD (2007) Incorporating positive interactions in aquatic restoration and conservation. Front Ecol Environ 5(3):153–160
Hanshue SK, Harrington AH (2017) Grand River assessment. www.michigan.gov/dnr/
Hardison BS, Layzer JB (2001) Relations between complex hydraulics and the localized distribution of mussels in three regulated rivers. Regul Rivers Res Manag 17(1):77–84. https://doi.org/10.1002/1099-1646(200101/02)17:1%3c77::AID-RRR604%3e3.0.CO;2-S
Hastie LC, Boon PJ, Young MR (2000) Physical microhabitat requirements of freshwater pearl mussels, Margaritifera margaritifera (L.). Hydrobiologia 429(1/3):59–71. https://doi.org/10.1023/A:1004068412666
Michigan Natural Features Inventory (2007) Moxostoma carinatum River Redhorse. Rare Species Explorer (Web Application). https://mnfi.anr.msu.edu/abstracts/zoology/Moxostoma_carinatum.pdf
IUCN (2019) The IUCN red list of threatened species. http://www.iucnredlist.org. Accessed 2 Apr 2019
Jenkins RE (1970) Systematic studies of the catostomid fish tribe Moxostomatini. Doctoral Dissertation
Jepsen N, Koed A, Thorstad EB, Baras E (2002) Surgical implantation of telemetry transmitters in fish: how much have we learned? In: Aquatic telemetry. Springer Netherlands, Dordrecht, pp 239–248. https://doi.org/10.1007/978-94-017-0771-8_28
Lamothe K, Dextrase A, Drake A (2019) Aggregation of two imperfectly detected imperilled freshwater fishers: understanding community structure and co-occurrence for multispecies conservation. Endanger Species Res 40:123–132. https://doi.org/10.3354/esr00982
Lee D, Gilbert CR, Hocutt CH, Jenkins RE, McAllister DE, Stauffer JR (1980) Atlas of North American freshwater fishes, 12th edn. North Carolina Biological Survey, Raleigh, North Carolina
Marcum CL, Loftsgaarden DO (1980) A nonmapping technique for studying habitat preferences. J Wildl Manag 44(4):963. https://doi.org/10.2307/3808336
Mongeau J-R, Dumont P, Cloutier L (1992) La biologie du suceur cuivré (Moxostoma hubbsi) comparée à celle de quatre autres espèces de Moxostoma (M. anisurum, M. carinatum, M. macrolepidotum, M. valenciennesi). Can J Zool 70(7):1354–1363. https://doi.org/10.1139/z92-191
Mueller G, Marsh PC, Knowles G, Wolters T (2000) Distribution, movements, and habitat use of razorback sucker (Xyrauchen texanus) in a lower Colorado River reservoir, Arizona-Nevada. West N Am Nat 60(2):7. https://scholarsarchive.byu.edu/cgi/viewcontent.cgi?article=1156&context=wnan
Mulcrone RS, Rathbun JE (2018) Pocket field guide to the freshwater mussels of Michigan. Michigan Department of Natural Resources
NatureServe (2018) Comprehensive report species—Moxostoma carinatum. http://explorer.natureserve.org/explorer/. Accessed 19 Nov 2018
O'Keefe D (2002) Range expansion of the river redhorse (Moxostoma carinatum) in michigan. Proj Completion Rep, Nat Heritage Grant Program
Parker BJ (1988) Status of the river redhorse, Moxostoma carinatum, in Canada. Can Field Nat 102:140–146
Pikitch EK, Santora C, Babcock EA, Bakun A, Bonfil R, Conover DO, Sainsbury KJ et al (2004) Ecosystem-based fishery management. Science. https://doi.org/10.1126/science.1098222
Reid SM (2003) River redhorse (Moxostoma carinatum) and channel darter (Percina copelandi) populations along the Trent-Severn waterway. Parks Research Forum of Ontario. Peterborough. http://casiopa.mediamouse.ca/wp-content/uploads/2010/05/PRFO-2005-Proceedings-p221-230-Reid.pdf
Reid SM, Gignac H, Mandrak NE, Vachon N, Dumont P (2006) Assessment and update status report on the river redhorse. Committee on the Status of Endangered Wildlife in Canada, pp 3–30. https://www.registrelep-sararegistry.gc.ca/virtual_sara/files/cosewic/sr_river_redhorse_e.pdf
Reynolds JD, Dulvy NK, Roberts CM (2008) Exploitation and other threats to fish conservation. In: Handbook of fish biology and fisheries, vol 2. Blackwell Science Ltd, Oxford, pp 319–341. https://doi.org/10.1002/9780470693919.ch15
