Abstract
Carbon and nitrogen stable isotope analyses were used to determine isotopic niche width of the invasive fish species Carassius gibelio to help assess the niche overlap and potential impact of this species on the native fish fauna in the Karamenderes River, northwest Turkey. C. gibelio had the highest niche area of the coexisting species. The greatest overlap of isotopic niche was between C. gibelio and Mugil cephalus in the river mouth. The freshwater species displayed similar patterns when taking into consideration their relative abundance and isotopic overlap. While C. gibelio is likely to outcompete some species at some localities, the species was found co-occurring with others by maximum tolerable overlap degree and apparently utilised vacant niche space at some stations. Overall our results indicate that C. gibelio has extensive niche overlap with the native fish species making it a strong competitor, and because of its high abundance and high niche width this invasive species represents a serious threat to the native fish fauna, particularly in the river mouth.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Niche width of a species is affected by several abiotic and biotic factors such as resource density and diversity, population density, competitors and predators (Fox 1981; Bearhop et al. 2004; Olsson et al. 2009). The relation between niche widths and abundance of species was formalized by early ecologists as a spatial model of the niche concept (Hutchinson 1957; Levins 1968; MacArthur 1968). Although a negative correlation between species abundance and niche width of a species in a community has been discussed by some authors (Seagle and Mccracken 1986), the general consensus is for a positive correlation between the abundance and the niche width of species which is explained by an increase in the variety of resources consumed and increased tolerance to environmental conditions (Rocha et al. 2018). Successful invaders tend to have wider niches with high abundance and higher plasticity in resource use than non-invasive species (Correia 2002), thus increasing their competitive capabilities (Blossey and Nötzold 1995; Tilman 1999). There are several hypotheses about the wide niche area of a species. For example; expansion of niche width has been explained as a result of decreased interspecific competition in order to maintain energy requirements (Robinson and Wilson 1994; Svanbäck and Persson 2004). As a superior competitor, an invasive species can compete for particular resources used by native species and cause competitive exclusion of that native species (Britton et al. 2018). On the other hand, co-existence of an invasive species with other species might be explained by availability of sufficient resources for all species and resource partitioning. In that respect, the degree of virtual and actual niche width of species (Colwell and Futuyma 1971), The maximum tolerable overlap among species in a community (Pianka 1974), is a useful tool to understand competition and the potential impact of an invasive species. Successful invaders may also occupy previously vacant niche space (Karlson et al. 2015). Therefore, the abundance of invasive fish species and degree of niche overlap with the native populations is a good indicator of possible impacts on a fish community. The impact of an invasive species with the abundance and overlap degree were documented recently for some fish species (Sakai et al. 2001; Ayala et al. 2007; Carey and Wahl 2010). However, revealing the quantitative ecological impacts of invasive species is challenging due to the complexity of ecological interactions (Miranda and Perissinotto 2012), and there are still gaps in the empirical examples of the impact of an invasive species in terms of community dynamics.
The Gibel carp, Carasius gibelio (Bloch 1782), with its rapid dispersion and high establishment success in both lentic and lotic inland waters is considered a nuisance species in Turkey and Europe (Özcan 2007; Özuluğ et al. 2004). The community-based impact of this species has only been reported to a limited extent (Crivelli 1995; Gaygusuz et al. 2007; Specziár and Rezsu 2009; Tarkan et al. 2012; Yalçın Özdilek and Jones 2014). Therefore studies of the spatio-temporal variation of niche width together with the niche overlap with native species along a river will improve understanding of community dynamics and potential impact for this invasive species.
Stable isotope analysis (SIA) offers an effective tool for understanding trophic niche widths of fish (Layman et al. 2007b; Schmidt et al. 2007; Syväranta and Jones 2008; Fink et al. 2012; Syväranta et al. 2013), the dietary overlap of species in a fish guild (DeNiro and Epstein 1978; Bootsma et al. 1996) and also the impact of invasive species (Vander Zanden et al. 1999; Simon et al. 2004; Yuille et al. 2015; Britton et al. 2018). We therefore used trophic (isotopic) niche width to understand the impact of invasive C. gibelio and the extent of dietary overlap with native species in the Karamenderes River in northwest Turkey. To explain the impact of this invasive species we tested the hypotheses that invasive C. gibelio have wider isotopic niche than co-existing species. C. gibelio may outcompete some co-existing species or may be found together by maximum tolerable overlap degree of co-existing species. In addition, invasive C. gibelio may occupy vacant niche space by having minor dietary overlap with co-existing species.
