Abstract
Eastern hemlock trees (Tsuga canadensis [L.] Carr.) often dominate riparian vegetation of central Appalachian headwater streams, and the invasive hemlock woolly adelgid (Adelges tsugae Annand; HWA) has decimated hemlock stands in this region. Although research concerning HWA impacts on soil, hydrology, and forest structure is emerging, associated changes in stream structure and function are not as well documented. We quantified HWA-invasion effects on benthic macroinvertebrate communities in 21 headwater streams across Ohio, West Virginia, and Virginia (USA) representing unimpacted, moderate invasion, and severe invasion, respectively. We observed differences in benthic macroinvertebrate community composition; severely invaded sites exhibited the highest diversity, whereas moderate sites had the lowest diversity. The composition of macroinvertebrate functional feeding groups exhibited shifts as well. For example, the relative abundance of herbivorous invertebrates increased from 4% (± 3%) at unimpacted sites to 23% (± 14%) at severely impacted sites. Changes in macroinvertebrate density, diversity, and functional-group composition were associated with sediment grainsize distribution (proportion bedrock and D84), large-wood characteristics (volume and density), and nutrient concentrations (PO4 and NH4). Our results suggest that in-stream physical and chemical alterations associated with HWA-invasion and subsequent hemlock decline are associated with changes in stream invertebrate diversity and trophic relationships. We demonstrate how a pervasive terrestrial invader can influence in-stream biotic communities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Invasive species can strongly influence, and potentially even restructure, ecosystems (Vilà et al. 2011; Simberloff et al. 2013). In aquatic ecosystems, alien invasive species have been implicated as an important factor in altering ecosystem functions [e.g., nutrient cycling (McDowell et al. 2017) and primary production; (Schindler et al. 2001)] and biodiversity (Rahel 2002; Dextrase and Mandrak 2006). Riparian zones adjacent to streams often support high abundances of invasive species, potentially due to high spatial and temporal habitat heterogeneity (Malanson 1993; Naiman and Decamps 1997; Sabo et al. 2005) and disturbance regimes (DeFerrari and Naiman 1994; Nakamura et al. 2000; Davies et al. 2005). Owing to the close ecological relationship between aquatic and riparian zones (Hynes 1975; Polis et al. 1997; Naiman et al. 2010), species invasions within the riparian zone can impact in-stream biota (Thompson and Townsend 2003; Kennedy and Hobbie 2004; Mineau et al. 2012). For example, caddisfly larvae fed a diet of invasive giant reed (Arundo donax L.) exhibited decreased growth rates compared to diets of native plants (Kennedy and Hobbie 2004). Additionally, highly productive riparian plant invaders can alter invertebrate trophic structure by shifting dominant basal aquatic resources from algae to detritus (Levin et al. 2006).
The hemlock woolly adelgid (HWA; Adelges tsugae Annand) is an insect pest that has invaded forests across the eastern United States (Evans and Gregoire 2007), leading to a pattern of death and decline of eastern hemlock (Tsuga canadensis [L.] Carr.) (Ward et al. 2004). In the central Appalachian Mountains, many riparian forests are characterized by eastern hemlock (Ellison et al. 2005), which are common in areas with low-nutrient and high-moisture soil (Rogers 1978). Replacement forests are predicted to comprise either previously occurring rhododendron (Rhododendron maximum L.) or mixed-hardwoods (Ford et al. 2012). Despite this widespread invasion, current understanding of the effects of riparian hemlock decline on the physical and chemical attributes of adjacent streams is currently incomplete and available evidence is somewhat contradictory.
Compared to deciduous forests, hemlock forests provide more stable thermal and hydrologic regimes (Snyder et al. 2002; Ford and Vose 2007; Brantley et al. 2013). For instance, Snyder et al. (1999) suggest that hemlock decline could lead to more frequent complete or partial stream dry-downs, whereas other studies predict that hemlock decline may result in long-term increases in discharge and depressed diurnal amplitudes of streamflow (Ford and Vose 2007; Kim et al. 2017; but also see Siderhurst et al. 2010). Physical habitat (microhabitat diversity) has not been found to be different between hemlock and deciduous forest streams (Snyder et al. 2002), although hemlock decline may be linked to an increase in large-wood loading in streams with advanced HWA infestation (Evans et al. 2012). Hemlocks also prevent more sunlight from reaching the stream substrate than mixed deciduous forests, suppressing benthic primary productivity (Hadley 2000; Rowell and Sobczak 2008). In general, water chemistry (including dissolved oxygen, pH, specific conductance, and some nutrients) is not different between hemlock and deciduous forest streams (Snyder et al. 2002), which is supported by the lack of long-term changes in water chemistry found in response to hemlock decline (Roberts et al. 2009). However, changes in nitrogen (N) mineralization, N turnover, and nitrification in riparian soils indicate that hemlock decline may facilitate increased N leaching to adjacent streams (Jenkins et al. 1999), at least until new forest growth can use the N (Cessna and Nielsen 2012).
In addition to these differences in physical and chemical characteristics, streams draining hemlock riparian forests tend to support different invertebrate communities than similar streams draining mixed hardwood deciduous forests (Snyder et al. 2002; Willacker et al. 2009; Adkins and Rieske 2015b). Hemlock streams have been found to exhibit more collector–gatherers and fewer shredders and grazing algivores than deciduous streams, although this can vary seasonally (Snyder et al. 2002; Willacker et al. 2009; Adkins and Rieske 2015a). Benthic invertebrate communities influenced by hemlocks tend to have higher diversity and species evenness, and lower abundance (Willacker et al. 2009), again supporting the notion that streams draining riparian areas with hemlocks harbor distinct benthic communities. Snyder et al. (2002) also suggest that HWA-induced hemlock decline could lead to reductions in both local (i.e., alpha) and landscape-level (i.e., gamma) diversity of benthic macroinvertebrate assemblages. Despite these studies that have compared mixed-hardwood forests with paired hemlock streams, the impacts of hemlock decline associated with HWA on aquatic biota remain largely unresolved.
Our objectives were to quantify and explain benthic community density, diversity, and composition at a suite of central Appalachian streams in Ohio, West Virginia, and Virginia (USA) that represent categories of hemlock-decline severity from HWA infestation. We predicted that hemlock decline would lead to lower overall diversity and density of aquatic macroinvertebrates, but an increased relative abundance of shredders and grazers owing to an expected shift in basal resources towards autochthony. To identify potential mechanisms driving changes in aquatic macroinvertebrate communities, we also considered potential relationships between nutrients, large wood, and stream geomorphic characteristics.
Methods
Study area
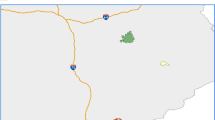
Our study was conducted at 21 sites across Appalachian regions of Ohio, West Virginia, and Virginia (Fig. 1). All sites were headwater streams (drainage area < 20 km2) and their adjacent forests were at least partially dominated by eastern hemlock prior to HWA invasion. Detailed descriptions of these sites are available in Costigan et al. (2015) and are presented here in brief. All sites were situated in 2nd- or 3rd-growth forests (except Carnifex Ferry 1, which is an old-growth hemlock forest). Streams were circumneutral in pH (7.0 ± 0.70) with generally low conductivity (0.08 ± 0.07 mS cm−2) and dissolved oxygen (DO) near saturation (89 ± 16%); see “Stream chemical parameters”. We selected sites representing a chronosequence of hemlock decline, ranging from invasions that initially occurred several decades ago to uninvaded (Table 1). We chose to use a chronosequence approach because the invasion and subsequent ecosystem impacts of HWA occur over relatively long-time scales (i.e., decades), allowing us to substitute space for time (Pickett 1989). Hemlock decline is evident primarily through canopy health (Orwig and Foster 1998) and is most strongly associated with the number of years of HWA presence, incident radiation, elevation, and percentage of eastern hemlock in the overstory (Martin and Goebel 2012, 2013). Using these factors, Martin and Goebel (2012, 2013) assigned Hemlock Decline Index (HDI) values ranging from 0 to 5 (0 no decline; 5 complete decline) to the 21 sites of the current study. Here, we collapsed these five indices into three Hemlock Decline Categories (HDCs): HDI 0 = HDC 1 = no decline, HDI 1, 2, and 3 = HDC 2 = moderate decline, HDI 4 and 5 = HDC 3 = severe decline. Within each HDC, there were seven study streams.
Map showing the locations and Hemlock Decline Categories (HDCs) of study sites across Appalachian Mountain regions of Virginia, West Virginia, and Ohio. Open triangles represent sites with no previous hemlock woolly adelgid invasion (i.e., reference); shaded/gray triangles represent moderate invasion; and filled/black triangles represent severe invasion. Insets show the relative locations of the clustered Ohio sites
The Ohio sites were located within the unglaciated Allegheny Plateau on multiple protected lands (Table 1) and were the northernmost of all sites. Initially selected as reference sites, HWA has since been found in Ohio counties Hocking and Jackson (Oh. Code §901:5–48), in which several of the reference (HDC1) sites were located. West Virginia sites were located in the Appalachian Plateau, where streams generally exhibited moderate decline severity (HDC2). Virginia sites were located within the Valley and Ridge province and included the most southern study sites; these were largely severely invaded sites (HDC3).
Study streams exhibited narrow bankfull widths (0.5–15.5, x̅ = 5.8 m), were moderately steep (0.058 ± 0.052 m m−1) with confined valleys (43 ± 44 m), and exhibited a mix of intermittent and perennial flows (Costigan et al. 2015). Geophysical, chemical, and biological parameters were measured over stream sites (i.e., reaches) of 90–190 m, corresponding with approximately 20 times bankfull width (Harrelson et al. 1994; Kondolf and Micheli 1995). Relevant physical stream characteristics (Table 2) were measured as part of a companion study during low flows in the summers of 2012 and 2013: see Costigan et al. (2015) for field methods and statistical analysis detailing streambed substrate, channel morphology, and large-wood metrics.
Stream chemical parameters
Temperature (°C), DO (%), conductivity (mS cm−2), and pH were measured at nine locations within each stream study site with a multiparameter sonde (YSI 600R, Yellow Springs, Ohio, USA), beginning at the downstream end and selecting sampling locations representative of the major flow habitats (riffle, run, and pool) found within each site. Sonde measurements were made in July or August 2012 for MB, CFX1, CFX2, KR, and SF; May, June, or July for all sites in 2013; and early August or September 2014 for all sites except LK2 and LK3, which were dry at the time of 2014 water sampling. Unfiltered streamwater samples (500 mL) were collected with opaque polyethylene bottles and stored on ice until analyzed by the STAR Laboratory (Wooster, Ohio) for total dissolved solids (TDS) and nutrient concentrations: total nitrogen (TN), total phosphorus (TP), phosphate (PO4), nitrate (NO3), ammonium (NH4). These grab samples were collected by rinsing out the bottles with streamwater three times and filling the bottle with a composite sample from three locations evenly spread along the study site. All grab samples were collected in August, September, or early October 2014.
Benthic macroinvertebrates
Following Sullivan et al. (2004), benthic macroinvertebrates were collected using a Surber sampler by agitating the substrate within a 0.09-m2 frame for 60 s and subsequently removing all invertebrates from the 500-µm mesh collecting net. Surber samples were collected from the principal flow habitats from each study site for a total of three samples per stream. Invertebrates were stored in 70% ethanol and subsequently enumerated. Macroinvertebrates were collected at least once during summers 2012–2014 at each site. All benthic invertebrates collected in 2013—because the Skidmore Fork site was entirely dry during sampling in 2013, the 2012 sample was used here; likewise, the Kephart Run sample used was from 2014—were identified to the lowest taxonomic resolution possible by Rhithron Associates, Inc. (Missoula, Montana) most frequently to genus (86.7%) and species (18.5%). Insects (96.8% of individuals) were then assigned functional feeding groups (FFG) according to Poff et al. (2006), Vieira et al. (2006), and Merritt et al. (2008): collector–grazer, collector–filterer, predator, shredder, and herbivore (including scrapers, piercers, and grazers).
Numerical and statistical analysis
We calculated Shannon–Weiner Diversity Index (H′; Shannon and Weaver 1949; Smith and Wilson 1996), taxon richness (S), taxonomic evenness (J; Pielou 1966), and Simpson’s Diversity Index (D; Simpson 1949) for each site using the diversity function in the vegan package (Oksanen et al. 2017). In the Shannon–Weiner Diversity Index, a greater S and J contribute to an increased H′:
where pi is the proportion of the total sample represented by taxon i. Taxon evenness (J) is the relative abundance of taxon within an assemblage and ranges from 0 (dissimilar) to 1 (highly similar):
where H′max is the natural log of taxon richness (S). Simpson’s Diversity Index (D) is less sensitive to density effects than the Shannon–Weiner Index; here we calculated 1 − D so that higher values correspond to greater diversity (i.e., akin to H′):
We used multivariate analysis of variance (MANOVA) to test for potential differences in density, diversity, relative abundance of benthic insect FFGs (i.e., proportions of total community), and water-chemistry characteristics (temperature, DO, pH, conductivity, and the biologically relevant nutrients NO3 and PO4) by HDCs. To improve normality and homogeneity of variance, log (conductivity), square-root (NO3 and PO4), logit (proportions of FFGs collector–filterer, herbivore, and shredder), and exponential (pH and 1 − D) transformations were applied. Additionally, some variables (e.g., temperature, taxonomic richness, H′, proportion collector–filterer) were removed to prevent variable collinearity as required by MANOVA assumptions, although two minor multivariate outliers for the water chemistry and five multivariate outliers for invertebrate measures were not removed to avoid reducing sample size. Following MANOVA, we used univariate analysis of variance (ANOVA) and post-hoc Tukey’s HSD tests to explore patterns between individual responses and HDCs. Subsequently, we used non-metric multidimensional scaling (NMS; metaMDS function in the vegan package) followed by permutational multivariate analysis of variance using distance matrices (PERMANOVA; adonis function in the vegan package) to compare benthic insect community composition among HDCs (Oksanen et al. 2017).
To investigate the influences of hemlock decline and physicochemical factors on the density, evenness, and diversity of benthic invertebrates, we used a model-selection approach based on least-squares regression and Akaike Information Criterion adjusted for small sample sizes (ΔAICc). In this way, we evaluated the relative support of each individual model in the set of candidate models, and included the null model for comparison. Predictor variables in the models included watershed-level characteristics (drainage area, elevation, hemlock basal area, total basal area), site-level characteristics (channel width, channel slope, proportion bedrock [BR], 84th percentile bed-material size [D84], density of large wood, volume of large wood), water temperature, and water chemistry (conductivity, dissolved oxygen, pH, TN, TP, PO4, NO3, NH4, TDS). Highly supported potential models (models with ΔAICc ≤ 2) were retained and their Akaike weights (ωi) were calculated to identify the probability that a model was the best-supported among all candidate models in the set. If assumptions for linear models were not met, appropriate transformations were applied. Highly correlated variables (ǀrǀ ≥ 0.80: drainage area, elevation, TP, TDS, temperature, NO3, channel width, and dissolved oxygen) were identified using the vifstep function in the usdm package (Naimi et al. 2014, as recommended by; Feld et al. 2016) and were not included in the same models (Burnham and Anderson 2004). Remaining transformed, non-collinear variables were standardized using the scale function. All statistical tests were run using R (version 3.3.0; R Project for Statistical Computing, Vienna, Austria). p < 0.05 was used as the threshold for statistical significance.
Results
Stream nutrient and chemical characteristics
Stream nutrient and chemical characteristics are summarized in Table 3. Multivariate ANOVA indicated differences among HDCs for nutrients but not for other parameters. Conductivity, pH, and DO were not different among HDCs (conductivity: F = 1.18, p = 0.213; pH: F = 0.12, p = 0.885; DO: F = 0.67, p = 0.526). In contrast, PO4 was greatest at HDC1 sites (0.015 ± 0.005 mg L−1) and was not different between HDC2 (0.006 ± 0.004 mg L−1) and HDC3 (0.008 ± 0.003 mg L−1) sites (Tukey’s HSD: p = 0.003). Similarly, NO3 was also greatest at HDC1 sites (0.42 ± 0.32 mg L−1), although it was lowest at HDC2 (0.08 ± 0.11 mg L−1) while HDC3 (0.21 ± 0.2 mg L−1) was not different from the other categories (Tukey’s HSD: p = 0.036).
Macroinvertebrate assemblages
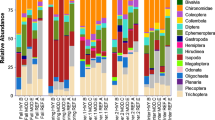
We collected 2157 invertebrates representing 122 genera across the 21 sites. The most common families were Chironomidae (5391 ind. m−2), Heptageniidae (2293 ind. m−2), and Leuctridae (2065 ind. m−2). The most abundant orders were Diptera, Ephemeroptera, Trichoptera, and Plecoptera, in decreasing abundance, respectively; these orders were represented at nearly all sites. See Online Resource 1 for complete presentation of aquatic invertebrate data.
The two-dimensional NMS ordination based on generic-level identification of invertebrates showed differences in community composition among HDCs (Fig. 2; PERMANOVA: p = 0.004). Reference sites (HDC1) had high relative abundances of the collector–filterer Microtendipes sp. and the collector–gatherers Tanytarsus sp. and Chironomus (12%, 11%, and 8% of insects by number, respectively). The most common genera at the HDC2 sites included the shredder Amphinemura sp. and the collector–gatherer Eurylophella sp. (12% and 11% of insects by number, respectively). At HDC3 sites, a wider range of FFGs were common: Epeorus sp. (herbivore), Dolophilodes sp. (collector–filterer), Ceratopsyche sp. (collector–filterer), Nigronia sp. (predator), and Cheumatopsyche sp. (collector–filterer) (10%, 9%, 5%, 4%, and 3% of insects by number, respectively). The shredder Leuctra sp. was among the most common genera across all three decline classes (HDC1 = 11%, HDC2 = 15%, HDC3 = 6%) and Ephemerella sp. was common at HDC2 and HDC3 sites (HDC2 = 9%, HDC3 = 8%) (Table 4).
Non-metric multidimensional scaling ordination of 21 sites based on benthic invertebrate community composition (stress value = 0.204). Reference sites (i.e., HDC1) are displayed as circles, sites with moderate decline (HDC2) are displayed as triangles, and sites with severe decline (HDC3) are displayed as crosses. Ellipses indicate location of centroids by decline category (α = 95%)
There were 69 individuals not classified by FFG (3.2% of samples; primarily omnivorous non-insect invertebrates, e.g., Cambaridae). Collector–gatherers were the most abundant FFG (dominated by Chironomidae, Leptophlebiidae, and Ephemerellidae; > 1300 ind. m−2 each), followed by shredders, collector–filterers, and predators; herbivores were the least abundant group. Multivariate ANOVA also indicated few differences among HDCs for FFG for proportions of collector–gatherers, shredders, and predators (Tukey’s HSD: p > 0.05). However, whereas herbivores made up the lowest proportion of macroinvertebrates across all sites, there was a 480% increase in proportion of herbivores from reference sites to sites in severe decline (Tukey’s HSD: p = 0.0129; Fig. 3). Based on our model selection (Table 5), the best-supported model for herbivore proportion included D84 and proportion bedrock (adj-R2 = 0.45, F = 8.38, p = 0.003).
Mean (± 1SE) proportion herbivorous benthic macroinvertebrates by Hemlock Decline Category (HDC). Subsamples were pooled to site-level prior to analysis. HDC categories are (1) reference (n = 7), (2) moderate decline (n = 7), and (3) severe decline (n = 7). Proportion herbivore was logit(x + 0.005) transformed for statistical analysis; raw data are displayed here. Different letters represent significant differences between group means at p < 0.05 based on Tukey’s HSD post-hoc tests
Mean invertebrate density and Simpson’s diversity were highest at the greatest level of hemlock decline and lowest at moderate levels of decline (Tukey’s HSD: p = 0.0154 and p = 0.0174, respectively; Fig. 4). Taxonomic evenness showed no trend across HDCs (Tukey’s HSD: p > 0.05). D84 and PO4 emerged as predictors for the strongest model for density (adj-R2 = 0.39, F = 6.86, p = 0.007; Table 5). The best-supported model predicting 1 − D included NH4, PO4, and large-wood volume (adj-R2 = 0.50, F = 7.06, p = 0.003).
Mean (± 1SE) values for measures relevant to diversity of benthic invertebrates by Hemlock Decline Category (HDC). Subsamples were pooled to site-level prior to analysis. a density ind. m−2 and b Simpson’s Index (1 − D). HDC categories are (1) uninvaded, n = 7 (2) moderate decline, n = 7 and (3) severe decline, n = 7. Simpson’s Index was ex transformed for statistical analysis; raw data are displayed here. Different letters represent significant differences between group means at p < 0.05 as determined by a Tukey’s HSD post-hoc test
Discussion
Our study provides evidence that a terrestrial invader can have strong indirect impacts on aquatic macroinvertebrate communities. Specifically, we found that the loss of eastern hemlock—a foundational tree species—via HWA invasion promoted shifts in the diversity, density, and functional-feeding group composition of benthic macroinvertebrate communities. We explored possible mechanisms for these changes and found that variability in bed material (proportion bedrock and D84), large-wood characteristics (volume and density), and nutrient concentrations (PO4, NH4) were important predictors. Together, our data suggest that headwater streams formerly surrounded by hemlock will likely support divergent macroinvertebrate assemblages with implications for both food webs and ecosystem functioning.
The loss of a foundational forest species such as hemlock is expected to have far-reaching consequences for streams because riparian hemlock stands can influence stream physicochemical characteristics in ways that are distinct from replacement forests (e.g., Brantley et al. 2013). Hemlock replacement relies in large part on the pre-existing forest composition. In the Northeast, hemlock is expected to be replaced with black birch (Betula lenta L.) monocultures, eastern white pine (Pinus strobus L.), and American beech (Fagus grandifolia Ehrh.) (Orwig et al. 2002; Case et al. 2017). In central Appalachia, the presence of rhododendron prior to decline strongly determines the eventual forest structure: if present, rhododendron can form monocultures following hemlock decline, but otherwise the replacement can be a mix of maple (Acer), birch (Betula), beech (Fagus), and oak (Quercus) species (Ford et al. 2012). Several studies have used a paired-watershed approach to compare hemlock vs. hardwood forest streams to assess and predict differences between current and post-invasion stream properties including water chemistry and hydrology (Snyder et al. 1999; Daley et al. 2007). In a companion study to ours, Costigan et al. (2015) showed that stream hydrologic and geomorphic characteristics differed by decline severity. For instance, log jams at sites in severe decline tended to be fewer in number, but larger in volume, possibly due to larger trees toppling and enabling recruitment of key pieces of large, but less mobile, wood. These jams can increase retention of flow, sediments, and nutrients (Ellison et al. 2005), ultimately altering benthic habitat and invertebrate assemblages (Benke and Wallace 2003). Additionally, bryophytes can trap sediment and increase microhabitat stability, providing important food sources for macroinvertebrates (Suren 1992). While bryophytes may be an important habitat for macroinvertebrates in some Appalachian streams (Glime 1968), they were not abundant at our sites (Diesburg, personal observation).
Consistent with our hypothesis, we observed differences in the characteristics of macroinvertebrate communities among HDCs. Specifically, measures of density and diversity were lowest in sites recently invaded by HWA. Conversely, severely invaded sites exhibited the highest density and diversity. Here, model-selection results suggest that invertebrate density was best predicted by PO4 and D84. Hemlock streams are known to be nutrient-limited (Northington et al. 2013), and increases in biologically available phosphorus stimulate primary and secondary productivity, increasing macroinvertebrate abundance (Cross et al. 2006). Regarding substrate, since the density of invertebrates is typically negatively related to bare surfaces and very fine substrates (Quinn and Hickey 1990; Wallace and Eggert 2009), we would expect, and did observe, positive correlations between D84 and invertebrate density. There are additional explanations for the relatively low diversity observed at our moderately impacted sites. Like other foundational species, hemlock exerts controls on and stabilizes ecosystem processes (Ellison et al. 2005), and in particular maintains a later successional forest system. The decline and death of a foundational species, even over several decades, could be considered a disturbance event of considerable impact. Notably, hemlock forests provide habitat for distinct communities, which is evident in the strong association of certain taxa (e.g., Hydropsyche ventura, Lanthus parvulus, etc.) with hemlock streams (Snyder et al. 2002). We found several benthic insect taxa that appeared to be associated with unimpacted hemlock streams: Microtendipes sp, Tanytarsus sp. and Chironomus sp. Microtendipes sp is a collector–filterer taxon that was common at the unimpacted sites but not at either moderately or severely impacted sites. Both Tanytarsus sp. and Chironomus sp. are collector–gatherer members of the ubiquitous family Chironomidae, subfamily Chironominae. The loss of such hemlock-associated taxa would be expected to cause a decrease in alpha biodiversity, at least until new colonizers are able to repopulate impacted areas.
In contrast to our predictions about general shifts in macroinvertebrate communities, we found the relative abundances of macroinvertebrate functional traits were altered in unexpected ways. For example, shredder densities did not differ among HDCs. Webster et al. (2012) suggests that rhododendron growth and litter inputs to streams may compensate for the loss of hemlock needles as a food resource. However, hemlock needles have low lability, so if few shredders feed on the needles, a change in needle availability would not be expected to make a large difference in consumer densities, especially if an alternate food source becomes available in similar quantities. Additionally, the major shredder genus, Leuctra, was present in relatively high numbers across sites in all three decline categories. Adkins and Rieske (2015a) found that, during summer months only, shredders were present in higher densities in hemlock streams than in hardwood streams, indicating that the timing of allochthonous inputs is important.
Instead of shredders, we observed the most distinct trends in non-shredder herbivores. These herbivores, driven entirely by the presence of Epeorus pleuralis in HDC3 sites, increased in both number and relative abundance from reference to severely impacted sites. Herbivore density in uninvaded sites may have been suppressed by a combination of light limitation to in-stream primary producers and microbial resources (Rowell and Sobczak 2008). Eastern hemlock needles and twigs likely provide poor-quality substrate for microbial colonization and growth (Morkeski 2007). Further, grazers can be more abundant in hardwood streams compared to eastern hemlock streams (Adkins and Rieske 2015b). Thus, the replacement of eastern hemlock with other plant species might be expected to alter in-stream microbial growth and, in turn, energetic resources to consumers like invertebrate herbivores. Additionally, the relative abundance of herbivores at our sites was controlled primarily by the substrate descriptors D84 and proportion bedrock. Substrate is influential in invertebrate responses to riparian disturbance; scraper densities increase on bedrock and decrease on smaller sediments following logging (Gurtz and Wallace 1984). Forest roads, which were common across our study regions (although less so near the Ohio sites), likely also contribute to sedimentation of streams and to invertebrate responses (Angermeier et al. 2004). Thus, a combination of resources and habitat structure may be responsible for the observed increase in relative abundance of non-shredder herbivores. The negative relationship between PO4 and relative abundance of herbivores in one of our models (Table 5) warrants consideration as well, in view of higher PO4 concentrations associated with increases in invertebrate densities overall. Heterotrophic microorganisms in detrital-based food webs increase production in response to greater nutrient availability, which can result in both increased nutrient content within the microbes and more rapid detritus mineralization (Cross et al. 2006). This can result in a lower detrital availability during seasons in which detritital inputs are low (Suberkropp et al. 2010). In this study, the seasonally-consistent contributions expected of hemlock detritus in our reference sites could overshadow these effects of elevated PO4 concentrations.
Our observations of stream biological and physicochemical characteristics across a chronosequence of HWA invasion represent further evidence for HWA-invasion effects on adjacent streams (e.g., Costigan et al. 2015). However, several limitations of this chronosequence approach need to be considered. For example, whereas chronosequences are useful in studying succession and predicting species abundance across multiple time scales, they can fail to predict species richness (Foster and Tilman 2000). Thus, applying a chronosequence approach may be less appropriate in cases of divergent succession, such as when considering the different trajectories of forest composition between rhododendron dominance and the growth of mixed hardwoods; this limitation can be mitigated with more intensive sampling at finer spatiotemporal scales (Walker et al. 2010). Likewise, while severe or frequent disturbances can introduce uncertainty into a chronosequences approach, well-documented disturbance events (i.e., their timing and magnitude) can help clarify succession (Walker et al. 2010). Another potential caveat to consider with the design of our study is the inherent geographic clustering of our sites, selected to coincide with the timing and severity of HWA invasion. This is a common issue with such chronosequence designs involving invasive species (e.g., Hartman and McCarthy 2008), and our study should be interpreted with appropriate caution given the potential confounding influence of other factors such as regional climate, elevation, and geology. Nevertheless, we sought to minimize variability in these features to the degree possible and carefully selected study streams that were similar in size, network position, slope, and confinement. Further, our model selection approach (see “Numerical and statistical analysis”) included watershed- and site- level predictors that could covary with geographic location, as a way to partially account for the effects of location vs. HWA decline.
Hemlock woolly adelgid is predicted to continue spreading throughout the range of eastern hemlock and has been found as far north as southern Maine and central New Hampshire (US Forest Service northeastern area data, as cited in Case et al. 2017), although it is expected to be limited by winter temperatures (Paradis et al. 2008). The magnitude and extent of the invasion is cause for conservation concern, not only for this foundational species, but also for the ecosystems that it influences, such as headwater forest streams. Our study emphasizes that the effects of HWA extend beyond the riparian zone to affect in-stream macroinvertebrate communities, and by extension, the ecosystem functions that they mediate such as nutrient cycling, productivity, and decomposition (Wallace and Webster 1996). We expect that similar changes could occur as the decline continues to spread. Indeed, the counties with several reference sites used in this study have now reportedly shown signs of HWA invasion. Therefore, greater attention needs to be afforded to the broader effects of HWA and other terrestrial riparian invaders on stream-riparian structure and function.
References
Adkins JK, Rieske LK (2015a) A terrestrial invader threatens a benthic community: potential effects of hemlock woolly adelgid-induced loss of eastern hemlock on invertebrate shredders in headwater streams. Biol Invasions 17(4):1163–1179. https://doi.org/10.1007/s10530-014-0786-y
Adkins JK, Rieske LK (2015b) Benthic collector and grazer communities are threatened by hemlock woolly adelgid-induced eastern hemlock loss. Forests 6(8):2719–2738. https://doi.org/10.3390/f6082719
Angermeier PL, Wheeler AP, Rosenberger AE (2004) A conceptual framework for assessing impacts of roads on aquatic biota. Fisheries 29(12):19–29
Benke A, Wallace JB (2003) Influence of wood on invertebrate communities in streams and rivers. In: Gregory SV, Boyer KL, Gurnell AM (eds) The ecology and management of wood in world rivers. American Fisheries Society 37, Symposium. Bethesda, Maryland, pp 149–177
Brantley S, Ford CR, Vose JM (2013) Future species composition will affect forest water use after loss of eastern hemlock from southern Appalachian forests. Ecol Appl 23(4):777–790. https://doi.org/10.1890/12-0616.1
Burnham KP, Anderson DR (2004) Multimodel inference: understanding AIC and BIC in model selection. Sociol Method Res 33(2):261–304. https://doi.org/10.1177/0049124104268644
Case BS, Buckley HL, Barker-Plotkin AA, Orwig DA, Ellison AM (2017) When a foundation crumbles: forecasting forest dynamics following the decline of the foundation species Tsuga canadensis. Ecosphere 8(7):e01893. https://doi.org/10.1002/ecs2.1893
Cessna JF, Nielsen C (2012) Influences of hemlock woolly adelgid–induced stand-level mortality on nitrogen cycling and stream water nitrogen concentrations in southern Pennsylvania. Castanea 77(2):127–135. https://doi.org/10.2179/11-025
Costigan KH, Soltesz PJ, Jaeger KL (2015) Large wood in central Appalachian headwater streams: controls on and potential changes to wood loads from infestation of hemlock woolly adelgid. Earth Surf Proc Land 40(13):1746–1763. https://doi.org/10.1002/esp.3751
Cross WF, Wallace JB, Rosemond AD, Eggert SL (2006) Whole-system nutrient enrichment increases secondary production in a detritus-based ecosystem. Ecology 87(6):1556–1565. https://doi.org/10.1890/0012-9658(2006)87[1556:WNEISP]2.0.CO;2
Daley MJ, Phillips NG, Pettijohn C, Hadley JL (2007) Water use by eastern hemlock (Tsuga canadensis) and black birch (Betula lenta): implications of effects of the hemlock woolly adelgid. Can J For Res 37(10):2031–2040. https://doi.org/10.1139/X07-045
Davies KF, Chesson P, Harrison S, Inouye BD, Melbourne BA, Rice KJ (2005) Spatial heterogeneity explains the scale dependence of the native-exotic diversity relationship. Ecology 86(6):1602–1610. https://doi.org/10.1890/04-1196
DeFerrari CM, Naiman RJ (1994) A multi-scale assessment of the occurrence of exotic plants on the Olympic Peninsula, Washington. J Veg Sci 5(2):247–258. https://doi.org/10.2307/3236157
Dextrase AJ, Mandrak NE (2006) Impacts of alien invasive species on freshwater fauna at risk in Canada. Biol Invasions 8:13–24. https://doi.org/10.1007/s10530-006-1833-0
Ellison AM, Banks MS, Clinton BD, Colburn EA, Elliott K, Ford CR et al (2005) Loss of foundation species consequences for the structure and dynamics of forested ecosystems. Front Ecol Environ 3:479–486. https://doi.org/10.2307/3868635
Evans AM, Gregoire TG (2007) A geographically variable model of hemlock woolly adelgid spread. Biol Invasions 9:369–382. https://doi.org/10.1007/s10530-006-9039-z
Evans DM, Dolloff CA, Aust WM, Villamagna AM (2012) Effects of eastern hemlock decline on large wood loads in streams of the Appalachian mountains. J Am Water Resour Assoc 48(2):266–276. https://doi.org/10.1111/j.1752-1688.2011.00610.x
Feld CK, Segurado P, Gutiérrez-Cánovas C (2016) Analysing the impact of multiple stressors in aquatic biomonitoring data: a ‘cookbook’ with applications in R. Sci Total Environ 573:1320–1339. https://doi.org/10.1016/j.scitotenv.2016.06.243
Ford CR, Vose JM (2007) Tsuga canadensis (L.) Carr. mortality will impact hydrologic processes in southern Appalachian forest ecosystems. Ecol Appl 17(4):1156–1167. https://doi.org/10.1890/06-0027
Ford CR, Elliott KJ, Clinton BD, Kloeppel BD, Vose JM (2012) Forest dynamics following eastern hemlock mortality in the southern Appalachians. Oikos 121(4):523–536. https://doi.org/10.1111/j.1600-0706.2011.19622.x
Foster BL, Tilman D (2000) Dynamic and static views of succession: testing the descriptive power of the chronosequence approach. Plant Ecol 146:1–10. https://doi.org/10.1023/A:1009895103017
Glime JM (1968) Ecological observations on some bryophytes in Appalachian Mountain streams. Castanea 33(4):300–325
Gurtz ME, Wallace JB (1984) Substrate-mediated response of stream invertebrates to disturbance. Ecology 65(5):1556–1569. https://doi.org/10.2307/1939135
Hadley JL (2000) Understory microclimate and photosynthetic response of saplings in an old-growth eastern hemlock (Tsuga canadensis L.) forest. Ecoscience 7:66–72. https://doi.org/10.1080/11956860.2000.11682573
Harrelson CC, Rawlins CL, Potyondy JP (1994) Stream channel reference sites: an illustrated guide to field 408 technique. Gen. Tech. Rep. RM-245. Fort Collins, CO: US Department of Agriculture, Forest Service, 409 Rocky Mountain Forest and Range Experiment Station 245, p 61
Hartman KM, McCarthy BC (2008) Changes in forest structure and species composition following invasion by a non-indigenous shrub, Amur honeysuckle (Lonicera maackii). J Torrey Bot Soc 135(2):245–259
Hynes HBN (1975) The stream and its valley. Verh Int Ver 19:1–15. https://doi.org/10.1080/03680770.1974.11896033
Jenkins JC, Aber JD, Canham CD (1999) Hemlock woolly adelgid impacts on community structure and N cycling rates in eastern hemlock forests. Can J For Res 29(5):630–645. https://doi.org/10.1139/x99-034
Kennedy TA, Hobbie SE (2004) Saltcedar (Tamarix ramosissima) invasion alters organic matter dynamics in a desert stream. Freshw Biol 49:65–76. https://doi.org/10.1046/j.1365-2426.2003.01166.x
Kim J, Hwang T, Schaaf CL, Orwig DA, Boose E, Munger JW (2017) Increased water yield due to the hemlock woolly adelgid infestation in New England. Geophys Res Lett 44(5):2327–2335. https://doi.org/10.1002/2016GL072327
Kondolf GM, Micheli ER (1995) Evaluating stream restoration projects. Environ Manage 19(1):1–15
Levin LA, Neira C, Grosholz ED (2006) Invasive cordgrass modifies wetland trophic function. Ecology 87(2):419–432. https://doi.org/10.1890/04-1752
Malanson GP (1993) Riparian landscapes. Cambridge University Press, New York
Martin KL, Goebel PC (2012) Decline in riparian Tsuga canadensis forests of the central Appalachians across an Adelges tsugae invasion chronosequence. J Torrey Bot Soc 139(4):367–378. https://doi.org/10.3159/TORREY-D-12-00012.1
Martin KL, Goebel PC (2013) The foundation species influence of eastern hemlock (Tsuga canadensis) on biodiversity and ecosystem function on the Unglaciated Allegheny Plateau. For Ecol Manag 289:143–152. https://doi.org/10.1016/j.foreco.2012.10.040
McDowell WG, McDowell WH, Byers J (2017) Mass mortality of a dominant invasive species in response to an extreme climate event: implications for ecosystem function. Limnol Oceanogr 62:177–188. https://doi.org/10.1002/lno.10384
Merritt R, Cummins K, Berg M (2008) An introduction to the aquatic insects of North America, 4th edn. Kendall Hunt, Dubuque
Mineau MM, Baxter CV, Marcarelli AM, Minshall GW (2012) An invasive riparian tree reduces stream ecosystem efficiency via a recalcitrant organic matter subsidy. Ecology 93(7):1501–1508. https://doi.org/10.1890/11-1700.1
Morkeski K (2007) In-stream hemlock twig breakdown and effects of reach-scale twig additions on Appalachian headwater streams. Thesis, Virginia Tech
Naiman RJ, Decamps H (1997) The ecology of interfaces: riparian zones. Annu Rev Ecol Syst 28:621–658. https://doi.org/10.1146/annurev.ecolsys.28.1.621
Naiman RJ, Decamps H, McClain ME (2010) Riparia: ecology, conservation, and management of streamside communities. Academic Press, Beijing
Naimi B, Hamm NA, Groen TA, