Abstract
Facilitation (enhancement of propagule retention in this case) is increasingly recognized as an important driver of biodiversity, but it is still unknown if facilitation during dispersal and colonization is affected by self-organized spatial pattern formation. We investigated the ability of in-stream submerged macrophyte patches to trap the vegetative propagules of three species (Berula erecta, Groenlandia densa, Elodea nuttallii in two size classes: 13–22 and 40–48 cm long), and to potentially benefit the colonization of these three species. We tested the effects of propagule traits, hydrodynamic forcing, and spatial patch configuration on propagule trapping. Propagule buoyancy was negatively correlated with trapping chance, while propagule size did not influence trapping. Species-specific differences in buoyancy were maintained for weeks after fragmentation. Propagule retention was interactive and conditional upon the interplay between incoming flow velocities and vegetation spatial patterning. In the flume experiment at low flows, a patchy configuration (one patch filling 66% of the flume width) retained more surface-drifting propagules (B. erecta, G. densa), than near-homogeneous cover (two patches close together, filling the entire flume width). In contrast, retention of sinking E. nuttallii propagules increased in the two-patch configurations. In flume and field releases where patches did not completely fill the channel width, water flowed around the patches rather than over or through them. This resulted in low-flow velocity areas within patches where canopies were upright and propagules were retained, and higher velocity flows around patches. In contrast, when vegetation filled the channel width, water could not be diverted laterally around the patches and preferentially flowed over them, causing the canopies to bend and reduce their trapping capacity. In flume experiments at high flows, retention of all species decreased, regardless of vegetation configuration, as propagules passed over the reconfigured vegetation canopies. These findings on the interplay of water movement and patch reconfiguration suggest that environmental heterogeneity generated by the self-organizing behavior of aquatic plants might enhance colonization of sessile organisms, calling for landscape-scale processes like dispersal to be better investigated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Understanding the drivers of biodiversity is a key research topic in ecology. Facilitation, or positive interactions between species, has strong effects on the diversity and species composition of communities (Bertness and Callaway 1994; Callaway 1994; Bruno et al. 2003; Brooker et al. 2008; McIntire and Fajardo 2014). Positive interactions are often performed by foundation species (Dayton 1972) or ecosystem engineers (Jones et al. 1994), which create stable conditions for other species and provide much of the structure of a community. Facilitation can increase diversity through well-studied mechanisms, such as enhanced resource availability, provision of refugia against physical stress and protection from predation or competition (Bertness et al. 1999; Borthagaray and Carranza 2007; Callaway 2007). The spatial component of facilitation is usually studied at the local scale of an individual patch, in locations under the protective influence of the facilitator [e.g. “nurse plant syndrome”; Niering et al. (1963), Padilla and Pugnaire (2006)], or along gradients of physical stress (Bertness and Callaway 1994; Bertness and Leonard 1997). However, many foundation species and ecosystem engineers generate striking spatial patterning at the landscape scale by self-organization processes, even in the absence of underlying abiotic gradients (Rietkerk and van de Koppel 2008). Understanding the role of patchiness at the landscape scale for inter-specific facilitation is critical in maintaining and restoring biodiversity.
Many self-organized spatial patterns in ecosystems emerge from scale-dependent feedbacks, whereby the interaction between the organisms and the environment leads to a positive feedback on a local scale, but a negative one inhibits their growth on larger scales (Rietkerk and van de Koppel 2008). These feedbacks arise through different mechanisms, such as concentration of limiting resources [e.g. nutrients in peatlands; Eppinga et al. (2009)] or divergence of physical stress [e.g. water flow or snow; Hiemstra et al. (2002), Larsen et al. (2007), Weerman et al. (2010)]. Here, the positive feedback of resource concentration or flow reduction within the patches is coupled with a negative feedback of resource depletion or increased flow stress outside the patches. Yet, it is unknown how the presence or absence of these underlying mechanisms affects facilitation. In such patchy systems, facilitative effects at the within-patch scale cannot be easily scaled up to facilitation at the larger, between-patch scale for the following two reasons. First, the spatial configuration or total cover of the patches may affect the environmental conditions in the gaps between them, by changing their feedback interaction with the stress factor (Fonseca et al. 1983; Granata et al. 2001; Larsen and Harvey 2010; Kondziolka and Nepf 2014). Secondly, the balance between competition and facilitation can be strongly scale-dependent (van de Koppel et al. 2006), as abiotic conditions are mitigated in the patches, but competition with the facilitator might be very high. Hence, it is important to consider how facilitation is affected by self-organized spatial patchiness and its underlying feedback mechanisms.
While self-organization can be due to a number of mechanisms, we focus here on the divergence of water flow. We define flow divergence as the lateral deflection of water flow around a patch of benthic organisms (plants or animals), rather than over or through it, resulting in an area of increased flow velocity adjacent to the patch (Fonseca et al. 1983; Gambi et al. 1990; Bouma et al. 2007; Follett and Nepf 2012). The accelerated flow around the patch may limit the further lateral expansion of the patch, creating a negative feedback: this is a common mechanism underlying the patchy distribution of foundation species in many aquatic ecosystems, such as rivers (Schoelynck et al. 2012), salt marshes (Temmerman et al. 2007; Bouma et al. 2009; Vandenbruwaene et al. 2011) and seagrass beds (Van Der Heide et al. 2010). In such physically-stressed environments, the arrival of dispersal units in favorable microsites within the patches of a facilitator species can be crucial (Aguiar and Sala 1997), especially for non-mobile organisms that require entrapment or stranding to establish (Rabinowitz 1978; Turner 1983; Nilsson et al. 2010). Here, any organism that enhances the arrival or retention of propagules can have a potential facilitative effect (Callaway 1995) and can influence colonization rates (Bruno et al. 2003; McKee et al. 2007). In many of these systems, the environmental stress may also be the dispersal vector (e.g. wind, water). Previous studies on transport and retention through vegetated environments often assumed homogeneous distribution or a single cover value of the facilitator (Chang et al. 2008; Peterson and Bell 2012; Gillis et al. 2014; Van der Stocken et al. 2015), despite its spatial patchiness. Considering only a single cover of the facilitator, overlooking its spatial structure in relation to environmental stressors, can tell us very little about the realized facilitative effects in a patchy landscape. Hence, we aim to test whether facilitation during dispersal and colonization depends on the flow divergence mechanism underlying spatial patchiness of the facilitator.
In lotic ecosystems, aquatic macrophytes are important foundation species (Carpenter and Lodge 1986). Submerged plants in rivers can grow in a patchy pattern due to local flow reduction within the vegetation and divergence of water flow around it (Sand-Jensen and Mebus 1996; Sand-Jensen 1998; Cotton et al. 2006; Wharton et al. 2006; Schoelynck et al. 2012). Water flow is both the stress factor that leads to vegetation patchiness and one of the main dispersal vectors of plant propagules (e.g. seeds, vegetative fragments, stolons, turions; Goodson et al. 2001, 2003; Bornette and Puijalon 2011; Nilsson et al. 2010). Among vegetative propagules, fragments are of clear importance for the colonization of stream reaches (Barrat-Segretain et al. 1998), and can account for up to 90% of new plant establishment in streams (Sand-Jensen et al. 1999; Riis 2008). Retention of vegetative fragments in streams is a necessary step before primary colonization and a bottleneck to vegetation establishment (Fig. 1), which relies on the availability of structures to entrap propagules (Riis and Sand-Jensen 2006; Riis 2008). In addition to physical river characteristics and abiotic structures such as boulders and large woody debris (Engström et al. 2009; Säumel and Kowarik 2013), existing macrophyte canopies are a potentially important biotic retention agent for plant fragments.

Modified from Riis (2008)
Consecutive processes involved in macrophyte colonization of lowland streams. Bars indicate the success rates based on the previous process (% of fragments).