Materials and methods
Study area and sampling
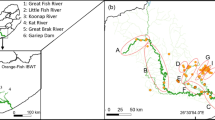
The Karamenderes River, which rises in the Ağı and Kaz Mountains and flows into the Çanakkale strait near the ancient city of Troy, is located in northwest Turkey (Fig. 1). The river is about 110 km long with discharge from 60 to 70 m3 to 1530 m3 per second throughout the year and is one of the biggest rivers in the Biga Peninsula (Sarı et al. 1999; Baba et al. 2007). The river flow is regulated by two reservoirs at Bayramiç and Pınarbaşı. The first record of invasive C. gibelio from this river was in a 2007 survey at Pınarbaşı station (Yalçın Özdilek 2008) after field studies performed in the Biga Peninsula in 2000 and 2001 (Sarı et al. 2006). The Karamenderes river has regional endemic species such as Salmo cf. coruhensis, Squalius cii (Richardson, 1857), Alburnus cf attalus, Barbus oligolepis Battalgil, 1941, Cobitis fahirae Erk’akan, Atalay-Ekmekçi-Nalbant, 1998. Cyprinus carpio has been introduced to reservoirs by aquaculture activities. Another introduced species Gambusia holbrooki Girard, 1859 had not been recorded from this river before this field study. Gobio kovatschevi Chichkoff, 1937 is also a regional endemic and is listed by the IUCN (International Union of Conservation of Nature) as vulnerable (Freyhof and Kottelat 2008). Anguilla anguilla (L., 1758) has a wide distribution, but the population of this species is decreasing and it is listed by the IUCN as critically endangered (Jacoby and Gollock 2014).

(Changed from Partal and Yalçın Özdilek 2017)
The study area showing the Karamenderes River and the sampling stations
Materials for the study were collected at five locations, Ahmetçeli (Ahm), Sarmısaklı (Srm), Kalafat (Klf), Kumkale Köprü (Kkop), Kumkale açık (Kka), from upstream to downstream along the river in Summer 2012, Fall 2012, and Spring 2013 (Fig. 1). Along the river each station has different characteristics such as depth (30 cm to 5 m) and width (5–30 m). Therefore, the fish sampling was performed using backpack electrofishing (SAMUS 725G) accompanied by cast net (10–16 mm), gill net (18–45 mm), and fyke net to cover all habitat types. The water temperature, (T, °C), dissolved oxygen (DO, mg L−1) and electrical conductivity (C, µS cm−1) were measured by WTW® 340i multimeter in the field. The relative abundance (%N) of each species was calculated as numerical percentage of all specimens collected (Table 1). The sum of relative abundances of all species was assumed to be one hundred for each sampling station. Fork length of each C. gibelio individual was recorded and dorsal muscle tissue samples of all fish specimens were taken for stable isotope analysis (SIA).
Stable isotope analyses
For isotopic analysis, muscle samples of all fish specimens were dried at 60 °C for 24 h and homogenized with a microdismembrator-U (2 min at 1500 rpm) into a fine powder. Stable isotope analyses were conducted using a FlashEA 1112 elemental analyser (Thermo Fisher Scientific Corporation, Waltham, MA, U.S.A.) coupled to a Thermo Finnigan DELTAplus at the University of Jyväskylä, Finland. Prior to analysis, 0.500–0.600 mg of homogenized powder from each sample was weighed into tin capsules. Standard delta notations (δ13C and δ15N) were used for stable carbon and nitrogen isotope ratios relative to the international standards for carbon (Vienna PeeDee Belemnite) and nitrogen (atmospheric nitrogen). Pike (Esox lucius L.) white muscle tissue with known isotopic composition was used as an internal working standard inserted in each run after every five samples. Standard deviation of the internal standards was less than 0.16‰ for δ13C and 0.12‰ for δ15N in each run. Lipid correction of muscle δ13C values was not performed because the C:N ratios (average = 3.4, range = 3.2–3.8) indicated very low lipid content (Kiljunen et al. 2006; Post et al. 2007).
Data analyses and statistics
The isotopic niche widths of the fish species were calculated from the δ13C or δ15N data as total area (TA), which means the total amount of isotopic niche area occupied, and as the standard ellipse area corrected for sample size (SEAc), which is less sensitive to outliers, using the SIAR package (Stable Isotope Analysis in R; Layman et al. 2007a; Parnell et al. 2010). The overlap between ellipses was calculated using the Bayesian method (SEA.B) and polygons were drawn using the code that underlines the overlap function in the SIBER package (Jackson et al. 2011). Bayesian-based determination of standard elipse area of each species (SEA.B) was used to compare the isotopic niche metrics of species statistically in the same package in R. The mean SEAc values of all specimens in the stations with and without C. gibelio were compared using Student t-Test. In the assessment of the degree of niche overlap between species (overlap degree), the fish species were grouped as < 1 and > 1 units of overlap degree. The highest overlap degree of C. gibelio with the other species were taken as the maximum tolerable overlap degree. The α value was calculated as α = 0.05/49 = 0.001 for multiple comparisons of SEA.B values of species pairs according to Bonferroni correction (Bland and Altman 1995). Statistics were performed using R, version 2.1.3 (Jackson et al. 2011) and Microsoft Excel version 2010.
