Abstract
Hydroperiod is considered an important aspect in shaping community structure and ecosystem patterns in temporary wetlands. However, most studies have focused on the community structure, demonstrating that biotic diversity increases with hydroperiod. Theory suggests that ecosystem patterns like trophic interactions and food web structure will also respond to hydroperiod in the same way. However, there are limited studies exploring ecosystem structure and patterns to changing hydroperiod in temporary wetlands. Maloti-Drakensberg Mountain region of southern Africa has a series of rock pools (shorter hydroperiod) and tarns (longer hydroperiod) that allowed us to explore the effect of hydroperiod on aquatic biodiversity and trophic interactions using stable isotope techniques. We hypothesised that tarns will have higher biotic diversity and complex food web structure as compared to rock pools, which we expected to exhibit low biotic diversity and simple food web structure. Our results were in agreement with our hypothesis, where tarns were characterised by longer food chain length, higher trophic level diversity, greater trophic divergence and even species distribution in the isotopic space. Thus, demonstrating a well-developed and complex food web structure. In contrary, rock pools were characterised by shorter food chain length, small trophic diversity with trophic redundancies and species clustering. Thus, representing a simple and a poorly developed food web structure. Further, macroinvertebrate biotic diversity was significantly higher in longer hydroperiods, also longer hydroperiod exhibited less dramatic changes in the physicochemistry characteristics, representing a more stable environment than shorter hydroperiods. This study demonstrates that hydroperiod not only affects aquatic biological diversity but ecosystem structure, as well.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hydroperiod, described as the exact length of the aquatic phase in ephemeral ecosystems, is a fundamental phenomenon characterised by an inundation–desiccation cycle (Williams 2006; Batzer 2013). It can be influenced by a number of physical properties, including hydrology (e.g. precipitation, evapotranspiration and ground water interaction), substrate geomorphology, geology and geography. These physical properties also affect a number of abiotic process, like water level, water nutrients and water physicochemistry (e.g. dissolved oxygen, pH and turbidity). For example, Magnusson and Williams (2006) demonstrated that longer hydroperiods resulted in relatively stable conditions, with steady dissolved oxygen concentration, pH, turbidity, and water nutrients compared to shorter hydroperiods. Studies, both field-based (e.g. Stenert and Maltchik 2007; Gleason and Rooney 2018) and mesocosms (e.g. Waterkeyn et al. 2010; Kneitel et al. 2017), have shown that hydroperiod is the predominant factor in structuring temporary wetland aquatic communities. Temporary wetlands with longer hydroperiod tend to have higher aquatic macroinvertebrate diversity compared to their counterparts with shorter hydroperiods (Schriever and Williams 2013; Nhiwatiwa et al. 2017; Gleason and Rooney 2018). However, hydroperiod not only plays an integral role in determining the composition, structure and biodiversity of temporary waters, but it also influences food webs and trophic interactions. The latter relationship is poorly understood in temporary waters (O’Neill and Thorp 2014).
Food webs, which include the number of trophic levels, energy resources abundance and utilisation among co-existing species, and energy flow from primary producers to top consumers, offer insights into the ecosystem patterns and their resultant community structure (Polis et al. 1997; Vander Zanden and Rasmussen 1999; Post 2002). As such, most attributes of food webs, including connectance, trophic complexity, trophic position, trophic niche of consumer groups, vary in space and time. For example, changes in food web architecture have been used to track the intensity of droughts (Ledger et al. 2013; DeColibus et al. 2017), impacts of invasive alien species (Vander Zanden et al. 1999; Jackson et al. 2012; Jackson and Britton 2014; Hill et al. 2015), anthropogenic disturbances (Kovalenko 2019; Jackson et al. 2020), aquatic ecosystem structure and functioning (Taylor et al. 2017; Peel et al. 2019) and restoration efforts (Vander Zanden et al. 2003; Yu et al. 2016).
In temporary waters, the dry phase facilitates the decomposition of the organic material accumulating on the dry surface and enhance nutrient release during the aquatic phase (Shumilova et al. 2019). As such, brown food webs (e.g. detritus-based), as opposed to green (e.g. autochthonous primary production) (Williams 2006; O’Neill and Thorp 2014), generally play a bigger role in temporary wetland ecosystems (Williams and Trexler 2006; Dalu et al. 2017a; DeColibus et al. 2017). Recent studies have shown that food webs increase in complexity with hydroperiod (Schriever and Williams 2013; O’Neill and Thorp 2014; Dalu et al. 2017a, b; Schalk et al. 2017). Generally, food web structure increases immensely after a wetland fills up before reaching equilibrium and then decreasing as the wetland dries out. This is partly due to the fact that species diversity is generally directly proportional to hydroperiod, and higher species richness (or more functional diversity) have been shown to result in complex food webs (Urban 2004). However, O’Neill and Thorp (2014) demonstrated that this relationship is context dependent.
Mountains are generally recognised as major repositories of regional biodiversity, water and ecosystem services (Körner and Spehn 2002; Taylor et al. 2016; Hoorn et al. 2018). As a source of the major rivers in southern Africa, the Maloti-Drakensberg Mountain catchment is a designated strategic water source area (Nel et al. 2013). This mountain range also constitutes a well-known centre of endemism for biodiversity (Clark et al. 2011, 2012; Carbutt 2019), harbouring many unique species and ecosystems (Travers et al. 2014; Taylor et al. 2020). Mountain lentic water bodies (e.g. lakes and pans) act as early warning systems for detecting ecological effects of global warming (Eggermont et al. 2010) and human pollution (Dunnink et al. 2016). Hamer and Martens (1998) conducted an extensive survey of the two main lentic water bodies of this mountain range, namely rock pools and afromontane tarns. They reported high levels of large brachiopod diversity and endemism. Similar results of high endemism are reported in other afromontane lentic waters (Van Damme and Eggermont 2011; Van Damme et al. 2013) and elsewhere (Coronel et al. 2007). However, Hamer and Martens (1998) hypothesised that the potential low levels of food resources in these water bodies could be shaping their community compositions, a proposal that has not been tested up to now. Using a sub-set of the water bodies studied in Hamer and Martens (1998)’s seminal study, this study intends to further investigate the relation between trophic interactions, food webs, aquatic macroinvertebrate biodiversity and hydroperiod regimes.
Afromontane tarns or pans are mountain top depressions that store water after rain events and a common feature of the alpine zone of the Maloti-Drakensberg Mountain range (Dunnink et al. 2016). They tend to have relatively longer hydroperiod (e.g. months of inundation), supporting a range of vegetation types (Sieben et al. 2010; Chatanga et al. 2019). In this study, we sampled afromontane tarns from the Golden Gate Highland National Park (hereafter referred to Golden Gate Highlands). Rock pools, on the other hand, formed through the weathering and erosion of the bedrock (Jocque et al. 2010), have a higher evaporation rate, usually devoid of vegetation, with virtually no groundwater exchange, and a shorter hydroperiod (e.g. days to few weeks of inundation) (Brendock et al. 2010). There were two different mountain areas sampled, namely QwaQwa and Fika-Patso Mountains representing two forms of rock pools with different hydroperiods (Hamer and Martens 1998). The rock pools in these areas differed in size, depth and character. QwaQwa Mountain had substantially deeper and bigger rock pools compared to Fika-Patso Mountain. As such, we consider Fika-Patso to have the shortest hydroperiod, with Qwaqwa having an intermediate hydroperiod. With rock pools and afromontane tarns representing different spectrums of hydroperiod, this study will explore the association of hydroperiod with aquatic macroinvertebrates diversity, trophic interactions and food web structure. We hypothesised that the Golden Gate Highlands tarns with longest hydroperiod will exhibit high biotic diversity and complex food web structure attributed by longer food chain, trophic diversity, divergence and even species spacing. While QwaQwa Mountain rock pools with intermediate hydroperiod and Fika-Patso Mountain rock pools with shortest hydroperiod to show intermediate and simplest food web complexities in comparison to the Golden Gate Highlands tarns.
Material and methods
Study area
This study was conducted within the Maloti-Drakensberg Mountain which represent a mountain range between 1800 to 3800 m.a.s.l, and a distance of approximately 400 km covering an area of 40,000 km2 (Dunnink et al. 2016; Carbutt 2019). The vegetation diversity and distribution of this region has been well studied and documented (Clark et al. 2011, 2012; Carbutt 2019; Chatanga et al. 2019). The rainfall season is from spring (September) to autumn (April), with annual average rainfall of between 1500 and 2000 mm and the ambient air temperature can range from 32 °C in summer to − 20 °C in winter (Hamer and Martens 1998; SANParks 2013).
The region is dominated by both rock pools and afromontane tarns (Hamer and Martens 1998). These vary in size, depth, hydroperiod and type of substratum. Rock pools form in depressions of rocks and boulders, they are small, usually less than 2 m in diameter and have depths less than 30 cm (Hamer and Martens 1998). Meanwhile, afromontane tarns are bigger, up to 30 m in diameter, and ≥ 1 m in water depth (Dunnink et al. 2016). They display heterogeneity in biotopes, some have sandy or muddy substratum, vegetation inside and on the margins of the tarns. More importantly, afromontane tarns hold water for longer periods as compared to rock pools, thus characterised with longer hydroperiods as compared to rock pools (Hamer and Martens 1998; Dunnink et al. 2016; Chatanga et al. 2019).
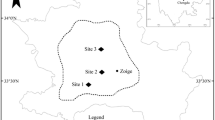
Eleven freshwater temporary wetlands from three study areas in the eastern Free State province, of the Maloti-Drakensberg region, South Africa were identified and sampled on the 20th–25th of January 2018, during the aquatic phase season. Sites included four rock pools from the Fika-Patso Mountain (− 28.679586 S, 28.840390 E) (Fig. 1a), three rock pools from the Qwaqwa Mountain (− 28.504795 S, 28.791542 E) (Fig. 1b) and three afromontane tarns at Golden Gate Highlands (− 28.487184 S, 28.637250 E) (Fig. 1c).
Hamer and Martens (1998) recognised three types of rock pools within the region which includes; (1) isolated, small depressions in boulders, (2) series of small rock pools, and (3) series of large and small rock pools. Despite some minor differences, e.g. water depth and size (see Fig. 1a, b), rock pools in Fika-Patso Mountain conform to the description by Hamer and Martens (1998), as isolated, small depressions, usually attaining less than 2 m in diameter, and less than 10 cm deep (Fig. 1a), while rock pools in QwaQwa Mountain were a series of large and small rock pools, that were 12 m in diameter, and ~ 10 cm deep (Fig. 1b). As such, QwaQwa Mountain rock pools represented an intermediate hydroperiod between the longer hydroperiod afromontane tarns of Golden Gate Highlands, and the shorter hydroperiod rock pools of Fika-Patso Mountain (Fig. 1).
Data collection
Physicochemistry
Physicochemical parameters such as pH, conductivity (EC: µS), salinity (ppm), water temperature (°C) and dissolved oxygen concentration (DO: mg/l) were measured using a portable water-chemistry multi-parameter (PCSTestr 35) and a DO Pen (Sper scientific, 850,045) meters. Water depth (cm) and total wetland area (m2) were measured using a custom-made water depth measuring stick and a Geographical Positioning System. Additionally, 500 ml (n = 3) water samples were collected and divided into two sub-samples (250 ml, n = 6) and were used to determine; (1) water nutrients concentration (n = 3), and (2) chlorophyll-a (Chl-a) biomass (n = 3). Water nutrients including nitrate (NO3: mg/l), ammonium (NH4: mg/l) and phosphate (PO4: mg/l) concentrations were measured using the Ion Specific Electrodes (range 1.0–100 mg/l) (Vernier LabQuest®2) and the HI 83,203 Multiparameter Bench Photometer for Aquaculture (range 0.0–30.0 mg/l) meters.
Chlorophyll-a biomass
Chlorophyll-a (Chl-a) sub-samples (250 ml, n = 3) were transferred into an opaque polyethylene water sample container and immediately stored on ice until they reached the laboratory. Prior Chl-a biomass analysis, samples were homogenised moderately by hand for five seconds and thereafter filtered through a Millipore nylon net filters (50 mm diameter, 20 µm mesh size) using a vacuum pump (Instruvac® Rocker 300) at 20 kiloPalscas (kPa). Any small unwanted invertebrates and plant litter were removed.
Acetone extraction method was used to determine Chl-a biomass fluorometrically following methods described by Holm-Hansen and Riemann (1978) and applied by Motitsoe et al. (2020). Briefly, each filtered nylon net was folded in half, placed in a reaction tube with screw and 10 ml of 90% acetone solution added. Reaction tube were then left for Chl-a extraction, in complete darkness at − 20 °C (freezer) for a minimum of 48 h. Thereafter, Chl-a wavelength reading was determined using 10AU Field and Laboratory fluorometer (Tuner Designs), noting the wavelength reading before and after the sample was acidified by adding 2/3 drops of 0.1 M hydrochloric acid. The final Chl-a biomass was estimated using the formula adopted from Lorenzen (1967) and Daemen (1986):
Biological data
Afromontane tarns were sampled by sweeping a hand held aquatic net (30 × 30 cm square frame, 1 mm mesh size) nine times on the open water and emergent aquatic vegetation biotopes or 18 times collectively per tarn as described by Mlambo et al. (2011). All biotopes were transverse in order to obtain a representative composite sample for each tarn. Rock pools on the other side, were sampled using small hand-held net with a round shape (15 cm diameter, 1 mm mesh size), and each pool was swept nine times (Hamer and Martens 1998). The sampling method was repeated twice in each system resulting into two samples of aquatic macroinvertebrates, for diversity and community analysis, and the for food web analysis. The aquatic macroinvertebrates community analysis sample was used to estimate biodiversity indices and assemblage composition between sites. Aquatic macroinvertebrates samples were identified to the lowest possible taxonomic level using a combination of identification guides for the Southern African region (Day et al. 2001a, b; Day and de Moor 2002a, b; de Moor et al. 2003a, b).