Interactions between vegetation and hydrodynamic stress can affect propagule retention in several ways. If hydrodynamic stress increases, patches of flexible vegetation can reconfigure by bending down closer to the substrate, creating less of an obstruction in the water column (Sand-Jensen and Pedersen 2008; Schoelynck et al. 2013). Propagule traits like buoyancy and size may also play a role in the dispersal process. For instance, buoyancy determines the propagule’s position within the water column and how likely it is to be retained by submerged macrophytes (Riis and Sand-Jensen 2006). And in the absence of in-channel vegetation, dispersed shoots are unlikely to be retained by bare river bed sediments (Riis 2008). In this study we focused on streams with self-organized patchy aquatic macrophytes which provided a unique opportunity to test how flow divergence mechanisms affect propagule retention, and how this depends on the landscape-scale setting of these vegetation patches.
We investigated the effects of water flow divergence on facilitation through propagule retention using a combination of mesocosm buoyancy tests, flume, and field studies. We examined the effects of the patchy submerged macrophyte Callitriche platycarpa Kütz on the dispersal and retention of vegetative fragments of other aquatic plant species, which have been found to significantly aggregate within and around Callitriche patches (Cornacchia et al. 2018). We examined the role of water flow divergence around vegetation patches on propagule retention by comparing different configurations of patchy vegetation where water could flow laterally around the patch and where water could only flow over or through the patches. We then examined the effects of propagule traits (i.e., buoyancy and size) and hydrodynamic forcing (i.e., flow velocity affecting the bending of the canopy) on the retention of propagules in these configurations.
Materials and methods
Studied species
The propagules of three freshwater macrophyte species, Berula erecta (Huds.) Coville, Groenlandia densa (L.) Fourr. and Elodea nuttallii (Planch.) St. John, were considered for this study (Fig. 2). Here, we focused on the dispersal of vegetative fragments, as the processes of interaction with vegetation patterns may be different for vegetative and sexual propagules, particularly due to differences in size or buoyancy (Cellot et al. 1998; Merritt and Wohl 2002; Chang et al. 2008; Carthey et al. 2016). Throughout this paper, the term propagule only refers to vegetative fragments, unless specified otherwise. Vegetative fragments are important for the recruitment of macrophyte species in streams as they can be viable for more than 10 weeks (Barrat-Segretain et al. 1998) and up to 6 months in the water (Sarneel 2013). Also, they can regrow into viable plants (i.e. regenerate) and develop new propagules (Barrat-Segretain et al. 1999). The propagules used in our experiments consisted of whole plants, comprising both aboveground and belowground parts. B. erecta has a rosette of petiolated-dissected leaves, G. densa is a caulescent species with opposite leaves, and E. nuttallii presents relatively rigid stems with short, densely packed whorls containing 3 leaves. This selection allowed us to compare propagules with different floating traits. For example, propagules of Elodea canadensis, which is morphologically similar to E. nuttallii (Riis and Sand-Jensen 2006), have lower buoyancy and tend to drift slightly below the water surface, rather than on the water surface as is the case for B. erecta and G. densa.
Sample collection
Whole plants of the three freshwater species B. erecta, G. densa and E. nuttallii were collected by hand from naturally occurring existing patches on 12 September 2014 in an artificial drainage channel located along the Rhône River near Serrières de Briord (France, 45.813551°N, 5.447440°E). Sample collection was performed at the end of the growing season to limit plant growth during storage or experiments. The whole plants, to be used as vegetative propagules in our experiments, were selected in two contrasting sizes for each species to represent their normal propagule size range (21.9 ± 2.6 and 48.4 ± 2.2 cm for B. erecta; 17.8 ± 1.3 and 41.4 ± 3.4 cm for G. densa; 12.8 ± 2.5 and 40.8 ± 4.2 cm for E. nuttallii). The propagules were stored in plastic bags and transported to the flume laboratory in the Royal Netherlands Institute for Sea Research (NIOZ), Yerseke (The Netherlands) within 24 h of collection, where they were kept outside in tanks with aerated tap water, with a water level of 20 cm and at natural light for one week before the experiments started.
Quantifying floating traits by a buoyancy test
Propagule buoyancy was monitored in the mesocosm to determine how plant floating capacity could influence retention within submerged vegetation. The plant samples (72 = 12 propagules × 2 size classes × 3 species) were stored in six large tanks (110 × 95 × 60 cm) with aerated tap water up to a depth of 20 cm, so that each tank contained two propagules per species and per size class. There was no water motion in the tanks, which were stored in an unheated greenhouse, where temperature fluctuations followed the outside ambient temperature. Propagule buoyancy was measured using a force transducer developed by WL Delft Hydraulics (Delft, The Netherlands). The transducer consisted of a solid platform, carried by two steel cantilever beams, with four temperature-corrected strain gauges mounted in pairs on opposite sides of each of the two steel cantilevers (Bouma et al. 2005). The voltage output for the force transducer was linear with forces up to 10 N. The buoyancy of all 72 samples was monitored weekly up to a month, after which there was no evidence of a decline in buoyancy, so the experiment was terminated. The force transducer was placed at the bottom of the tank and each plant was mounted on top of it. We measured the buoyant force, i.e. the upward pulling force exerted on the plant. Voltage readings were collected on a data logger at a frequency of 100 Hz and expressed as the mean value for 1 min.
Quantifying the dispersal and retention of plant propagules by a flume experiment
The ability of submerged aquatic vegetation to trap propagules of other species was assessed by modelling the patch morphology of the aquatic macrophyte Callitriche platycarpa in a flume setup. Although Callitriche patches are often monospecific (Sand-Jensen et al. 1999; Demars and Gornall 2003), ‘mixed’ patches with individuals of different species have been observed frequently at our field sites. We chose to focus on Callitriche as this species forms very dense patches, showing a high capacity to trap both plant propagules and small drifting debris (e.g. decomposing tree leaves, twigs and small branches) (L. Cornacchia, personal observation). The experiments were conducted in the racetrack flume (17.5 m long, 0.6 m wide and 0.3 m of water depth) at NIOZ using a smooth flume bottom. Patches of C. platycarpa (1.2 m in length) were modelled using commercial polyethylene fishing rope (Ymuiden Stores; diameter: 1.44 mm, buoyancy: 0.0275 N, bending Young’s modulus: 94.5 MPa), which was mounted on boards in a staggered pattern and cut to recreate the typical patch morphology of this submerged macrophyte. Plants are rooted at the upstream end and form a trailing canopy just beneath the water surface. In addition, C. platycarpa has gradually increasing canopy height from upstream to downstream (Licci et al. 2016). For an average sized C. platycarpa patch, the upstream part of the patch canopy is more exposed to flow pressure and is compressed near the sediment bed, whereas the shoots in the middle and downstream parts of the patch have higher biomass and reach the water surface, where they form floating leaf rosettes. We chose to model Callitriche patches and thereby control for biological characteristics (vegetation density, flexibility, shoot length and morphology) while only changing their spatial configuration.
After the month-long buoyancy experiments, we released the propagules in the flume to examine the role of water flow divergence around vegetation on propagule retention. Ten replicates were completed for a combination of 48 treatments: 4 vegetation configurations, 3 species (B. erecta, G. densa, E. nuttallii), 2 propagule sizes (small and large individuals), and 2 flow velocities (0.1 and 0.3 m s−1). Each replicate involved the same six propagules for each size of a given species. The four vegetation configurations consisted of two single-patch configurations (‘W’: wide patch, 0.4 m wide, i.e. 66% of the flume width; ‘N’: narrow patch, 0.2 m wide, i.e. 33% of the flume width) and two multiple-patch configurations (‘W–N’: W patch upstream of N patch, 0.75 m distance between their leading edges, creating a flume cross-section with 100% vegetation cover; ‘W—N’: W patch upstream of N patch, 1.90 m distance between their leading edges; Fig. 3a). For comparison with the field releases, the percentage of the flume bed occupied by vegetation in the horizontal plane was also calculated for each configuration (‘N’: 20%; ‘W’: 41%; ‘W—N’: 39%; ‘W–N’: 62%; see Fig. 7d). The patch mimics were placed next to the flume wall, rather than in the middle, to follow the vegetation patch distributions observed at previously studied field sites (i.e., channels along the Rhône River, France and in the Frome and Piddle catchments in Dorset, UK), with an empty (unvegetated) zone next to the patch due to water flow deflection and acceleration around it. In the two single-patch configurations and the ‘W—N’ multiple-patch configuration, the flow divergence mechanism was maintained by keeping an unvegetated channelled flow area next to the vegetation. Instead, flow divergence was prevented in the ‘W–N’ configuration by placing the patches close together to create an almost fully vegetated cross-section, with no areas for lateral flow redistribution. Both spatial arrangements have been observed at the field sites and in other freshwater streams (Cotton et al. 2006; Sand-Jensen and Pedersen 2008; Cornacchia et al. 2016). Within each configuration, we measured the canopy height and water height at three points along the patch central axis, using a reinforced meter rule. The free-flowing space within each configuration was then expressed as the minimum difference observed between water depth and canopy height over all points across the patches in the section.
a Schematic top view of the four single- and multiple-patch spatial configurations of Callitriche platycarpa mimics in the racetrack flume tank. ‘W’ indicates the wide patch, corresponding to 66% of the flume width; ‘N’ is the narrow patch, corresponding to 33% of the flume width. Water flow direction is from bottom to top of the figure. b Percentage of propagules trapped within single or multiple patch configurations at the 0.1 m s−1 velocity treatment, for E. nuttallii, c B. erecta and d G. densa. (E) Percentage of propagules trapped within single or multiple patch configurations at the 0.3 m s−1 velocity treatment, for E. nuttallii, f B. erecta and g G. densa. Propagules trapped (%) are means (+ 1 SE) of 12 propagules for n = 10 runs. Hashed bars indicate the propagules trapped in patch ‘W’, and solid bars indicate the propagules trapped in patch ‘N’. The sum of the propagules trapped in both patches was used in the analyses. Letters denote significant differences (Tukey’s contrasts, p < 0.05)
Propagules were released onto the water surface 1 m upstream of the patch mimics. We measured the time for propagules to move through the vegetated section and recorded the total time they were retained within the patch canopy. If this time exceeded 2 min, we considered the propagules to be trapped in submerged vegetation, as longer-term preliminary tests showed no propagule release once the retention time exceeded 2 min. Hence, the trapping capacity inside each patch configuration was determined as the percentage of propagules retained within a patch for more than 2 min. For the two multiple-patch configurations (‘W–N’ and ‘W—N’), the sum of the propagules trapped within each patch was the value used in the analyses.
Quantifying the role of vegetation cover and structure on propagule retention in the field
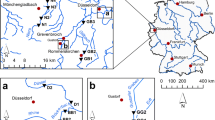
Field release experiments were conducted in two naturally-vegetated channels along the Rhône River (France), near Serrières-de-Briord (45.815°N, 5.427°E) and Flévieu (45.767°N, 5.480°E). The channels are uniform in terms of width and water depth, with relatively straight banks. The two channels present similar length (3.19 and 4.26 km for Flévieu and Serrières-de-Briord channels, respectively), width (5.8–8.0 m), depth (0.75–1.00 m) and substrate characteristics (fine to coarse gravel bed). We examined the impact of the natural macrophyte structure in the water column (presence of floating vegetation vs. fully submerged vegetation) and increasing vegetation cover on propagule retention in natural conditions. The average flow velocities during the experiments (July 2015) were 0.18 and 0.25 m s−1 for Flévieu and Serrières-de-Briord respectively, with a discharge of 0.73 and 1.30 m3 s−1. Here, we selected different 10-m sections along the channels to represent different percentage cover of either fully submerged or both submerged and floating-leaved Callitriche platycarpa stands. Callitriche platycarpa was the most abundant species within each section (80–90% of the total vegetation cover), and it represented ca. 70% of the total vegetation cover at the reach scale (Cornacchia et al. 2018). The remaining 30% was composed of G. densa, B. erecta, E. nuttallii, E. canadensis, Potamogeton crispus. Within each section, we measured the canopy height and water height at three points along the patch central axis, using a reinforced meter rule. The free-flowing space within each section was then expressed as the minimum difference observed between water depth and canopy height over all points across the macrophyte beds in the section. Five propagules of each species (23.5 ± 1.0 cm for B. erecta; 20.9 ± 0.5 cm for G. densa; 20.4 ± 0.9 cm for E. nuttallii) were collected from neighboring patches to represent the standard size range found drifting in small and intermediate-sized streams (Riis and Sand-Jensen 2006). Ten replicates were completed for each propagule, which were released one by one by placing them in the water at a distance of 3 m from the bank. We measured the time for propagules to move through the section and recorded whether they were retained in submerged vegetation for more than 2 min (as in the flume experiments). If the propagule was trapped by a retention agent other than submerged vegetation (e.g. emergent riparian vegetation), the retention agent was recorded but the propagule was considered as ‘not trapped’. Hence, the percentage of trapped propagules was calculated as the percentage of propagules retained inside the C. platycarpa patches for more than 2 min.
Statistical analyses
All statistical analyses were performed in R 3.1.2 (R Core Team 2015). We used repeated-measures ANOVA to analyse changes in propagule buoyancy over time. A one-way ANOVA was used to test for differences in buoyant force between species after 1 month in the water. The effects of propagule size on trapping capacity could not be tested for E. nuttallii, as the larger propagules of this species fragmented during the mesocosm monitoring. Therefore, we used a repeated-measures generalized linear mixed model (GLMM) with a logit link function and binomial error distribution to test the effects of two propagule species (G. densa and B. erecta) and their propagule size, spatial configuration, flow velocity and their interactions on trapping capacity (average percentage of trapped propagules per configuration) in the flume study. As the effect of propagule size was not significant, we used a repeated-measures GLMM to test the inter-relationships between the floating traits of the propagule species, spatial configuration of submerged vegetation and flow velocity with trapping capacity. Within the high velocity treatment in the flume experiment, the data for B. erecta and G. densa showed complete separation (i.e., 0% trapped propagules). To enable convergence of the GLMM, we therefore added one trapped propagule of B. erecta and G. densa per configuration in the high velocity treatment (a change of trapping capacity to 1%). For the field study, a repeated-measures GLMM was constructed to test the effects of propagule species, vegetation type (submerged/emerged), vegetation cover and their interactive effects on trapping capacity. A random effect of individual propagule was included in all models to account for non-independence between the repeated observations for each propagule. Significance of predictors was determined using likelihood ratio tests to compare the full model with reduced models using the ‘anova’ function. Tukey’s contrasts for multiple comparisons were performed using the ‘glht’ function in the package ‘multcomp’. Linear regression was used to test for the relationship between buoyant force and trapping capacity in the flume experiment, and between free-flow space over the canopy and trapping capacity in the field study.
Results
Effects of propagule traits on propagule trapping
Changes in propagule buoyancy since dislodgement—mesocosm measurements
Propagule buoyancy for the three species did not change significantly during the four weeks spent in the water column after fragmentation (repeated-measures ANOVA, F2,66 = 0.879, p = 0.42 for E. nuttallii, F2,66 = 1.327, p = 0.27 for B. erecta, F2,63 = 2.405, p = 0.098 for G. densa; Fig. 4). Hence, the time spent in the water column after detachment could be regarded as a marginal factor in terms of dispersal and trapping over this time scale. However, the buoyant force differed significantly between species (one-way ANOVA, F2,61 = 28.3, p < 0.001). E. nuttallii showed significantly lower buoyant force than B. erecta and G. densa (Tukey’s HSD p < 0.05 for both pairwise comparison). Buoyancy values also differed between the two surface floating species, with significantly higher values for B. erecta than G. densa (Tukey’s HSD p < 0.001). Both B. erecta and G. densa were positively buoyant and floated on the water surface, while E. nuttallii was neutrally buoyant and thus drifted 10–20 cm below the water surface. All propagules were viable after four weeks in the water and formed new leaves and shoot ramifications.
The influence of propagule size and buoyancy on propagule trapping—flume experiments
Propagule buoyancy, but not propagule size, affected the chance of being trapped by submerged vegetation. Testing the average trapping capacity (percentage of trapped propagules) in all flume configurations with a repeated-measures GLMM revealed no significant interactons between propagule size, species, flow velocity and patch spatial configuration on trapping of G. densa and B. erecta propagules (Table 1, p = 0.99). No difference in trapping was found between small and large propagules of the two species (likelihood ratio test, \(\chi _{1}^{2}\) = 1.524, p = 0.22), thus rejecting our hypothesis that large propagules have a greater chance of being trapped. However, buoyancy (as measured at the end of the monitoring period in the buoyancy test) was negatively correlated with the percentage of propagules trapped in the flume experiments at the 0.1 m s−1 velocity treatment (r2 = 0.56, p < 0.05; Fig. 5).
Percentage of retained propagules of Elodea nuttallii (diamonds), Groenlandia densa (triangles) and Berula erecta (squares) for two single-patch configurations (66% and 33% of vegetation in the cross-section) and two multiple patch configurations (short and large spacing between the patches) at the 0.1 m s−1 velocity treatment, in relation with their buoyant force (N) measured at the end of the 4-week monitoring in the mesocosm buoyancy test. Error bars are standard error of the mean
Effects of spatial vegetation patterns and vegetation cover on propagule trapping
Patch size and spatial configuration—flume experiments
Propagule trapping in the flume was strongly affected both by changes in vegetation patch size (in terms of width in the cross-section) and their spatial distribution (in terms of distance between vegetation patches) (Table 2). The net-effect was, however, strongly conditional upon the interaction between flow velocity and the propagule species. Thus, we discuss the results separated by velocity treatment in this section. First, we describe the results for the two surface-floating species G. densa and B. erecta that showed similar responses, and then the results for the sinking species E. nuttallii.
Within the 0.1 m s−1 velocity treatment, there was a statistically significant two-way interaction between the effects of species and configuration on propagule trapping (\(\chi _{6}^{2}\) = 39.747, p < 0.001). When submerged vegetation cover in the cross section was halved, by decreasing patch width from 66 to 33% of the flume width, the chance of propagules getting trapped decreased more than twofold for the two surface-floating species G. densa and B. erecta (Tukey’s contrasts, z = 2.845, p = 0.022 and z = 3.502, p = 0.002, respectively; Fig. 3c, d, W and N). When two patches were positioned a short distance apart (0.75 m between their leading edges), and therefore partially next to each other leading to a cross section with 100% vegetation cover, trapping chance significantly dropped compared to the W configuration (Tukey’s contrasts, p < 0.001 for both species), as the flow was confined to a narrow channel in between the two patches (Fig. 3c, d, W–N). As the distance between the patches increased to a gap of 70 cm (Fig. 3c, d, W—N), trapping ability was significantly higher than when patches were closely aligned (Tukey’s contrasts, z = 4.276, p < 0.001 for G. densa, z = 2.994, p = 0.01 for B. erecta), but not significantly different from the W treatment (z = 0.597, p = 0.93 for G. densa, z = − 1.154, p = 0.64 for B. erecta). Patch configuration significantly affected propagule trapping also for the neutrally buoyant species, E. nuttallii (\(\chi _{3}^{2}\) = 29.485, p < 0.001). No significant difference in propagule trapping of E. nuttallii was found between the two single-patch configurations (Tukey’s contrasts, z = 2.315, p = 0.09), or between the two multiple-patch configurations (z = -0.939, p = 0.78; Fig. 3b, W–N); however, the two multiple-patch configurations retained a significantly higher percentage of propagules than the single-patch configurations (p ≤ 0.05; Fig. 3B, W—N).
Within the 0.3 m s−1 velocity treatment, trapping significantly decreased as the patch canopy became compressed to the substrate forming the bed of the flume, thus leading to very low trapping compared to the 0.1 m s−1 treatment (\(\chi _{1}^{2}\)= 45.992, p < 0.001 for G. densa, \(\chi _{1}^{2}\) = 28.029, p < 0.001 for B. erecta, \(\chi _{1}^{2}\) = 57.81, p < 0.001 for E. nuttallii; Fig. 3F-G; Table 3). Only sinking propagules of E. nuttallii, which had an average buoyant force of 0.0014 N, were trapped in this treatment, and no significant difference in trapping was found between the different configurations (\(\chi _{3}^{2}\) = 6.627, p = 0.08; Fig. 3e).
Vertical structure of macrophyte vegetation—flume and field experiments
Flume and field release experiments showed that a critical canopy height in the water column is needed for patches to be able to act as trapping agents for propagules. We found a significant negative relationship between the number of trapped propagules (averaged over all three species) in each section, and the minimum amount of free-flowing space measured between the water surface and canopy height over all patches in the section (R2 = 0.50, p = 0.014, R2 = 0.67, p = 0.01; Fig. 6a). Trapped propagules in our field and flume experiments were typically oriented parallel to the main flow direction. The floating propagules of G. densa and B. erecta generally remained on top of the patches and became entangled in the downstream (floating) part of the Callitriche canopy. The sinking propagules of E. nuttallii entangled within the upstream half of the patch. In the two multiple-patch configurations, the neutrally buoyant propagules drifted in a confined space, where they would more frequently collide with either one of the two patches. However, the stream cross-section in the field releases was never fully occupied by vegetation. Therefore, as these flume configurations created a fully constrained situation for neutrally buoyant propagules to drift, which was never found in the field, they were considered outliers and excluded from the comparison between flume and field results (red diamonds in Fig. 6a).
a The number of propagules trapped (%) averaged over the three aquatic plant species, for different amounts of free-flow space over the canopy (i.e., the difference between the canopy height and the height of the water surface; cm). Black circles are field releases and show the minimum amount of free-flowing space measured over all vegetation patches in the section, for each of the submerged and mixed vegetation sites where field releases were carried out (same locations as in Fig. 7). Grey diamonds are flume releases and show the minimum amount of free-flowing space over the canopy during the flume releases. Red diamonds are outliers in the flume release of neutrally buoyant propagules, where the patch configuration created a fully constrained situation that was not found in the field. Outliers were not included in the averaged measurements. For both field and flume releases, the number of propagules trapped within the canopy is inversely correlated with the distance between the patch canopy and the water surface (R2 = 0.50, p = 0.014; R2 = 0.67, p = 0.01). b Changes in free-flow space over the canopy with increasing vegetation cover in the flume releases, for low (0.1 m s−1) and high (0.3 m s−1) flow velocity treatments
a Schematic planform representation of two example sections of a submerged vegetation site (left) and a mixed vegetation site (right), which were selected as locations for the field releases. These two types of locations show contrasting vegetation types: in the submerged vegetation site, the whole canopy is submerged and does not float on the water surface; in the mixed vegetation site, a certain portion of the canopy is composed of floating leaves reaching the water surface. For each location selected for the field releases, vegetation cover (%) was calculated as the cover over the whole section. b Relationship between fully submerged and mixed (floating and submerged) C. platycarpa vegetation cover (%) in the section and propagules trapped (%) in the field releases. Each point denotes a different site along the channels where field releases were conducted; labels M1 to M4 indicate mixed vegetation sites. c Relationship between the ratios of floating/submerged C. platycarpa cover in the section for the mixed vegetation sites (M1 to M4), and number of propagules trapped in each site in the field releases. d Relationship between vegetation cover (%) and propagules trapped (%) in the flume releases. Labels (N, W, W–N, W—N) indicate flume configurations. e Relationship between the ratios of floating/submerged vegetation cover and number of propagules trapped in each flume configuration
Percentage cover of macrophyte vegetation—field and flume experiments
Field releases showed that propagule retention was significantly affected by vegetation cover, propagule species, and the presence of either fully submerged or mixed (submerged and floating-leaved) vegetation patches (GLMM, Table 4; Fig. 7). Propagule species had a significant interactive effect with both vegetation type and total macrophyte cover. No significant interactive effects were found between vegetation type and total macrophyte cover.
In the fully submerged vegetation case, changes in vegetation cover did not significantly influence propagule retention (\(\chi _{6}^{2}\) = 3.44, p = 0.75; Fig. 7B), with no significant differences in trapping between species (\(\chi _{2}^{2}\) = 2.68, p = 0.26). However, both vegetation cover, propagule species and their interaction were significant in the mixed vegetation case, where part of the in-stream vegetation was emergent and part was submerged (\(\chi _{8}^{2}\) = 20.752, p = 0.008; Fig. 7b, c). Most propagules were trapped when in-stream macrophyte cover was intermediate (45–70%). At higher vegetation cover (86%), vegetation patches started to reconfigure as they were compressed to the river bed, thereby transforming their floating canopy into a submerged canopy and changing the ratio of floating to submerged vegetation cover (locations M1 to M4 in Fig. 7b, c). Significant differences in trapping between species were found with 45% and 70% vegetation cover in the mixed vegetation case, while no significant differences were found with no vegetation (0% cover), sparse vegetation (25% cover) and full reconfiguration of the vegetation (86% cover), where only a few propagules were retained for all three species. In the 45% cover release, propagule retention for E. nuttallii (36 ± 4%) and G. densa (22 ± 3.6%) was significantly higher than for B. erecta (2 ± 2%) (Tukey’s contrasts, z = 3.091, p = 0.005 and z = 2.414, p = 0.04). In the 70% cover release, G. densa propagule retention (66 ± 7.3%) was significantly higher than both E. nuttallii (32% ± 8%) and B. erecta (38% ± 6.3%) (Tukey’s contrasts, z = 3.340, p = 0.002 and z = 2.485, p = 0.03), while no significant differences were found between the latter two species. Field and flume releases both showed that trapping was highest at intermediate cover (40%) and declined at higher cover (> 60%) (Fig. 7d). As observed in the field, the reduced propagule trapping at the highest vegetation cover in the flume was due to canopies being pushed over by the flow towards the river bed (Figs. 6b, 7e). The only other retention agent during the field releases was emergent riparian vegetation. Among all the releases, propagules of E. nuttallii, G. densa and B. erecta were trapped by emergent riparian vegetation 0.5, 1 and 6% of the time, respectively.
Discussion
Facilitation has been increasingly recognized as an important driver of the structure and dynamics of natural communities (McIntire and Fajardo 2014). Despite the patchy distribution of many facilitator species at the landscape scale, it is largely unknown how facilitation is affected by self-organized spatial patchiness and its underlying feedback mechanisms in such a landscape setting. Using aquatic macrophytes as a model system, we showed that the feedback between vegetation and water flow diversion around patches, leading to self-organization, is crucial for the retention of propagules of other plant species. When the patches do not completely fill the width of the channel, the water flows around the vegetation patch rather than through it, resulting in increased flow velocities adjacent to the patch. By diverting the flow laterally around the patch and into the unvegetated areas, macrophytes locally create areas of reduced flow velocity where their canopies are upright, occupying the water column and reaching the water surface. This, in turn, can potentially benefit other plant species during the dispersal and colonization phase, as most propagules are retained in such low-flow areas within upright plant canopies that are not too close together. In contrast, when the patches almost completely fill the width of the channel, water cannot be diverted laterally around the patches but flows preferentially over the patches, causing the canopies to bend and depress towards the riverbed (Fig. 8). This causes propagules to float over the submerged vegetation, preventing facilitation as the plants are unable to overcome an important bottleneck in colonization. Our results highlight that self-organization and its underlying feedback processes are essential to enhance propagule retention and might affect species colonization.
Conceptual framework showing the main factors affecting canopy emergence of the flexible submerged macrophyte Callitriche platycarpa, and the resulting outcome for trapping chance of aquatic plant vegetative propagules. Conditions leading to floating or bending of the canopy include both direct and indirect effects on flow velocity (e.g. increase in channel flow velocity due to higher discharge vs. changes in flow patterns due to bio-physical interactions). In the planform representations of the stream, green shapes represent aquatic macrophyte patches, blue arrows are flow patterns between the canopy, and white arrows are flow patterns on top of the canopy (arrow length and width proportional to flow velocity). Bottom graphs are longitudinal sections through a Callitriche patch and show changes in bending behaviour of the canopy, and the consequences for propagule trapping. The buoyancy characteristics of the dispersal units also influence the final outcome in terms of trapping chance, with stronger effects for buoyant propagules
Is propagule retention a good proxy for facilitation during plant dispersal and colonization?
It is largely acknowledged that facilitation can improve survival or growth of organisms once they have reached a location under the protective influence of the facilitator [e.g. nurse plant syndrome; Niering et al. (1963), Callaway (1995)]. Facilitation studies generally focus on the number of seedlings that establish within a patch vs. the bare interspaces between patches (Padilla and Pugnaire 2006). Far less attention is given to whether facilitation may enhance the arrival of organisms in such suitable sites. Previous studies found a close correlation between seed and sediment deposition in rivers (Goodson et al. 2003; Gurnell 2007). Further, the sediment retained within stands of the emergent macrophyte Sparganium erectum contained a larger number of viable seeds and more species than unvegetated areas (O’Hare et al. 2012; Gurnell et al. 2013). In our study, which is focused on the dispersal of vegetative fragments rather than seeds, we reveal that submerged aquatic vegetation in the middle of the channel can similarly enhance the arrival and retention of propagules of other species.
Propagule retention represents a suitable proxy for facilitation during plant dispersal and colonization, for a variety of reasons. Once trapped in submerged vegetation, propagules are prevented from being lost at sea or in the river system and are retained in a favourable slow-flow site, indicating a facilitative effect (Callaway 1995). When high flows depress the canopy, propagules retained in the downstream part of the patch might move closer to the streambed where they can re-root (Minckley 1963). Aquatic plant fragments can grow roots for more than 20 cm through the water column and penetrate the substrate (Chadwell and Engelhardt 2008), suggesting another way for fragments to re-establish once they are locally retained by submerged vegetation. Moreover, among all field releases, less than 6% of propagules per species were trapped by emergent riparian vegetation. This occurred when flow deviated laterally around a submerged patch, transporting the propagules towards the banks, where root development in shallow water depth can increase the probability of establishment (Barrat-Segretain et al. 1998). Future studies are needed to measure the retention capacity of Callitriche on its own propagules. Assuming successful establishment after retention, a high trapping ability of Callitriche for its own propagules might be a mechanism decreasing biodiversity, whereas a low trapping ability might promote a more diverse plant community.
Water level fluctuations might also play a role in the retention and further establishment of propagules. Emergent obstacles can be effective retention agents during low flows, but retention is strongly reduced when the obstacles are completely submerged at higher discharges (Engström et al. 2009). Similarly, patches of flexible submerged vegetation bend closer to the bed substrate as discharge increases (Schoelynck et al. 2013; this study), retaining a significantly lower number of propagules at higher velocities in our flume experiments. Propagules trapped during low flows might be released again during high flows, suggesting a stepwise manner of reaching and colonizing new sites (Engström et al. 2009). And as colonization times for macrophyte shoots usually range between 1 and 10 days (Barrat-Segretain et al. 1998, 1999), establishment is likely to be more successful if timing between high flow events is long enough to allow re-rooting of fragments (Riis and Biggs 2003; Riis 2008).
Previous studies found that seed dispersal increased with high flows (Merritt and Wohl 2002), and the number of generative and vegetative propagules increased significantly with discharge (Boedeltje et al. 2004). Thus, there might be a temporal shift between the timing of vegetative dispersal (high flows) and the timing where submerged vegetation is more efficient at trapping (low flows). However, the main constraint for establishment does not seem to be the number of propagules, but rather the very low chance of primary colonization (< 5% of retained shoots; Riis 2008, Fig. 1). Although retention does not necessarily equal establishment, drifting propagules might not be able to root in the sediment without an agent or feature that retains them in one place. Therefore, retention patterns as shown in this study can play a large part in determining where plants can establish.
While our study focused on vegetative fragments, the interaction between vegetation patterns and physical forces is likely to affect the dispersal of other propagules, such as seeds. Although aquatic plants have reduced flowering and seed production under flowing conditions (Sculthorpe 1967; Haslam 1978; Sand-Jensen et al. 1999), low velocity areas can be a source of seeds that then drift towards faster flowing areas of the river. The transport of seeds and other propagules is likely influenced by differences in size, buoyancy (Cellot et al. 1998; Merritt and Wohl 2002; Chang et al. 2008) and other physical traits that determine their transport at the surface, as suspended load or in bedload (Carthey et al. 2016). Since the seeds of many aquatic plants have limited buoyancy (Boedeltje et al. 2003) and have been found to follow sediment deposition patterns (Gurnell 2007; O’Hare et al. 2012), seed retention might be expected to increase with the amount of vegetation cover in the stream.
Bio-physical stress divergence and implications for abiotic dispersal vectors
Our study reveals that vegetation patchiness due to flow divergence feedbacks can create optimal conditions for retention of dispersal units (e.g. vegetative fragments). As such, it reinforces the importance of foundation species in creating heterogeneity and habitats for many other species (Dayton 1972; Jones et al. 1994). Vegetative fragments provide aquatic plants with opportunities for long-distance dispersal (Johansson and Nilsson 1993; Boedeltje et al. 2003) and our study shows that propagule traits, hydrodynamic stress, and pre-existing vegetation cover interact to determine the dispersal distance of propagules in a stream landscape. Propagules of G. densa and B. erecta have important traits for effective dispersal in water, namely high floating capacity and viability for months in water (Barrat-Segretain et al. 1998; Sarneel 2013). In contrast, E. nuttallii is neutrally buoyant and fragmented easily during our experiment, traits which are likely to enhance its propagation and invasive ability (Cook and Urmi-König 1985; Barrat-Segretain et al. 2002; Thomaz et al. 2015).
The interplay of water movement and the spatial patterning of vegetation has additional feedbacks on propagule retention. The increase in vegetation abundance at the peak of the growing season might provide species with the opportunity for long distance dispersal [supporting high gene flow and longitudinal connectivity; Nilsson et al. (2010); Soomers et al. (2013)]. In contrast, a patchy vegetation arrangement (as found early in the growing season) allows more than one species to be retained in suitable low-flow areas where they can establish. These findings on the arrival of propagules in suitable locations support the hypothesis of facilitation between species (Callaway 1995; Bruno et al. 2003) and directed dispersal in aquatic plants (Soons et al. 2017). As water is a very common dispersal vector for plants (Nilsson et al. 2010) and animals [e.g. passive drift of motile invertebrate fauna or larvae of sessile organisms; Malmqvist (2002)] in both marine and freshwater environments, the effects of self-organized patterning on dispersal and retention are likely to affect a large number of species at different trophic levels within a community.
Beyond aquatic ecosystems, similar processes may be generalized to other self-organized environments where species have patchy distributions. In terrestrial environments, such as grasslands, prairies or arid ecosystems, patchy vegetation creates a mosaic of suitable and unsuitable sites for establishment (Aerts et al. 2006; Pueyo et al. 2008). Although the stress divergence feedback may involve other dispersal vectors (e.g. wind), facilitative interactions occurring in this stage are in a similar way crucial for colonization. These bio-physical interactions have a strong spatial component, which argues for landscape-scale processes like dispersal to be better investigated in future studies.
Conclusions
Our study extends the body of literature on both self-organized pattern formation and facilitation in natural communities, by linking these processes at the landscape scale. Whereas previous studies have focused on local amelioration of physical conditions by ecosystem engineers, we show that the stress divergence mechanisms underlying spatial pattern formation promote facilitation processes. That is, when facilitation is mediated by a pattern-forming species (a submerged aquatic macrophyte in our study), the self-organizing feedbacks underlying these patterns are crucial in maintaining the facilitative effects. If the spatial pattern is absent, the facilitation effect is also lost. Hence, river restoration and management must consider bio-physical feedbacks underlying spatial pattern formation with the potential for further research to specify an optimum patch configuration promoting flow and sediment conveyance whilst maintaining high biodiversity.
Data Availabitiy
Data associated with this manuscript will be available from https://doi.org/10.4121/uuid:ce95f637-3487-4b24-81d4-2549c81e0ac0.
References
Aerts R, Maes W, November E, Behailu M, Poesen J, Deckers J, Hermy M, Muys B (2006) Surface runoff and seed trapping efficiency of shrubs in a regenerating semiarid woodland in northern Ethiopia. CATENA 65:61–70. https://doi.org/10.1016/j.catena.2005.09.004
Aguiar MR, Sala OE (1997) Seed distribution constrains the dynamics of the Patagonian steppe. Ecology 78:93–100
Barrat-Segretain M-H, Bornette G, Hering-Vilas-Bôas A (1998) Comparative abilities of vegetative regeneration among aquatic plants growing in disturbed habitats. Aquat Bot 60:201–211
Barrat-Segretain M-H, Henry CP, Bornette G (1999) Regeneration and colonization of aquatic plant fragments in relation to the disturbance frequency of their habitats. Archiv für Hydrobiologie 145:111–127
Barrat-Segretain M-H, Elger A, Sagnes P, Puijalon S (2002) Comparison of three life-history traits of invasive Elodea canadensis Michx. and Elodea nuttallii (Planch.) H. St John. Aquat Bot 74:299–313
Bertness MD, Callaway R (1994) Positive interactions in communities. Trends Ecol Evol 9:191–193
Bertness MD, Leonard GH (1997) The role of positive interactions in communities: lessons from intertidal habitats. Ecology 78:1976–1989
Bertness MD, Leonard GH, Levine JM, Schmidt PR, Ingraham AO (1999) Testing the relative contribution of positive and negative interactions in rocky intertidal communities. Ecology 80:2711–2726
Boedeltje G, Bakker JP, Bekker RM, Van Groenendael JM, Soesbergen M (2003) Plant dispersal in a lowland stream in relation to occurrence and three specific life-history traits of the species in the species pool. J Ecol 91:855–866
Boedeltje G, Bakker JP, Ten Brinke A, Van Groenendael JM, Soesbergen M (2004) Dispersal phenology of hydrochorous plants in relation to discharge, seed release time and buoyancy of seeds: the flood pulse concept supported. J Ecol 92:786–796
Bornette G, Puijalon S (2011) Response of aquatic plants to abiotic factors: a review. Aquat Sci 73:1–14
Borthagaray AI, Carranza A (2007) Mussels as ecosystem engineers: their contribution to species richness in a rocky littoral community. Acta Oecol 31:243–250
Bouma T, De Vries M, Low E, Peralta G, Tánczos I, van de Koppel J, Herman PMJ (2005) Trade-offs related to ecosystem engineering: a case study on stiffness of emerging macrophytes. Ecology 86:2187–2199
Bouma T, Van Duren L, Temmerman S, Claverie T, Blanco-Garcia A, Ysebaert T, Herman P (2007) Spatial flow and sedimentation patterns within patches of epibenthic structures: combining field, flume and modelling experiments. Cont Shelf Res 27:1020–1045
Bouma T, Friedrichs M, Klaassen P, van Kasenbeeck B, Brun F, Temmerman S, Van Katwijk M, Graf G, Herman P (2009) Effects of shoot stiffness, shoot size and current velocity on scouring sediment from around seedlings and propagules. Mar Ecol Prog Ser 388:293–297
Brooker RW, Maestre FT, Callaway RM, Lortie CL, Cavieres LA, Kunstler G, Liancourt P, Tielbörger K, Travis JM, Anthelme F (2008) Facilitation in plant communities: the past, the present, and the future. J Ecol 96:18–34
Bruno JF, Stachowicz JJ, Bertness MD (2003) Inclusion of facilitation into ecological theory. Trends Ecol Evol 18:119–125
Callaway RM (1994) Facilitative and interfering effects of Arthrocnemum subterminale on winter annuals. Ecology 75:681–686
Callaway RM (1995) Positive interactions among plants. Bot Rev 61:306–349
Callaway RM (2007) Positive interactions and interdependence in plant communities. Springer, Dordrecht, The Netherlands
Carpenter SR, Lodge DM (1986) Effects of submersed macrophytes on ecosystem processes. Aquat Bot 26:341–370
Carthey AJ, Fryirs KA, Ralph TJ, Bu H, Leishman MR (2016) How seed traits predict floating times: a biophysical process model for hydrochorous seed transport behaviour in fluvial systems. Freshw Biol 61:19–31
Cellot B, Mouillot F, Henry CP (1998) Flood drift and propagule bank of aquatic macrophytes in a riverine wetland. J Veg Sci 9:631–640
Chadwell TB, Engelhardt KA (2008) Effects of pre-existing submersed vegetation and propagule pressure on the invasion success of Hydrilla verticillata. J Appl Ecol 45:515–523
Chang E, Veeneklaas R, Buitenwerf R, Bakker J, Bouma T (2008) To move or not to move: determinants of seed retention in a tidal marsh. Funct Ecol 22:720–727
Cook CD, Urmi-König K (1985) A revision of the genus Elodea (Hydrocharitaceae). Aquat Bot 21:111–156
Cornacchia L, Licci S, van de Koppel J, van der Wal D, Wharton G, Puijalon S, Bouma TJ (2016) Flow velocity and morphology of a submerged patch of the aquatic species Veronica anagallis-aquatica L. In: Rowiński PM, Marion A (eds) Hydrodynamic and mass transport at freshwater aquatic interfaces. Springer, Cham, pp 141–152
Cornacchia L, van de Koppel J, van der Wal D, Wharton G, Puijalon S, Bouma TJ (2018) Landscapes of facilitation: how self-organized patchiness of aquatic macrophytes promotes diversity in streams. Ecology 99:832–847. https://doi.org/10.1002/ecy.2177
Cotton J, Wharton G, Bass J, Heppell C, Wotton R (2006) The effects of seasonal changes to in-stream vegetation cover on patterns of flow and accumulation of sediment. Geomorphology 77:320–334
Dayton PK (1972) Toward an understanding of community resilience and the potential effects of enrichments to the benthos at McMurdo Sound, Antarctica. In: Proceedings of the colloquium on conservation problems in Antarctica, 1972. Allen Press Lawrence, KS, pp 81–96
Demars B, Gornall R (2003) Identification of British species of Callitriche by means of isozymes. Watsonia 24:389–400
Engström J, Nilsson C, Jansson R (2009) Effects of stream restoration on dispersal of plant propagules. J Appl Ecol 46:397–405
Eppinga MB, De Ruiter PC, Wassen MJ, Rietkerk M (2009) Nutrients and hydrology indicate the driving mechanisms of peatland surface patterning. Am Nat 173:803–818
Follett EM, Nepf HM (2012) Sediment patterns near a model patch of reedy emergent vegetation. Geomorphology 179:141–151
Fonseca MS, Zieman JC, Thayer GW, Fisher JS (1983) The role of current velocity in structuring eelgrass (Zostera marina L.) meadows Estuarine. Coast Shelf Sci 17:367–380
Gambi MC, Nowell AR, Jumars PA (1990) Flume observations on flow dynamics in Zostera marina (eelgrass) beds. Mar Ecol Prog Ser 61:159–169
Gillis L, Bouma T, Kiswara W, Ziegler A, Herman P (2014) Leaf transport in mimic mangrove forests and seagrass beds. Mar Ecol Prog Ser 498:95–102
Goodson J, Gurnell A, Angold P, Morrissey I (2001) Riparian seed banks: structure, process and implications for riparian management. Prog Phys Geogr 25:301–325
Goodson J, Gurnell A, Angold P, Morrissey I (2003) Evidence for hydrochory and the deposition of viable seeds within winter flow-deposited sediments: the River Dove, Derbyshire. UK River Res Appl 19:317–334
Granata T, Serra T, Colomer J, Casamitjana X, Duarte C, Gacia E (2001) Flow and particle distributions in a nearshore seagrass meadow before and after a storm. Mar Ecol Progress Ser 218:95–106
Gurnell AM (2007) Analogies between mineral sediment and vegetative particle dynamics fluvial systems. Geomorphology 89:9–22. https://doi.org/10.1016/j.geomorph.2006.07.012
Gurnell AM, O’Hare MT, O’Hare JM, Scarlett P, Liffen TM (2013) The geomorphological context and impact of the linear emergent macrophyte, Sparganium erectum L.: a statistical analysis of observations from. Br Rivers Earth Surf Process Landf 38:1869–1880
Haslam SM (1978) River plants: the macrophyte vegetation of watercourses. Cambridge Univer Press, Cambridge
Hiemstra CA, Liston GE, Reiners WA (2002) Snow redistribution by wind and interactions with vegetation at upper treeline in the Medicine Bow Mountains, Wyoming, USA. Arct Antarctic Alp Res 34:262–273
Johansson ME, Nilsson C (1993) Hydrochory, population dynamics and distribution of the clonal aquatic plant Ranunculus lingua. J Ecol 81:81–91