Results
A total of 106 individuals of the invasive C. gibelio together with individuals of other species were caught with various nets along the Karamenderes River. C. gibelio were caught from all the stations below the Bayramiç reservoir dam along the river. The mean length of C. gibelio with the standard deviation and some habitat characteristics for each station are given in Table 1. While small specimens were caught at the upper stations, larger specimens were caught from the river mouth in all three seasons. In the river mouth with the low temperature and high salinity, smaller specimens were found in fall than in the other seasons (Table 1). The relative abundances of C. gibelio and of the other species are given Table 2 as N%. C. gibelio had highest relative abundance in the river mouth stations in Summer 2012 (Table 2).
The other fish species were categorised into three groups: (1) native freshwater fish species, which were (A) anguilla, S. cii, (B) oligolepis, (A) cf. attalus, G. kovatschevi, C. fahirae, Rhodeus amarus (Bloch, 1782); (2) introduced species, which were C.carpio and G. holbrooki; and (3) marine-freshwater transitional fish species, which were L. aurata (Risso, 1810), Liza ramada (Risso, 1827), Chelon saliens (Risso, 1810), C. labrossus (Risso, 1827), Mugil cephalus L., 1758, and Platichthys flesus (L., 1758) (Table 2). While C. gibelio were collected from all stations below the Bayramiç reservoir in summer, they were not sampled at the Srm station in fall and were collected only from the two lower stations, Klf and KKop in spring. C. gibelio shared the last two stations KKop and KKa with transitional fish species such as Mugilidae family members and P. flesus. Specimens of S. cii, (B) oligolepis, R. amarus together with (C) gibelio were caught only from Klf and KKop stations in all three seasons (Table 2). Sparus aurata with low abundance were recorded only from KKa station in Summer 2012 (7.3%) and Spring 2013 (1.4%). Similarly, A. anguilla were recorded from Srm (1.5% in Summer 2012), KKop (10% Spring 2013), KKa (3.3% in Summer 2012 and 12.2% in Spring 2013) stations.
Carassius gibelio exhibited wide variation in δ13C and δ15N values which encompassed the range of values of nearly all the native species (Fig. 2). Total isotopic niche area (TA) of C. gibelio varied from 2.2 to 25.7‰ and exceeded that of all the other species. The highest recorded value of TA was for C. gibelio in spring at the KKop station, when there were no mugilids at that station. In general, the TA of C. gibelio increased downstream, the downstream KKa site having the highest TA (Table 3).
The indigenous freshwater fish species shared similar isotopic niche area with the three most freshwater tolerant transition fish species (Fig. 3). Interestingly, C. gibelio appears to occupy a similar isotopic niche area as nearly all the other freshwater and transitional fish species. However, C. gibelio occupied a wider isotopic overall niche area than the others, exhibited higher plasticity in isotopic niche width, and occupied a particular isotopic area which was about − 26‰ to − 27‰ and about 14‰ to 16‰ for δ13C and δ15N values, respectively.
Trophic niche widths and overlaps of native fish species and invasive C. gibelio along the Karamenderes River (black, C. gibelio; red, S. cii; green, B. oligolepis; cyan, A. cf. attalus; darkviolet, C. carpio; blue, G. kovatschevi ; grey, R. amarus; midnightblue, G. holbrooki; orange, L. ramada; slateblue, M. cephalus; pink, P. flesus)
Carassius gibelio had higher values of standard ellipse area than the native fish species except for Mugilidae family members. We compared two ellipses for significant differences in SEA.B to test whether C. gibelio isotopic niche area differed from that of other fish groups (Table 3).The SEA.B values for C. gibelio showed a much larger area than the other species particularly at the river mouth stations and particularly for native freshwater fish species, but also for other introduced species (Table 3). However, the SEA.B value of C. gibelio was not significantly larger than that of the mugilids (p > 0.05).
The corrected mean standard ellipse areas (SEAc) of all specimens for the stations with and without C. gibelio were 25.1 ± 14.6 and 10.8 ± 5.6 respectively, and there was a significant difference between these mean values (t = 2.74; p < 0.05). However, at the level of individual species, although SEAc values of S. cii (1.7 times) and B. oligolepis (1.5 times) were higher in the absence of C. gibelio, there were no significant differences between SEAc values of these native fish species, which are the dominant freshwater fish in the Karamenderes River, in the presence and absence of C. gibelio (p > 0.05).
The niche overlap degrees between species and their significance varied according to season and station (Table 3; Fig. 3). The species which had < 1 overlap degrees any time with C. gibelio were P. flesus, G. holbrooki, and C. fahirae. B. oligolepis, G. kovatschevi and L. ramada followed having < 1 overlap degrees with C. gibelio on more than 75% of co-occurrences of these species. About 50–60% of co-occurrence of the species of S. cii, A. cf. attalus, R. amarus, C. carpio, L. aurata and M. cephalus were < 1 overlap degrees with C. gibelio. No overlap was observed with C. labrossus. Moreover, there were no, or only very low, overlap degrees between C. gibelio and other species at KKop and Ahm stations in Summer 2012 and Fall 2012 seasons, respectively (Table 3).