Stable isotope analysis
Primary producers, including dominant aquatic plant species leaves (n = 3, per species) and detritus (n = 3) were collected by hand, together with surface water samples (500 ml, n = 3). Particulate organic matter (POM) was extracted by filtering the surface water samples through the pre-combusted Whatman Glass microfiber filters papers (GFFs; 0.7-micron pore, 47 mm diameter), using a vacuum pump (Instruvac® Rocker 300) at 20 kiloPalscas (kPa). Consumers, included all aquatic macroinvertebrates stable isotope sub-samples which were identified to species level as described above. For each aquatic macroinvertebrate species present, a minimum of three–five individual aquatic macroinvertebrate species were used for stable isotope sample. All collected samples; dominant aquatic plant species (n = 3, per species), detritus (n = 3), POM (n = 3) and aquatic macroinvertebrates (n = 3, per species) were stored in aluminium foil envelopes and oven dried for 48 h at 50 °C.
The oven dried aquatic plants, detritus and aquatic macroinvertebrates tissues samples (n = 3) were further grounded into fine homogenous powder using pestle and mortar expect the POM Whatman Glass microfiber filters. Thereafter, about 0.5–0.6 mg of aquatic macroinvertebrates homogenised tissues and 1.0–1.2 mg of aquatic plants tissues and detritus were weight into separate tin capsules (8 × 5 mm). Thereafter, all samples were sent for δ13C and δ15N isotope analysis using a Flash EA 1112 Series coupled to a Delta V Plus stable light isotope ratio mass spectrometer via a ConFlo IV system (all equipment supplied by Thermo Fischer, Bremen, Germany), at the Stable Isotope Facility, University of Pretoria, South Africa. The δ13C and δ15N isotopic values were reported as ‰ vs air, normalised to internal standards (Merck and DL-Valine) calibrated to the International Atomic Energy reference materials (IAEA-CH-3 and IAEA-CH-6 for δ13C, IAEA-N1 and IAEA-N2 for δ15N). Results were expressed in standard delta notation using per mil scale, δX (‰) = \(\left( {\frac{Rsample}{{Rstandard}} - 1} \right) \times 1000\), where X = 15N or 13C, and R is the ratio of the heavy over the light isotope (15N/14N or 13C/12C). Average analytical precision for δ13C, and δ15N was + 0.08 and + 0.07 respectively.
Data analysis
Physicochemistry
Physicochemical parameters were tested for normality using the Shapiro-Wilks normality test and Levene’s test for homogeneity of variances. Data was found to be normally distributed (Shapiro–Wilks test, P > 0.05) and the variances homogenous (Levene’s test, P < 0.05), then a parametric test, in this case one-way analysis of variance (ANOVA) with post-hoc Tukey HSD test was used to test for significant difference in physicochemical parameters between rock pools and tarns.
Aquatic macroinvertebrate biodiversity patterns and assemblage composition
To investigate aquatic macroinvertebrate biodiversity between rock pools and tarns, relative species abundance (N), species richness (S), Shannon–Weaver diversity index; H′ = \(- \sum_{i = 1}^{s} pi lnpi,\) (where pi is the proportional abundance of taxa i in the sample given s taxa) and Pielou’s evenness; J′ = \( \frac{H^{\prime}}{{{\text{ln}}\left( S \right)}}\) (where H′ is Shannon diversity and S species richness) indices were computed in PRIMER version 6.1.13 & PERMANOVA+ version 1.0.3 (Clarke and Gorley 2006). Similarly, ANOVA was further used to test for significant difference in biodiversity indices between study sites.
Then to demonstrate aquatic macroinvertebrate community assemblages between rock pools and tarns, unconstrained ordinations were completed using principal coordinate ordination (PCO) on Bray–Curtis similarity matrix to visualise aquatic macroinvertebrates assemblage composition. This was followed by a constrained canonical analysis of principal coordinate (CAP) to emphasise assemblage differences between sites (e.g. Fika-Patso, QwaQwa and Golden Gate Highland) and habitat type (e.g. rock pools and tarns). Aquatic macroinvertebrate abundances were fourth-root transformed to meet normality and to recognise the abundance of rare taxa. Canonical analysis (δ21 and δ22) with permutation test (No. of permutation 999) were used to test the significance difference in explained aquatic macroinvertebrate assemblage variation between sites, while Pearson’s correlation (r > 0.7) was used to identify taxa driving the differences in aquatic macroinvertebrate assemblage structure between sites (Anderson and Wills 2003). Analysis was conducted in PRIMER version 6.1.13 & PERMANOVA+ version 1.0.3 (Clarke and Gorley 2006).
Trophic interactions and food web structure
Primary consumers are generally used to baseline ecosystems, making them comparable across spatial and temporal gradient (Cabana and Rasmussen 1996; Vander Zanden et al. 1997). These organisms are long-lived organisms that generally feed on plankton or detritus, thus provide a time integrated measure of the ecosystems’ primary productivity (Cabana and Rasmussen 1996; Post 2002). This study used δ15N and δ13C isotopes values of freshwater snails including Physidae, Lymnaeidae and Planorbidae (Gastropoda) collected from all sites with an average δ15Nbaseline = 4.63‰ ± 2.11; δ13Cbaseline = − 20.34‰ ± 3.73; δ13Cmax = − 17.18; δ13Cmin = − 27.44 and a carbon range CRbaseline = 10.26‰.
δ15N and δ13C isotopes values of all organisms collected from each site were then adjusted using pre-treated freshwater snails baseline values based on the following equations:
where 2 represents the trophic position of the primary consumer of the baseline organisms, δ15N represents the fractionation factor calculated as 3.23‰, \(\delta^{15} N_{organism}\) is the isotope ratio of the organism, \(\delta^{15} N_{baseline}\) is the isotope ratio (4.63‰) of the primary consumers used for the baseline (Post 2002).
where \(\delta^{13} C_{corrected} \) is the corrected carbon isotope ratio of the consumer, \(\delta^{13} C_{organism}\) is the uncorrected isotope ratio of the organism, \(\delta^{13} C_{baseline}\) is the mean primary consumer isotope ratio (− 20.34‰), and \(CR_{baseline}\) is the primary consumer carbon range (δ13Cmax–δ13Cmin = 10.26‰) (Olsson et al. 2009; Jackson and Britton 2014).