Ricciardi A, Rasmussen JB (1999) Extinction rates of North American freshwater fauna. Conserv Biol 13(5):1220–1222. https://doi.org/10.1046/J.1523-1739.1999.98380.X
Ross MJ, Kleiner CF (1982) Shielded-needle technique for surgically implanting radio-frequency transmitters in fish. Progres Fish Cult 44(1):41–43. https://doi.org/10.1577/1548-8659(1982)44[41:STFSIR]2.0.CO;2
Schlosser IJ (1991) Stream fish ecology: a landscape perspective. BioScience 41(10):704–712. https://www.jstor.org/stable/pdf/1311765.pdf?refreqid=excelsior%3A6b54455418354adb515bb56f601b411f
Scott WB, Crossman EJ (1973) Freshwater fishes of Canada. Fisheries Research Board of Canada, Ottawa
Stanford JA, Ward JV (1993) An ecosystem perspective of alluvial rivers: connectivity and the hyporheic corridor. J N Am Benthol Soc 12(1):48–60. https://doi.org/10.2307/1467685
Strayer DL (2014) Understanding how nutrient cycles and freshwater mussels (Unionoida) affect one another. Hydrobiologia 735(1):277–292. https://doi.org/10.1007/s10750-013-1461-5
Trautman MB (1981) Discovery of the river redhorse, Moxostoma carinatum, in the Grand River, an Ohio tributary to Lake Erie. Ohio J Sci 81:45–46. http://hdl.handle.net/1811/22757
USGS (2019) Temperature gauge 04119400 near Eastmanville, MI, provisional data. https://nwis.waterdata.usgs.gov/nwis/uv?. Accessed 28 Mar 2019
Vaughn CC, Nichols SJ, Spooner DE (2008) Community and foodweb ecology of freshwater mussels. J N Am Benthol Soc 27(2):409–423. https://doi.org/10.1899/07-058.1
Ward JV (1989) The four-dimensional nature of lotic ecosystems. J N Am Benthol Soc 8(1):2–8. https://doi.org/10.2307/1467397
Wenger SJ, Isaak DJ, Luce CH, Neville HM, Fausch KD, Dunham JB, Williams JE et al (2011) Flow regime, temperature, and biotic interactions drive differential declines of trout species under climate change. Proc Natl Acad Sci 108(34):14175–14180
Wentworth CK (1922) A scale of grade and class terms for clastic sediments. J Geol 30(5):377–392. http://www.journals.uchicago.edu/t-and-c
Williams JD, Warren ML, Cummings KS, Harris JL, Neves RJ (1993) Conservation status of freshwater mussels of the United States and Canada. Fisheries 18(9):6–22. https://doi.org/10.1577/1548-8446(1993)018%3c0006:CSOFMO%3e2.0.CO;2
Winter JD (1996) Advances in underwater biotelemetry. Fisheries techniques, 2nd edn. American Fisheries Society, Bethesda
Yoder CO, Beaumier RA (1986) The occurrence and distribution of river redhorse, Moxostoma carinatum and greater redhorse, Moxostoma valenciennesi in the Sandusky River. Ohio J Sci 86(1):18–21. http://hdl.handle.net/1811/23114
Acknowledgements
We would like to thank Sarah Lamar, Barney Boyer, Katy Sheets, Ana Wassilak, Hailee Pavisich, and all our other field work volunteers. Funding for this project was provided by GVSU’s presidential research grant, Michigan Space Grant Consortium’s graduate research grant, and the GVSU Metropolitan council.
Funding
Funding for this project was provided by the Michigan Space Grant Consortium and Grand Valley State University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
Collection and surgery of river redhorse took place in accordance with the Grand Valley State University Institutional Animal Care and Use Committee, study number 18-12-a, and with the Michigan Department of Natural Resources Threatened and Endangered Species permit number 2231.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Preville, N.M., Snyder, E.B., O’Keefe, D. et al. Habitat use of the threatened river redhorse (Moxostoma carinatum) in the Grand River, MI, USA. Aquat Sci 84, 43 (2022). https://doi.org/10.1007/s00027-022-00870-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00027-022-00870-7