The greatest extent of isotopic niche overlap was between C. gibelio and M. cephalus at the Kka station in summer and the Kkop station in fall. Despite the low abundance of M. cephalus, SEA.B of C. gibelio was not larger than M. cephalus at these sites (p > 0.05; Fig. 4). In addition, there were high overlaps with L. ramada at the Kac station in fall and with B. oligolepis at the Sar station in summer (Fig. 4). At these stations the SEA.B value of C. gibelio was also statistically lower than those of these other species (Table 3). However, these species were more abundant than C. gibelio at those stations.
The abundance and isotopic overlap relationships of fish species at different C. gibelio abundances. a These species had significantly larger SEA.B values than that of C. gibelio. b These species had significantly smaller SEA.B values than that of C. gibelio. The circle indicates freshwater fish species which have low abundance and low overlap with C. gibelo
The relation between abundance and isotopic overlap of particularly freshwater fish species indicated that the species represented similar patterns rather than station (Fig. 4b). For instance, S. cii and R. amarus were more abundant and had high overlap degree at various stations even under the condition of higher isotopic niche area of C. gibelio. These species are freshwater species which had mostly smaller isotopic niche areas than that of C. gibelio and the extent of overlap at different stations related to their abundance relative to C. gibelio.
Discussion
The isotopic niche concept, which is widely used by ecologists, is a useful tool for indicating the potential impact of invasive species. In a river ecosystem, the dynamics of the fish community is flexible and environmental dependent. Even though the possible consequences of invasive fish introductions to natural ecosystems is well known, the possible effects of invasive species on dynamic stream ecosystems are poorly understood. This study presents some explanatory arguments on the possible impacts of invasive species on native fish species in a dynamic lotic system. The most important findings of this study are that C. gibelio has a large niche width and high niche overlap with native fish species. The hypotheses that successful invaders have a large niche width (Elton 1958; Shea and Chesson 2002) is supported by our results. In addition, this invasive species occupies vacant niche space particularly in unfavourable environmental conditions for freshwater fish.
The isotopic niche width and the isotopic niche overlap of coexisting species in the Karamenderes river showed spatial and temporal variation. It is known that interspecific competition is a major factor determining the trophic niche width of coexisting species (MacArthur 1972; Pianka 1974; Cody 1974). According to optimal foraging theory, niche width will increase as the availability of foraging resources decreases (Mac Arthur and Pianka 1966, 2011). Therefore, high niche width with the lower overlap of C. gibelio might be explained by a decrease in the optimal prey of C. gibelio. These limited resource conditions promote the consumption of a wide range of suboptimal prey types which are shared by all freshwater fish species particularly at the river mouth stations (Klf, Kka and Kkop) in fall and spring, where the maximum tolerable overlap of C. gibelio was 2.33, 0.16, 1.63, 0.35, 2.77 for S. cii, B. oligolepis, A. cf. attalus, G. kovatschevi and R. amarus, respectively. We suggest that C. gibelio has a greater advantage in habitat use than the other freshwater fish species, particularly less abundant species like C. fahirae and G. kovatschevi, and the fact that these rare species were not found from some stations may reflect competitive exclusion. The importance of impact by C. gibelio is clear when taking into consideration the vulnerability of G. kovatschevi.
The total niche area of fish communities generally increased from upstream to the river mouth except in spring. The fluctuations in the total niche area in that season might be explained by low abundance of C. gibelio at the Srm (1.8%) and KKa (4.1%) stations. The dominance of transitional fish species in that season suggests that high salinity (7.44 mS cm−1) might have limited the abundance of C. gibelio in the river mouth in Spring 2013. On the other hand, C. gibelio appeared to occupy high salinity (6730 µS cm−1) environmental conditions which other freshwater fish species could not survive at KKa station in Summer 2012. As explained by Hubbell (2001), new individuals cannot enter the community, either by birth or by immigration, unless there is a vacant niche left by individuals. Environmental fluctuation at the river mouth may result in disappearance of freshwater fishes because of intolerance to salinity. Then the vacant niches might be used by C. gibelio and transitional fish species under high salinity conditions. The chemistry of a river has dynamic patches through time and space with more fluctuations in the lower reaches than in the headwaters (Sabater et al. 1991). The more time-dependent chemistry of the lower reaches might represent a marked advantage for survival of C. gibelio.
The variance in the population stable isotope values of species can be used as an indicator of feeding niche widths (Syväranta and Jones 2008). Bearhop et al. (2004) recommended using SEAc for estimating the niche width from small sample sizes (Bearhop et al. 2004; Jackson et al. 2011). However, even SEAc is still susceptible to sample size effects (Syväranta et al. 2013), and we acknowledge that our sample sizes are small. However, SEA.B, which provides the 95% CI of the ellipse, is used in SIBER when comparing the ellipses of species. Therefore, the statistical test results give unbiased comparison of isotopic niche area of C. gibelio. In fact, the highest TA value for C. gibelio actually derived from a small sample size (n = 6) in KKop in Spring 2013. Moreover, the rather close clustering of replicate individuals from each species suggests that our calculated SEAc values are unlikely to be overestimates and we are confident that the larger value for C. gibelio than for the other species is a true reflection that this species has a wider trophic niche than the native freshwater species.
The isotopic niche width of C. gibelio was relatively small compared to other fish species at Ahm and Sar stations. The small niche width at these sites might be explained by less diverse resources, with species adapting to the availability of the most suitable foods (Gordon and Illius 1989). On the other hand, smaller niche area with a high degree of overlap with sympatric species might be explained by Pianka’s maximum tolerable overlap degrees of these species (Pianka 1974). In the Karamenderes river, the smaller and even zero degree of overlap support Pianka’s hypothesis that limited resources result in smaller degree of overlap among sympatric species before competitive exclusion occurs. This would suggest that, C. fahirae is the most susceptible species in the presence of invasive C. gibelio. However, elimination of these vulnerable species because of competitive exclusion should take place slowly for the reasons given by Hubbell (2001) relating to density dependence.
There are different microhabitat characteristics along the river and depending on their adaptations different kinds of fishes respond with greater or lesser foraging niche width and abundance. In general, and despite having a small isotopic niche area than C. gibelio, S. cii is abundant probably due to its better adaptability to any particular microhabitat throughout the river. S. cii has a generalist feeding strategy, may outcompete other species under limited resource conditions (Yalçın Özdilek 2017) and is also widespread through the Biga peninsula (Bakaç 2018). C. gibelio is also a generalist feeder and mostly utilises similar food items as S. cii (Partal and Yalçın Özdilek 2017; Yalçın Özdilek 2017). It was notable that C. gibelio mostly covered at least 50% of the SEAc of S. cii and the two species compete for some foods. S. cii outnumbers C. gibelio only at the upper station (Srm). However, C. gibelio did not outcompete S. cii at any other station or season. Therefore, the competitive pressure of C. gibelio on at least the dominant fish species, S. cii, is not as large as expected. On the other hand the river provides many suitable microhabitats for both fish species with their high spatial and temporal dietary plasticity.
C. gibelio has a wide diet compared to native species in the Karamenderes river (Partal and Yalçın Özdilek 2017), so some degree of isotopic niche overlap with other freshwater fish is expected. In this study the isotopic overlaps were assessed for fish of all length groups combined and there is a lack of data regarding possible length-based niche overlaps between fish species. However, the degree of overlap may vary according to length groups; Specziar & Rezsu (Specziár and Rezsu 2009) reported that C. gibelio diet overlapped only with 41–120 mm Rutilus rutilus. Studies of possible size-specific overlap will be required to understand fully the competitive interactions between C. gibelio and the native freshwater fish species. C. carpio is another species which is regularly introduced into the Bayramiç Dam which is a potential site for entry of C. gibelio to the lower sections of the river system. C. carpio and G. holbrooki are not common in the river system and the abundances of these species were very low (Cc, 6.1% in Ahm 3.4% in Klf and Gh, 3.4% in Klf). The high isotopic niche area C. gibelio with the low degree of overlap might be explained by high competition with these introduced species.
C. gibelio has herbivore-omnivore characteristics and the trophic position of C. gibelio is lower than that of the other dominant fish species such as S. cii, B. oligolepis and A. cf attalus (Yalçın Özdilek and Jones 2014). Therefore, the population of C. gibelio might be regulated by piscivore species such as A. anguilla at the river mouth stations. The large mean fork length of C. gibelio recorded at the KKa and Kkop stations might support this finding being a consequence of size-dependent prey selectivity by piscivore species on small C. gibelio species. If we can assume C. gibelio is a potential prey for European eel, even though no data in this study can be used to support that assumption, the decreasing trend in A. anguilla populations (ICES 2016) might be advantageous for C. gibelio particularly at the river mouth.
The introduction of C. gibelio is assumed to arise from escapes from reservoirs, and the Ahm station is the station for entrance of C. gibelio to the river system. The results indicate that C. gibelio has not successfully established at these first two stations but when moving to the lower part of the river they find the most suitable conditions at Klf station where they can establish by resource partitioning with the other dominant fish species such as S. cii and B. oligolepis according to the maximum tolerable niche overlaps. However, at the river mouth stations, very variable environmental parameters, such as conductivity, restricts survival of the freshwater fish species and the more tolerant C. gibelio can occupy the resulting vacant niche space, outcompeting or sharing the resources with the transition fish species in terms of resource partitioning.
In addition to typical advantages for successful invasion, the wide isotopic niche area and competition ability of C. gibelio was assessed in this study. Less than a decade from the first introduction into the river system (Yalçın Özdilek 2008) may be too soon to see the full potential impact of this species on the native river fish community in terms of any extinction of species compared to previous data (Sarı et al. 2006). However, it is clear that C. gibelio has an important functional role in the community dynamics, having high dominance, high niche area and some degree of niche overlap with many freshwater and transitional fish species, including new introductions. We suggest that the different microhabitat characteristics of the river system should be maintained and long-term monitoring studies are needed for the river management plan.
References
Ayala JR, Rader RB, Belk MC, Schaalje GB (2007) Ground-truthing the impact of invasive species: spatio-temporal overlap between native least chub and introduced western mosquitofish. Biol Invas 9:857–869. https://doi.org/10.1007/s10530-006-9087-4
Baba A, Deniz O, Gülen O, Gülen O (2007) Effects of mining activities on water around the Çanakkale Plain, Turkey. In: Zaidi MK (ed) Wastewater reuse–risk assessment, decision-making and environmental security. Springer, Netherlands, pp 3–10
Bakaç İ (2018) Assessment of distribution and abundances of freshwater fish in Çanakkale, Turkey. Çanakkale Onsekiz Mart University, Çanakkale
Bearhop S, Adams CE, Waldron S et al (2004) Determining trophic niche width: a novel approach using stable isotope analysis. J Anim Ecol 73:1007–1012. https://doi.org/10.1111/j.0021-8790.2004.00861.x
Bland JM, Altman DG (1995) Multiple significance tests: the Bonferroni method. BMJ 310:170
Blossey B, Nötzold R (1995) Evolution of increased competitive ability in invasive nonindigenous plants: a hypothesis author (s): Bernd Blossey and Rolf Notzold source. J Ecol 83:887–889
Bootsma HA, Hecky RE, Hesslein RH, Turner GF (1996) Food partitioning among Lake Malawi nearshore fishes as revealed by stable isotope analysis. 77:1286–1290
Britton JR, Ruiz-Navarro A, Verreycken H, Amat-Trigo F (2018) Trophic consequences of introduced species: comparative impacts of increased interspecific versus intraspecific competitive interactions. Funct Ecol 32:486–495. https://doi.org/10.1111/1365-2435.12978
Carey MP, Wahl DH (2010) Native fish diversity alters the effects of an invasive species on food webs. Ecology 91:2965–2974. https://doi.org/10.1890/09-1213.1
Cody ML (1974) Optimization in ecology: natural selection produces optimal results unless constrained by history or by competing goals. Science 183:1156–1164. https://doi.org/10.1126/science.183.4130.1156
Colwell RK, Futuyma DJ (1971) On the measurement of niche breadth and overlap. Ecology 52:567–576. https://doi.org/10.2307/1934144
Correia AM (2002) Niche breadth and trophic diversity: feeding behaviour of the red swamp cray sh (Procambarus clarkii) towards environmental availability of aquatic macroinvertebrates in a rice eld (Portugal). Acta Oecol 23:421–429
Crivelli A (1995) Are fish introductions a threat to endemic freshwater fishes in the Northern Mediterranean region? Biol Conserv 72:311–319
DeNiro MJ, Epstein S (1978) Influence of diet on the distribution of carbon isotopes in animals. Geochim Cosmochim Acta 42:495–506
Elton CS (1958) The ecology of invasions by animals and plants, first. Chapman and Hall, London
Fink P, Reichwaldt ES, Harrod C, Rossberg AG (2012) Determining trophic niche width: an experimental test of the stable isotope approach. Oikos 121:1985–1994. https://doi.org/10.1111/j.1600-0706.2012.20185.x
Fox BJ (1981) Niche parameters and species richness. Source Ecol 62:1415–1425
Freyhof J, Kottelat M (2008) Gobio kovatschevi. In: IUCN Red List Threat. Species 2008 e.T135615A4162824. http://dx.doi.org/10.2305/IUCN.UK.2008.RLTS.T135615A4162824.en. Accessed 27 Apr 2018
Gaygusuz Ö, Tarkan AS, Gaygusuz ÇG (2007) Changes in the fish community of the Ömerli Reservoir (Turkey) following the introduction of non-native gibel carp Carassius gibelio (Bloch, 1782) and other human impacts. Aquat Invas 2:117–120. https://doi.org/10.3391/ai.2007.2.2.6
Gordon IJ, Illius AW (1989) Resource partitioning by ungulates on the Isle of Rhum. Oecologia 79:383–389. https://doi.org/10.1007/BF00384318
Hubbell SP (2001) The unified neutral theory of biodiversity and biogeography. Princeton University Press, New Jersey
Hutchinson GE (1957) Concluding Remarks. In: Cold Spring Harbor Symposia on Quantitative Biology. pp 415–427
ICES (2016) ICES WGEEL report 2016 report of the working group on Eels WGEEL9. ICES, Cordoba
Jackson AL, Inger R, Parnell AC, Bearhop S (2011) Comparing isotopic niche widths among and within communities: SIBER—Stable Isotope Bayesian Ellipses in R. J Anim Ecol 80:595–602. https://doi.org/10.1111/j.1365-2656.2011.01806.x
Jacoby D, Gollock M (2014). Anguilla anguilla. The IUCN Red List of Threatened Species 2014:e.T60344A45833138. https://doi.org/10.2305/IUCN.UK.2014-1.RLTS.T60344A45833138.en. Accessed 08 Feb 2019
Karlson AML, Gorokhova E, Elmgren R (2015) Do deposit-feeders compete? Isotopic niche analysis of an invasion in a species-poor system. Sci Rep 5:1–8. https://doi.org/10.1038/srep09715
Kiljunen M, Grey J, Sinisalo T et al (2006) A revised model for lipid-normalizing δ13C values from aquatic organisms, with implications for isotope mixing models. J Appl Ecol 43:1213–1222. https://doi.org/10.1111/j.1365-2664.2006.01224.x
Layman CA, Arrington AD, Montana CG, Post DM (2007a) Can stable isotope ratios provide for community-wide measures of trophic structure ? 88:42–48. https://doi.org/10.1002/jae.l200
Layman CA, Quattrochi JP, Peyer CM, Allgeier JE (2007b) Niche width collapse in a resilient top predator following ecosystem fragmentation. Ecol Lett 10:937–944. https://doi.org/10.1111/j.1461-0248.2007.01087.x
Levins R (1968) Evolution in changing environments: some theoretical explorations. Princeton University Press, New Jersey
Mac Arthur RH, Pianka ER (1966) On optimal use of a patchy environment. Am Nat 100:603–609
MacArthur RH (1968) The theory of the niche. In: Lewontin RC (ed) Population biology and evolution. Syracuse University Press, Syracuse, pp 159–176
MacArthur RH (1972) Patterns in the distribution of species. In: MacArthur RH (ed) Geographycal ecology, first. Princeton University Press, New York
Miranda NAF, Perissinotto R (2012) Stable isotope evidence for dietary overlap between alien and native gastropods in coastal lakes of Northern KwaZulu-Natal, South Africa. PLoS One 7:e31897. https://doi.org/10.1371/journal.pone.0031897
Olsson K, Stenroth P, Nyström P, Graneli W (2009) Invasions and niche width: does niche width of an introduced crayfish differ from a native crayfish? Freshw Biol 54:1731–1740. https://doi.org/10.1111/j.1365-2427.2009.02221.x
Özcan G (2007) Distribution of non-indigenous fish species, Prussian carp Carassius gibelio (Bloch, 1782) in the Turkish freshwater systems. Pakistan J Biol Sci 10:4241–4245
Özuluğ M, Meriç N, Freyhof J (2004) The distribution of Carassius gibelio (Bloch, 1782) (Teleostei: Cyprinidae) in Thrace (Turkey). Zool Middle East 31:63–66. https://doi.org/10.1080/09397140.2004.10638023
Parnell AC, Inger R, Bearhop S, Jackson AL (2010) Source partitioning using stable isotopes: coping with too much variation. PLoS One 5:1–5. https://doi.org/10.1371/journal.pone.0009672
Partal N, Yalçın Özdilek Ş (2017) Feeding ecology of invasive Carassius gibelio (Bloch, 1782) in Karamenderes Stream, Turkey. Ege J Fish Aquat Sci 34:157–167. https://doi.org/10.12714/egejfas.2017.34.2.07
Pianka ER (1974) Niche overlap and diffuse competition. Proc Nat Acad Sci USA 71:2141–2145
Pianka ER (2011) Evolutionary ecology, vol 486. Eric R. Pianka, Siskiyou County. https://doi.org/10.2307/2257971
Post DM, Layman CA, Arrington DA et al (2007) Getting to the fat of the matter: models, methods and assumptions for dealing with lipids in stable isotope analyses. Oecologia 152:179–189. https://doi.org/10.1007/s00442-006-0630-x
Robinson BW, Wilson DS (1994) Character release and displacement in fishes: a neglected literature. Am Nat 144:596–627. https://doi.org/10.1086/285696
Rocha MP, Bini LM, Siqueira T et al (2018) Predicting occupancy and abundance by niche position, niche breadth and body size in stream organisms. Oecologia 186:205–216. https://doi.org/10.1007/s00442-017-3988-z
Sabater F, Armengol J, Sabater S (1991) Physico-Chemical disturbances associated with spatial and temporal variation in a Mediterranean river. J N Am Benthol Soc 10:2–13. https://doi.org/10.2307/1467759
Sakai AK, Allendorf FW, Holt JS et al (2001) The population biology of invasive specie source: annual review of ecology and systematics. Annu Rev Ecol Syst 32:305–332
Sarı HM, Balık S, Bilecenoğlu M, Türe G (1999) Recent changes in the fish fauna of Lake Bafa, Aegean region of Turkey. Zool Middle East 18:67–76. https://doi.org/10.1080/09397140.1999.10637783
Sarı HM, Balık S, Ustaoğlu R, İlhan A (2006) Distribution and ecology of freshwater ichthyofauna of the Biga Peninsula, North-western Anatolia, Turkey. Zool Turk J 30:35–45
Schmidt SN, Olden JD, Solomon CT, Vander Zanden MJ (2007) Quantitative approaches to the analysis of stable isotope food web data. Ecology 88:2793–2802. https://doi.org/10.1890/06-0937.1
Seagle SW, Mccracken GF (1986) Species abundance, niche position, and niche breadth for five terrestrial animal assemblages. Ecology 67:816–818
Shea K, Chesson P (2002) Community ecology theory as a framework for biological invasions. Trends Ecol Evol 17:170–176. https://doi.org/10.1016/s0169-5347(02)02495-3
Simon KS, Townsend CR, Biggs BJF et al (2004) Habitat-specific nitrogen dynamics in New Zealand streams containing native or invasive fish. Ecosystems 7:777–792. https://doi.org/10.1007/s10021-004-0024-z
Specziár A, Rezsu ET (2009) Feeding guilds and food resource partitioning in a lake fish assemblage: an ontogenetic approach. J Fish Biol 75:247–267. https://doi.org/10.1111/j.1095-8649.2009.02283.x
Svanbäck R, Persson L (2004) Individual diet specialization, niche width and population dynamics: implications for trophic polymorphisms. J Anim Ecol 73:973–982. https://doi.org/10.1111/j.0021-8790.2004.00868.x
Syväranta J, Jones RI (2008) Changes in feeding niche widths of perch and roach following biomanipulation, revealed by stable isotope analysis. Freshw Biol 53:425–434. https://doi.org/10.1111/j.1365-2427.2007.01905.x
Syväranta J, Lensu A, Marjomäki TJ et al (2013) An empirical evaluation of the utility of convex hull and standard ellipse areas for assessing population niche widths from stable isotope data an empirical evaluation of the utility of convex hull and standard ellipse areas for assessing population niche. PLoS One 8:e56094. https://doi.org/10.1371/journal.pone.0056094
Tarkan AS, Gaygusuz O, Gürsoy Gaygusuz C et al (2012) Circumstantial evidence of gibel carp, Carassius gibelio, reproductive competition exerted on native fish species in a mesotrophic reservoir. Fish Manag Ecol 19:167–177. https://doi.org/10.1111/j.1365-2400.2011.00839.x
Tilman D (1999) The ecological consequences of changes in biodiversity: a search for general principles. Ecology 80:1455–1474. https://doi.org/10.2307/176540
Vander Zanden MJ, Casselman JM, Rasmussen JB (1999) Stable isotope evidence for the food web consequences of species invasions in lakes. Nature 401:464–467. https://doi.org/10.1038/46762
Yalçın Özdilek Ş (2017) Turkish Journal of Zoology Seasonal and ontogenetic diet shift of two sympatric cyprinid fish species from the temperate Karamenderes River, Çanakkale, Turkey. Turk J Zool 41:67–81. https://doi.org/10.3906/zoo-1603-34
Yalçın Özdilek Ş, Jones RI (2014) The diet composition and trophic position of introduced prussian Carp Carassius gibelio (Bloch, 1782) and native fish species in a Turkish River. Turk J Fish Aquat Sci 14:769–776. https://doi.org/10.4194/1303-2712-v14_3_19
Yalçın Özdilek Ş (2008) Karamenderes’in Doğal ve istilaci baliklari. In: Akdemir A, Demircan O, Yılmaz S et al (eds) Ezine değerleri sempozyumu. Olay Matbaası, Çanakkale, pp 129–139
Yuille MJ, Fisk AT, Stewart T, Johnson TB (2015) Evaluation of Lake Ontario salmonid niche space overlap using stable isotopes. J Great Lakes Res 41:934–940. https://doi.org/10.1016/j.jglr.2015.05.011
Acknowledgements
This project was supported by The Scientific and Technological Research Council of Turkey (TUBITAK) 111Y280 coded project. We thank fisherman Selahattin EROL for help sampling with the B.12.0BSÜ.0.01.00.00/140.03.03-460 and 65465693-605/20.08.2014 numbered legal permissions. We thank the project team for valuable support in the field studies. We also thank two anonymous reviewers for their helpful comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yalçın Özdilek, Ş., Partal, N. & Jones, R.I. An invasive species, Carassius gibelio, alters the native fish community through trophic niche competition. Aquat Sci 81, 29 (2019). https://doi.org/10.1007/s00027-019-0623-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00027-019-0623-6