Then to investigate difference in trophic interactions and food web structure measured by food chain length, primary resources abundances, trophic diversity and divergence between rock pools and tarns, corrected δ13C (corrected Carbon) and δ15N (trophic position) values were investigated using the Stable Isotope Bayesian Ellipses in R using the “SIBER” package (Jackson et al. 2011). Layman’s metrics (Layman et al. 2007) were used to describe trophic food web characteristics which included nitrogen range (NRb), which describes trophic community length (or food web length); carbon range (CRb), which represents basal resources diversity; mean distance to the centroid (CDb), which indicates trophic diversity and species spacing; total area/convex hull area (TAb) indicating food web niche width; mean nearest neighbour distance (MNNDb), which estimates density and clustering of species within the community and trophic redundancy; the standard deviation of nearest neighbour distance (SDNNDb), measuring evenness of spatial density and packing of species in the isotopic space, and standard ellipse area (SEAc), which provides a bivariate measure of mean core isotopic niche (Layman et al. 2007; Jackson et al. 2011). The calculation of SEAc further allows for a measure of niche overlap (%; with a maximum of 100% indicating complete overlap between trophic food web niche), which can then be used as a quantitative measure of trophic food web niche similarity between sites following Jackson et al. (2012) and Jackson and Britton (2014). Stable isotope ratios from all individual samples were used to estimate differences in trophic interactions and food web structure between sites. However due to differences in samples sizes between sites and for comparison purposes, all metrics were bootstrapped (N = 10,000, indicated with a subscript ‘b’) as seen in Jackson et al. (2012), Jackson and Britton (2014), Hill et al. (2015) and Taylor et al. (2017). The SIBER modelling (Stable Isotope Bayesian Ellipses in R; Jackson et al. 2011) and all statistical tests expect when stated, were conducted in R version 3.6.1 (R Core Team 2018).
Biodiversity and trophic community metrics response to physicochemical variables
Multiple linear regression analysis using the lm function in ‘MASS’ package was used to examine which predictor variables e.g. physicochemical variables, influenced explanatory variables e.g. aquatic macroinvertebrate biodiversity indices and selected trophic community metrics. The initial model included the following variables: pH, EC, salinity, water temperature, [DO], [NO3], [NH4], [PO4], water depth, total wetland area and Chl-a biomass, and prior to analysis, all physicochemical variables were log(x + 1) transformed. The StepAIC function from the package ‘MASS’ performed forward–backward selection of the predictor variables, and the best model, that is, the one with the lowest Akaike’s information criterion (AIC) score was selected (Venables and Ripley 2002).
Results
Physicochemistry
The following measures were significantly different between the three areas (Golden Gate Highlands, Qwaqwa Mountain and Fika-Patso Mountain) representing variable hydroperiods: pH (ANOVA, F1, 15 = 2.18, P = 0.025), EC (ANOVA, F1, 15 = 4.96, P = 0.04), DO (ANOVA, F1, 15 = 5.84, P = 0.029), water temperature (ANOVA, F1, 15 = 14.29, P = 0.002), total wetland area (ANOVA, F1, 15 = 5.88, P = 0.029) and water depth (ANOVA, F1, 15 = 7.12, P = 0.018) (Table 1). Total wetland area was highest in Golden Gate Highlands, representing the longest hydroperiod, followed by Qwaqwa Mountain rock pools, representing intermediate hydroperiod and then Fika-Patso Mountain rock pools having the smallest, thus representing the shortest hydroperiod (Table 1). Similar pattern was also seen with water depth, thus demonstrating that both the physical measures of hydroperiod (e.g. total wetland area and water depth) aptly characterised these three studied areas (Table 1). Contrary, water chemistry parameters, e.g. DO and water temperature, exhibited the opposite trend, were Golden Gate Highlands tarns with longest hydroperiod recorded lower values and lesser variation (e.g. standard deviation) compared to QwaQwa and Fika-Patso Mountain rock pools with intermediate and short hydroperiod respectively (Table 1). QwaQwa Mountain rock pools had the intermediate physicochemistry parameters readings, while Fika-Patso Mountain rock pools had the highest value and higher variation (Table 1).
Biodiversity and assemblage patterns
Relative species abundance (ANOVA: F1, 8 = 7.1, P = 0.029) and species richness (F1, 8 = 3.55, P = 0.044) were the two biological diversity indices that where significantly different between rock pools and tarns (Fig. 2). Again, sites with longer hydroperiod, Golden Gate Highlands tarns had the significantly higher relative species abundance and richness, followed by sites with intermediate hydroperiod, QwaQwa Mountain rock pools, and Fika-Patso Mountain rock pools with the shorted hydroperiod having the least relative species abundance and richness (Fig. 2). However, the significant difference between QwaQwa and Fika-Patso Mountain rock pools were seen only with the relative species abundance while species richness was indifferent. Shannon–Weaver diversity index (F1, 8 = 0.20, P = 0.67) and Pielou’s evenness (F1, 8 = 0.88, P = 0.38) were not significantly different between sites (Fig. 2).
Aquatic macroinvertebrate biodiversity indices (mean ± standard error) between the Fika-Patso Mountain rock pools (FF), Qwaqwa Mountain rock pools (QQ) and Golden Gate Highlands tarns (GG) in the Eastern Free State, South Africa. Bar represent mean biodiversity values and error bars represent standard error values. Different lowercase letters represent significant differences (ANOVA, P < 0.05)
CAP ordination revealed three very distinct aquatic macroinvertebrates assemblage clusters, were each cluster represented study area (Fig. 3). Canonical correlation (δ2) explained 99% (CAP 1) and 94% (CAP 2) total aquatic macroinvertebrate assemblage variation between Golden Gate Highlands tarns, QwaQwa and Fika-Patso Mountain rock pools, thus indicating complete and significant difference (PERMUTATION TEST: trace statistics = 1.94, P = 0.004) in aquatic macroinvertebrate community assemblage between study areas (Fig. 3). Two aquatic macroinvertebrate species including Trithemis sp. and Agraptocorixa sp. were positively associated with QwaQwa Mountain rock pools, and seven species Taeniochauliodes sp., Mesovelia sp., Berosus sp., Bulinus sp., Culex sp., Marsupiobdella africana Goddard and Malan, 1913 and Orthocladinae were positively associated with the Golden Gate Highlands tarns (Fig. 3).
Canonical analysis of principal coordinate (CAP) ordination indicating distinct differences in aquatic macroinvertebrate assemblage structure found between Fika-Patso Mountain rock pools (FF), QwaQwa Mountain rocks pools (QQ) and Golden Gate Highlands tarns (GG) in the Eastern Free State, South Africa. CAP1 eigenvector σ2 = 0.99, CAP2 eigenvector σ2 = 0.94, where σ2 = the amount of variation explained by the canonical axis
Trophic interactions and food web structure
Layman’s community-wide metrics illustrated a more complex, longer and diverse trophic food web structure for Golden Gate Highlands tarns, followed by QwaQwa Mountain rock pools, and Fika-Patso Mountain rock pools with simple, smaller and least diverse trophic food web structure. In addition, the Layman’s community-wide metric results supported isotope core niche width results (Table 2, Fig. 4). Golden Gate Highlands tarns with longer hydroperiod recorded high nitrogen range (dNRb), which was indicative of a large trophic community length (or food chain length). QwaQwa Mountain rock pools with intermediate hydroperiod had the second highest dNRb, whereas Fika-Patso Mountain rock pools recorded the smallest dNRb (Table 2, Fig. 4). QwaQwa Mountain rock pools and Golden Gate Highlands tarns showed similar carbon range (dCRb) values, which were substantially higher than those recorded at Fika-Patso Mountain rock pools. This suggests QwaQwa Mountain rock pools and Golden Gate Highlands tarn that at intermediate and long hydroperiod respectively, had diverse primary resources as compared to Fika-Patso Mountain rock pools (shorter hydroperiod) which showed the least diverse food sources (Table 2, Fig. 4). As a result, QwaQwa Mountain rock pools had the largest food web total area/convex hull area (TAb) and this was attributed by the high diversity of food sources (dCRb) recorded on sites, while Fika-Patso Mountain rock pools illustrated the smallest food web total area, thus limited food sources (Table 2, Fig. 4). Further, all the other three metrics; CDb, MNNDb and SDNNDb were high at Golden Gate Highlands tarns and moderate at QwaQwa Mountain rock pools, demonstrating high to moderate trophic level diversity, trophic levels divergence and even distribution of species. Whereas the Fika-Patso Mountain rock pools showed the least trophic level diversity, high trophic level redundancy and trophic species clustering (Table 2).
Golden Gate Highlands tarns and Fika-Patso rock pools showed completely different trophic community niches in the isotopic space (Fig. 4). While QwaQwa Mountain rock pools had the largest core niche width, supported by diverse basal resources (dCRb) and total food web area (TAb), it was followed by the Golden Gate Highlands tarns and Fika-Patso Mountain rock pools had the least trophic community core niche width (Fig. 4). Standard ellipses area (SEAc) results showed similar trends as in core niche width, where QwaQwa Mountain rock pools showed the largest SEAc followed by Golden Gate Highlands tarns and the smallest niche area been Fika-Patso Mountain rock pools (Figs. 4, 5).
Density plots showing the confidence interval of the standard ellipses areas (SEAc) for Fika-Patso Mountain rock pools (FF), QwaQwa Mountain rock pools (QQ) and Golden Gate Highlands tarns (GG) in the Eastern Free State, South Africa. The black points correspond to the mean standard ellipse area for each community. The grey to light grey-boxed areas reflect the 95, 75 and 50% confidence interval for the overall aquatic community niche area respectively
The Fika-Patso Mountain rock pools SEAc overlapped by 91.4% with the QwaQwa Mountain rock pools, thus showing high similarity in trophic community structure and ecologies, whereas QwaQwa Mountain rock pools SEAc overlapped by 8.6% with Golden Gate Highlands tarns, indicating different trophic community structure and ecologies (Fig. 4, Table 3). Golden Gate Highlands tarns showed no overlap to Fika-Patso Mountain rock pools, that is the two systems had completely different trophic community structure, thus function differently (Fig. 4, Table 3).
Biodiversity and trophic structure response to physicochemical variables
Wetland total area, [PO4], [NO3], EC, water temperature, [DO] and Chl-a biomass as predictor variables explained 88.3% variation in aquatic macroinvertebrates relative species abundance (Table 4). Wetland total area and EC were significant and positively affected relative species abundance, whereas both [NO3] and Chl-a biomass negatively affected relative species abundance. [PO4], [NO3], EC, [DO] and Chl-a biomass, explained 79.4% variation in species richness. [NO3] and [DO] showed a negative relationship to species richness, but [PO4] was positive (Table 4). The Shannon–Weaver diversity index, on the other hand, was affected by pH, EC, water temperature, [DO] and salinity, which explained 57.9% aquatic macroinvertebrates diversity, and only [DO] negatively affected diversity and EC, water temperature, salinity showed positive association to Shannon–Weaver diversity index (Table 4). Wetland total area, pH, EC, salinity, water temperature and [DO] collectively explained 72.6% in aquatic macroinvertebrates Pielou’s evenness, where EC, salinity and water temperature showed positive association to evenness and only [DO] was negative (Table 4).
Comparatively, food chain length (dNRb) was influenced by [NH4], [NO3], EC, salinity, water temperature, [DO], water depth and Chl-a biomass, explaining 79.1% variation in food chain length. [NH4], EC, [DO] positively contributed to food chain length whereas salinity, and Chl-a biomass showed a negative effect (Table 4). Wetland total area, [NH4], EC, [DO], water depth and Chl-a biomass collectively explained 53.1% of ecosystem basal resources (dCRb), where EC showed a negative association, and both [DO] and Chl-a biomass showed a positive relationship to basal resources diversity. Trophic diversity (CDb) was influenced by wetland total area, [NH4], [PO4], EC, [DO] and Chl-a biomass which collectively explained 63.1% variation, but only [DO] and Chl-a biomass positively contribute to trophic diversity (Table 4). Total wetland area, [NO3], EC, water temperature and Chl-a biomass collectively explained 56.4% of food web convex hull area (TAb), where only EC showed negative effect and [NO3], water temperature and Chl-a biomass had a positive effect to food web convex hull area (Table 4).
Discussion
In the current study, we report about the variation in the hydroperiod of temporary wetlands, rock pools and afromontane tarns, from the Maloti-Drakensberg Mountain range in relation to three variables: physicochemistry, biotic diversity and food web dynamics. Research on hydroperiod in pond/wetland ecosystems has largely involved sampling of the first week(s) and the last week of inundation (e.g. Schalk et al. 2017) or a continuous sampling of the aquatic phase (e.g. Dalu et al. 2017a, b). However, given the remote nature of our study area and natural variation of wetland area and water depth, known proxies of hydroperiod (Stenert and Maltchik 2007; Gleason and Rooney 2018), we categorised these temporary wetlands into three levels of hydroperiods (e.g. long, intermediate and short), and sampled each category once. We hypothesised that temporary wetlands with long hydroperiods, represented by three afromontane tarns from Golden Gate Highlands, will have more stable physicochemistry (Magnusson and Williams 2006), higher aquatic macroinvertebrate diversity (Schriever and Williams 2013; Gleason and Rooney 2018), and food web structures that are longer, diverse and more complex (Dalu et al. 2017a; Schalk et al. 2017). Whereas, slightly bigger and deeper rock pools at QwaQwa Mountain, were predicted to have intermediate levels, and then shallow and smaller rock pools as seen in Fika-Patso Mountain will have the least stable physicochemistry, the lowest aquatic macroinvertebrate diversity and a simple food web structure. Results from this study largely supported all these hypotheses.
Our study confirms that there was a relatively low productivity in these temporary water bodies compared to similar systems in lower altitudes (e.g. Roussouw et al. 2018; de Necker et al. 2020). Results showed low levels of Chl-a concentration, and this was a general trend within the region, where all sampled areas of different hydroperiods showed similar Chl-a levels, which were oligotrophic in nature (Dunnink et al. 2016). Hamer and Martens (1998) also predicted that these low levels could be responsible for shaping aquatic communities compositions in the region. However, we found out that Chl-a and [DO] concentrations negatively affected biodiversity indices, but positively contributing to community structures. While, [PO4], EC and salinity were more important for biodiversity indices. Although, we did not continuously measure physicochemistry, but the relatively small variation in some water chemistry variables (e.g. pH and water temperature) in the longer hydroperiod sites, even though this could also be due to small sample size and other reasons, indicates that longer hydroperiod had more stable physicochemical characteristics, as reported by Magnusson and Williams (2006).
Food web studies have largely been conducted in permanent water bodies, with relatively little work done in temporary water bodies (but see Schriever and Williams 2013; O’Neill and Thorp 2014; Dalu et al. 2017a; de Necker et al. 2020). This is unfortunate, given that the processes of basal resource accumulation, community development and food web complexity are fundamentally different to those of permanent water bodies. There are, at least, two major hypotheses, applicable to temporary waters that predict a linear relationship between food chain length and ecosystem properties, reviewed by Post et al. (2002). The first one is ecosystem size hypothesis, which predicts that food chain length should be longer in larger ecosystems, as they often have higher species diversity, more heterogeneous habitat and more available resources. This was in agreement with our findings, were longer hydroperiod study sites e.g. Golden Gate Highlands tarns, where attributed by large total wetland area and high water depth showed more biotic diversity, longer trophic community length (food chain length), diverse trophic levels and food sources (second to QwaQwa Mountains rock pools). This was also true following O’Neill and Thorp (2014) study, were authors demonstrated that insect diversity and not that of large branchiopods, lengthen the food chain in their studied temporary water bodies (e.g. playas from Colorado, USA). Large brachiopod diversity does contribute to biological diversity but not functional diversity, whereas insect’s diversity affects both biological and functional diversity in systems. Thus, biological and functional diversity are important aspects of ecosystems structure and their loss have knock-on effects on the ecosystems function. Similarly, Dalu et al. (2017b) study also showed that low functional diversity (functional redundancy) in pond ecosystems result to low trophic levels divergence (or low MNNDb), which indicate that more species occupied similar trophic level positions. When we look at the present study, QwaQwa Mountain rock pools were diverse in primary resources and thus species had different and diverse sources of energy to exploit as compared to the Golden Gate Highlands tarns (moderate CRb) and Fika-Patso Mountain rock pools (low CRb). In terms of species orientation in the food web structure, the Golden Gate Highlands tarns (long hydroperiod) constituted more divergent trophic levels (high MNNDb) that were evenly distributed (low SDNNDb) throughout the food web structure, thus indicative of more specialised aquatic macroinvertebrate community.
Our results also supported findings from Schriever and Williams (2013) that systems with less-stable environmental variables as seen in Fika-Patso Mountain rock pools will have shorter food chain, as predicted by the dynamic constraints hypothesis. The dynamic constraints hypothesis predicts that food chain length should be shorter in highly variable habitats. In our study, we demonstrated that the disturbance represented by the variable hydroperiod, affected this important measure of food web structure. Specifically, the results of our study showed that temporary wetlands with longer hydroperiods had the longest food chain length (dNRb), whereas those with the shortest hydroperiod had the least. This further adds to the fact that temporary wetlands with longer hydroperiods have stable biotic and abiotic characteristics, allowing the system to reach maturity during the aquatic phase, with high biological and functional diversity before the systems begins dries out.
Tampering with the hydroperiod has been employed by environmental managers all over the world for various goals, including mosquitoes and invasive species control (Hanford et al. 2020) and for increasing biodiversity (Martens and de Moor 1995), however this have triggered unintended consequences. For example, deepening the temporary pools to increase inundation phase in order to hopefully attract more bird species for tourism purposes have led to extirpation of a unique monotypic genera of fairy shrimps (Anostraca), Rhinobranchipus martensi Brendonck 1995 (Martens and de Moor 1995). This study demonstrates that hydroperiod does not only impact biological diversity and community structure, but it also affects ecosystems processes like food web dynamics, that also need to be considered when thinking about tampering with hydroperiod. As such, we recommend that future management and conservation studies should incorporate trophic interactions (ecosystems structure and functioning) as an early warning indication of wetland deterioration.
References
Anderson MJ, Willis TJ (2003) Canonical analysis of principal coordinates: an ecologically meaningful approach for constrained ordination. Ecology 84:511–525
Batzer DP (2013) The seemingly intractable ecological responses of invertebrates in North American wetlands: a review. Wetlands 33:1–15
Brendock L, Jocque M, Hulsman A, Vanschoewinkel B (2010) Pools on the rocks: freshwater rock pools as model systems in ecological and evolutionary research. Limnetica 29:25–40
Cabana G, Rasmussen JB (1996) Comparison of aquatic food chains using nitrogen isotopes. Proc Natl Acad Sci USA 93:10844–10847
Carbutt C (2019) The Drakensberg Mountain Centre: a necessary revision of southern Africa’s high-elevation centre of plant endemism. S Afr J Bot 124:508–529
Chatanga P, Kotze DC, Janks M, Sieben EJJ (2019) Classification, description and environmental factors of montane wetland vegetation of the Maloti-Drakensberg region and the surrounding areas. S Afr J Bot 125:221–233
Clark VR, Barker NP, Mucina L (2011) The great escarpment of Southern Africa: a new frontier for biodiversity exploration. Biodivers Conserv 20:2543–2561
Clark VR, Barker NR, Mucina L (2012) Taking the scenic route the southern Great Escarpment (South Africa) as part of the Cape to Cairo floristic highway. Plant Ecol Divers 4:313–328
Clarke KR, Gorley RN (2006) Plymouth Routines in Multivariate Ecological Research. PRIMER v6: use manual/Tutorial. PRIMER-E: Plymouth
Coronel JS, Declerck S, Brendonck L (2007) High-altitude peatland temporary pools in Bolivia house a high cladoceran diversity. Wetlands 27:1166–1174
Daemen EAMJ (1986) Comparison of methods for the determination of chlorophyll in estuarine sediments. Neth J Sea Res 20:21–28
Dalu T, Wasserman RJ, Froneman PW, Weyl OLF (2017a) Trophic isotopic carbon variation increases with pond’s hydroperiod: evidence from an Austral ephemeral ecosystem. Sci Rep 7:7572
Dalu T, Wasserman RJ, Vink TJF, Weyl OLF (2017b) Sex and species-specific isotopic niche specialisation increases with trophic complexity: evidence from an ephemeral pond ecosystem. Sci Rep 7:43229
Day JA, de Moor IJ (2002a) Guides to the Freshwater Invertebrates of Southern Africa. Volume 5: Non-Arthropods. Water Research Commission, Pretoria
Day JA, de Moor IJ (2002b) Guides to the Freshwater Invertebrates of Southern Africa. Volume 6: Arachnida and Mollusca. Water Research Commission, Pretoria
Day JA, de Moor IJ, Stewart BA, Louw A (2001a) Guides to the Freshwater Invertebrates of Southern Africa. Volume 3: Crustacea II. Water Research Commission, Pretoria
Day JA, de Stewart B, Moor IJ, Louw A (2001b) Guides to the freshwater invertebrates of southern Africa. Volume 4: Crustacea III. Water Research Commission, Pretoria
de Moor IJ, Day JA, de Moor FC (2003a) Guides to the freshwater invertebrates of Southern Africa. Volume 7: Insecta I. Water Research Commission Report no. TT 207/03. Pretoria, South Africa
de Moor IJ, Day JA, de Moor FC (2003b) Guides to the Freshwater Invertebrates of Southern Africa. Volume 8: Insecta II. Water Research Commission Report no. TT 214/03. Pretoria, South Africa
de Necker L, Manfrin A, Ikenaka Y, Ishizuka M, Brendonck L, van Vuren JHJ, Sures B, Wepener V, Smit NJ (2020) Using stable δ13C and δ15N isotopes to assess foodweb structures in an African subtropical temporary pool. Afr Zool 55:79–92
DeColibus DT, Rober AR, Sampson AM, Shurzinske AC, Walls JT, Turetsky MR, Wyatt KH (2017) Legacy effects of drought alters the aquatic food web of a northern boreal peatland. Freshw Biol 62:1377–1388
Dunnink JA, Curtis CJ, Beukes JP, van Zyl PG, Swartz J (2016) The sensitivity of Afromontane tarns in the Maloti-Drakensberg region of South Africa and Lesotho to acidic deposition. Afr J Aquat Sci 41:413–426
Eggermont H, Verschuren D, Audenaert L, Lens L, Russell J, Klaassen G, Heiri O (2010) Limnological and ecological sensitivity of Rwenzori mountain lakes to climate warming. Hydrobiologia 648:123–142
Gleason JE, Rooney RC (2018) Pond permanence is a key determinant of aquatic macroinvertebrate community structure in wetlands. Freshw Biol 63:264–277
Hamer ML, Martens K (1998) The large Branchiopoda (Crustacea) from temporary habitats of the Drakensberg region, South Africa. Hydrobiologia 384:151–165
Hanford JK, Webb CE, Hochuli DF (2020) Management of urban wetlands for conservation can reduce aquatic biodiversity and increase mosquito risk. J Appl Ecol 57:794–805
Hill JM, Jones RW, Hill MP, Weyl OLF (2015) Comparisons of isotopic niche widths of some invasive and indigenous fauna in a South African river. Freshw Biol 60:893–902
Holm-Hansen O, Riemann B (1978) Chlorophyll-a determination: improvements in methodology. Oikos 30:438–447
Hoorn C, Perrigo A, Antonelli A (2018) Mountains, climate and biodiversity. Wiley, Oxford
Jackson MC, Britton JR (2014) Divergence in the trophic niche of sympatric freshwater invaders. Biol Invasions 16:1095–1103
Jackson AL, Inger R, Parnell AC, Bearhop S (2011) Comparing isotopic niche widths among and within communities: SIBER—stable isotope Bayesian ellipses in R. J Anim Ecol 80:595–602
Jackson MC, Donohue I, Jackson AL, Britton JR, Harper DM, Grey J (2012) Population-level metrics of trophic structure based on stable isotopes and their application to invasion ecology. PLoS One 7:1–12. https://doi.org/10.1371/journal.pone.0031757
Jackson MC, Fourie HE, Dalu T, Woodford DJ, Wasserman RJ, Zengeya TA, Ellender BR, Kimberg PK, Jordaan MS, Chimimba CT, Weyl OLF (2020) Food web properties vary with climate and land use in South African streams. Funct Ecol. https://doi.org/10.1111/1365-2435.13601
Jocque M, Vanschoewinkel B, Brendock L (2010) Freshwater rock pools: a review of habitat characteristics, faunal diversity and conservation value. Freshw Biol 55:1587–1602
Kneitel JM, Samiylenko N, Rosas-Saenz L, Nerida A (2017) California vernal pool endemic responses to hydroperiod, plant thatch, and nutrients. Hydrobiologia 801:129–140
Körner C, Spehn EM (eds) (2002) Mountain biodiversity: a global assessment. Parthenon Publication Group, Boca Raton
Kovalenko KE (2019) Interactions among anthropogenic effects on aquatic food webs. Hydrobiologia 841:1–11
Layman CA, Arrington DA, Montana CG, Post DM (2007) Can stable isotope ratios provide for community-wide measures of trophic structure? Ecology 88:42–48
Ledger ME, Brown LE, Edwards FK, Milner AM, Woodward G (2013) Drought alters the structure and functioning of complex food webs. Nat Clim Change 3:223–227
Lorenzen CJ (1967) Determination of chlorophyll and phaeophytin: spectrophotometric equations. Limnol Oceanogr 12:343–346
Magnusson AK, Williams DD (2006) The roles of natural temporal and spatial variation versus biotic influences in shaping the physicochemical environment of intermittent ponds: a case study. Archiv für Hydrobiologie 165:537–556
Martens K, De Moor F (1995) The fate of the Rhino Ridge pool at Thomas Baines Nature Reserve: a cautionary tale for conservationists. S Afr J Sci 91:385–387
Mlambo MC, Bird MS, Reed C, Day JA (2011) Diversity patterns of temporary wetland macroinvertebrate assemblages in the south-western Cape, South Africa. Afr J Aquat Sci 36:299–308
Motitsoe SN, Coetzee JA, Hill JM, Hill MP (2020) Biological control of Salvinia molesta (D.S. Mitchell) drives aquatic ecosystem recovery. Diversity 12:204
Nel J, Colvin C, Le Maitre D, Smith J, Haines I (2013) Defining South Africa’s Water Source Areas. WWF-SA, Cape Town
Nhiwatiwa T, Brendonck L, Dalu T (2017) Understanding factors structuring zooplankton and macroinvertebrate assemblages in ephemeral pans. Limnologica 64:11–19
O’Neill BJ, Thorp JH (2014) Untangling food-web structure in an ephemeral ecosystem. Freshw Biol 59:1462–1473
Olsson K, Stenroth P, Nyström P, Granéli W (2009) Invasions and niche width: does niche width of an introduced crayfish differ from a native crayfish? Freshw Biol 54:1731–1740
Peel RA, Hill JM, Taylor GC, Weyl OLF (2019) Food web structure and trophic dynamics of a fish community in an ephemeral floodplain lake. Front Environ Sci 7:192
Polis GA, Anderson WB, Holt RD (1997) Toward an integration of landscape and food web ecology: the dynamics of spatially subsidized food webs. Annu Rev Ecol Syst 28:289–316
Post DM (2002) The long and short of food-chain length. Trends Ecol Evol 17:269–277
Post DM, Pace ML, Hairston NG Jr (2002) Ecosystem size determines food-chain length in lakes. Nature 405:1047–1049
R Core Team (2018) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Roussouw N, Bird MS, Perissinotto R (2018) Microalgal biomass and composition of surface waterbodies in a semi-arid region earmarked for shale gas exploration (Eastern Cape Karoo, South Africa). Limnologica 72:44–56
SANParks (2013) Golden Gate Highlands National Park: Park Management Plan 2013–2023
Schalk CM, Montana CG, Winemiller KO, Fitzgerald LA (2017) Trophic plasticity, environmental gradients and food-web structure of tropical pond communities. Freshw Biol 62:519–529
Schriever TA, Williams DD (2013) Influence of pond hydroperiod, size, and community richness on food-chain length. Freshw Sci 32:964–975
Shumilova O, Zak D, Datry T, von Schiller D, Corti R, Foulquier A et al (2019) Simulating rewetting events in intermittent rivers and ephemeral streams: a global analysis of leached nutrients and organic matter. Glob Change Biol 25:1591–1611
Sieben EJJ, Kotze DC, Morris CD (2010) Floristic composition of wetlands of the South African section of the Maloti-Drakensberg Transfrontier Park. Bothalia 40:117–134
Stenert C, Maltchik L (2007) Influence of area, altitude and hydroperiod on macroinvertebrate communities in southern Brazil wetlands. Mar Freshw Res 58:993–1001
Taylor SJ, Ferguson JWH, Engelbrecht FA, Clark VR, Van Rensburg S, Barker N (2016) The Drakensberg Escarpment as the Great Supplier of Water to South Africa. In: Greenwood GB, Shroder JF (eds) Mountain ice and water: investigations of the hydrologic cycle in alpine environments. Elsevier, Oxford, pp 1–46
Taylor GC, Weyl OLF, Hill JM, Peel RA, Hay CJ (2017) Comparing the fish assemblages and food-web structures of large floodplain rivers. Freshw Biol 62:1891–1907
Taylor PJ, Kearney T, Dalton DL, Chakona G, Kelly CMR, Barker NP (2020) Biomes, geology and past climate drive speciation of laminate-toothed rats on South African mountains (Murinae: Otomys). Zool J Linnean Soc. 10.1093
Travers SL, Jackman TR, Bauer AM (2014) A molecular phylogeny of Afromontane dwarf geckos (Lygodactylus) reveals a single radiation and increased species diversity in a South African montane center of endemism. Mol Phylogenet Evol 80:31–42
Urban MC (2004) Disturbance heterogeneity determines freshwater metacommunity structure. Ecology 85:2971–2978
Van Damme K, Eggermont H (2011) The Afromontane Cladocera (Crustacea: Branchiopoda) of the Rwenzori (Uganda–D.R. Congo): taxonomy, ecology and biogeography. Hydrobiologia 676:57–100
Van Damme K, Bekker E, Kotov AA (2013) Endemism in the Cladocera (Crustacea: Branchiopoda) of Southern Africa. J Limnol 72:440–463
Vander Zanden MJ, Rasmussen JB (1999) Primary consumer δ13C and δ15N and the trophic position of aquatic consumers. Ecology 80(4):1395–1404
Vander Zanden MJ, Cabana G, Rasmussen JB (1997) Comparing trophic position of freshwater fish calculated using stable nitrogen isotope ratios (d15N) and literature dietary data. Can J Fish Aquat Sci 1158:1142–1158
Vander Zanden MJ, Casselman JM, Rasmussen JB (1999) Stable isotope evidence from the food web consequences of species invasion in lakes. Nature 401(6752):464–467
Vander Zanden MJ, Chandra S, Allen BC, Reuter JE, Goldman CR (2003) Historical food web structure and restoration of native aquatic communities in the Lake Tahoe (California-Nevada) Basin. Ecosystems 6:274–288
Venables WN, Ripley BD (2002) Random and mixed effects. In: Venables W, Ripley BD (eds) Modern applied statistics with S. Statistics and Computing, New York, pp 271–300
Waterkeyn A, Vanschoenwinkel B, Grillas P, Brendonck L (2010) Effect of salinity on seasonal community patterns of Mediterranean temporary wetland crustaceans: a mesocosm study. Limnol Oceanogr 55:1712–1722
Williams DD (2006) The biology of temporary waters. Oxford Biology, Oxford, p 268
Williams AJ, Trexler JC (2006) A preliminary analysis of the correlation of food-web characteristics with hydrology and nutrient gradients in the southern Everglades. Hydrobiologia 569:493–504
Yu J, Liu Z, He H, Zhen W, Guan B, Chen F, Li K, Zhong P, Mello FT, Jeppesen E (2016) Submerged macrophytes facilitates dominance of omnivorous fish in a subtropical shallow lake: implications from lake restoration. Hydrobiologia 775:97–107
Acknowledgements
This research was funded through the Rhodes University Research Council Fund. Further funding for this work was provided by the National Research Foundation of South Africa and the Department of Higher Education and Training: The New Generation of Academics Programme. Any opinion, finding, conclusion or recommendation expressed in this material is that of the authors and the NRF does not accept any liability in this regard. We are grateful to the South African National Parks (SANParks): Golden Gate Highlands National Park Eastern Free State especially Hendrik Sithole and Dr Charlene Bissett for facilitating our research permit (No: MLAMC1466) and staff at the Golden Gate Highlands National Park in particular Mr Dhiraj Nariandas and section rangers Mr Mokoena and Tshabalala for providing access and logistical support to undertake this research within the park. Daniel Rogers is also thanked for his assistance in the field.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mdidimba, N.D., Mlambo, M.C. & Motitsoe, S.N. Trophic interactions and food web structure of aquatic macroinvertebrate communities in afromontane wetlands: the influence of hydroperiod. Aquat Sci 83, 36 (2021). https://doi.org/10.1007/s00027-021-00792-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00027-021-00792-w