Jones CG, Lawton JH, Shachak M (1994) Organisms as ecosystem engineers. In: Samson FB, Knopf FL (eds) Ecosystem management. Springer, New York, NY, pp 130–147
Kondziolka JM, Nepf HM (2014) Vegetation wakes and wake interaction shaping aquatic landscape evolution Limnology and Oceanography. Fluids Environ 4:106–119
Larsen LG, Harvey JW (2010) How vegetation and sediment transport feedbacks drive landscape change in the Everglades and wetlands worldwide. Am Nat 176:E66–E79
Larsen LG, Harvey JW, Crimaldi JP (2007) A delicate balance: ecohydrological feedbacks governing landscape morphology in a lotic peatland. Ecol Monogr 77:591–614
Licci S, Delolme C, Marmonier P, Philippe M, Cornacchia L, Gardette V, Bouma T, Puijalon S (2016) Effect of aquatic plant patches on flow and sediment characteristics: the case of Callitriche platycarpa and Elodea nuttallii. In: Rowiński M, Marion P A (eds) Hydrodynamic and mass transport at freshwater aquatic interfaces: 34th International School of Hydraulics. Springer International Publishing, Cham, pp 129–140. https://doi.org/10.1007/978-3-319-27750-9_11
Malmqvist B (2002) Aquatic invertebrates in riverine landscapes. Freshw Biol 47:679–694
McIntire EJ, Fajardo A (2014) Facilitation as a ubiquitous driver of biodiversity. New Phytol 201:403–416
McKee KL, Rooth JE, Feller IC (2007) Mangrove recruitment after forest disturbance is facilitated by herbaceous species in the Caribbean. Ecol Appl 17:1678–1693. https://doi.org/10.1890/06-1614.1
Merritt DM, Wohl EE (2002) Processes governing hydrochory along rivers: hydraulics, hydrology, and dispersal phenology. Ecol Appl 12:1071–1087
Minckley W (1963) The ecology of a spring stream: Doe Run, Meade County, Kentucky. Wildl Monogr 11:3–124
Niering W, Whittaker R, Lowe C (1963) The saguaro: a population in relation to environment. Science 142:15–23
Nilsson C, Brown RL, Jansson R, Merritt DM (2010) The role of hydrochory in structuring riparian and wetland vegetation. Biol Rev 85:837–858
O’Hare JM, O’Hare MT, Gurnell AM, Scarlett PM, Liffen T, McDonald C (2012) Influence of an ecosystem engineer, the emergent macrophyte Sparganium erectum, on seed trapping in lowland rivers and consequences for landform colonisation. Freshw Biol 57:104–115
Padilla FM, Pugnaire FI (2006) The role of nurse plants in the restoration of degraded environments. Front Ecol Environ 4:196–202
Peterson JM, Bell SS (2012) Tidal events and salt-marsh structure influence black mangrove (Avicennia germinans) recruitment across and ecotone. Ecology 93:1648–1658
Pueyo Y, Kefi S, Alados C, Rietkerk M (2008) Dispersal strategies and spatial organization of vegetation in arid ecosystems. Oikos 117:1522–1532
R Core Team (2015) R: a language and environment for statistical computing (Version 3.1. 2): R Foundation for Statistical Computing
Rabinowitz D (1978) Dispersal properties of mangrove propagules. Biotropica 10:47–57
Rietkerk M, van de Koppel J (2008) Regular pattern formation in real ecosystems. Trends Ecol Evol 23:169–175
Riis T (2008) Dispersal and colonisation of plants in lowland streams: success rates bottlenecks. Hydrobiologia 596:341–351
Riis T, Biggs BJF (2003) Hydrologic and hydraulic control of macrophyte establishment and performance in streams. Limnol Oceanogr 48:1488–1497
Riis T, Sand-Jensen K (2006) Dispersal of plant fragments in small streams. Freshw Biol 51:274–286
Sand-Jensen K (1998) Influence of submerged macrophytes on sediment composition and near-bed flow in lowland streams. Freshw Biol 39:663–679
Sand-Jensen K, Mebus JR (1996) Fine-scale patterns of water velocity within macrophyte patches in streams. Oikos 76:169–180
Sand-Jensen K, Pedersen ML (2008) Streamlining of plant patches in streams. Freshw Biol 53:714–726
Sand-Jensen K, Andersen K, Andersen T (1999) Dynamic properties of recruitment, expansion and mortality of macrophyte patches in streams. Int Rev Hydrobiol 84:497–508
Sarneel J (2013) The dispersal capacity of vegetative propagules of riparian fen species. Hydrobiologia 710:219–225
Säumel I, Kowarik I (2013) Propagule morphology and river characteristics shape secondary water dispersal in tree species. Plant Ecol 214:1257–1272
Schoelynck J, De Groote T, Bal K, Vandenbruwaene W, Meire P, Temmerman S (2012) Self-organised patchiness and scale-dependent bio-geomorphic feedbacks in aquatic river vegetation. Ecography 35:760–768
Schoelynck J, Meire D, Bal K, Buis K, Troch P, Bouma T, Meire P, Temmerman S (2013) Submerged macrophytes avoiding a negative feedback in reaction to hydrodynamic stress. Limnol Ecol Manag Inland Waters 43:371–380
Sculthorpe CD (1967) Biology of aquatic vascular plants. St. Martin’s, New York
Soomers H, Karssenberg D, Soons MB, Verweij PA, Verhoeven JT, Wassen MJ (2013) Wind and water dispersal of wetland plants across fragmented landscapes. Ecosystems 16:434–451
Soons MB, Groot GA, Cuesta Ramirez MT, Fraaije RG, Verhoeven JT, Jager M (2017) Directed dispersal by an abiotic vector: Wetland plants disperse their seeds selectively to suitable sites along the hydrological gradient via water. Funct Ecol 31:499–508
Temmerman S, Bouma T, Van de Koppel J, Van der Wal D, De Vries M, Herman P (2007) Vegetation causes channel erosion in a tidal landscape. Geology 35:631–634
Thomaz SM, Mormul RP, Michelan TS (2015) Propagule pressure, invasibility of freshwater ecosystems by macrophytes and their ecological impacts: a review of tropical freshwater ecosystems. Hydrobiologia 746:39–59
Turner T (1983) Facilitation as a successional mechanism in a rocky intertidal community. Am Nat 121:729–738
van de Koppel J, Altieri AH, Silliman BR, Bruno JF, Bertness MD (2006) Scale-dependent interactions and community structure on cobble beaches. Ecol Lett 9:45–50
Van Der Heide T, Bouma TJ, Van Nes EH, Van De Koppel J, Scheffer M, Roelofs JG, Van Katwijk MM, Smolders AJ (2010) Spatial self-organized patterning in seagrasses along a depth gradient of an intertidal ecosystem. Ecology 91:362–369
Van der Stocken T, De Ryck D, Vanschoenwinkel BB, Bouma T, Dahdouh-Guebas F, Koedam N (2015) Impact of landscape structure on propagule dispersal in mangrove forests. Mar Ecol Prog Ser 524:95–106
Vandenbruwaene W, Temmerman S, Bouma T, Klaassen P, De Vries M, Callaghan D, Van Steeg P, Dekker F, Van Duren L, Martini E (2011) Flow interaction with dynamic vegetation patches: implications for biogeomorphic evolution of a tidal landscape. J Geophys Res Earth Surf 116:F01008
Weerman EJ, Van de Koppel J, Eppinga MB, Montserrat F, Liu QX, Herman PM (2010) Spatial self-organization on intertidal mudflats through biophysical stress divergence. Am Nat 176:E15–E32
Wharton G, Cotton JA, Wotton RS, Bass JA, Heppell CM, Trimmer M, Sanders IA, Warren LL (2006) Macrophytes and suspension-feeding invertebrates modify flows and fine sediments in the Frome and Piddle catchments, Dorset (UK). J Hydrol 330:171–184
Acknowledgements
This work was supported by the Research Executive Agency, through the Seventh Framework Programme of the European Union, Support for Training and Career Development of Researchers (Marie Curie - FP7-PEOPLE-2012-ITN), which funded the Initial Training Network (ITN) HYTECH ‘Hydrodynamic Transport in Ecologically Critical Heterogeneous Interfaces’, N.316546. We thank the CNR (Compagnie Nationale du Rhône) for providing access to field sites. We thank the Associate Editor and two anonymous referees for their helpful comments that have improved the quality of our manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cornacchia, L., van der Wal, D., van de Koppel, J. et al. Flow-divergence feedbacks control propagule retention by in-stream vegetation: the importance of spatial patterns for facilitation. Aquat Sci 81, 17 (2019). https://doi.org/10.1007/s00027-018-0612-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00027-018-0612-1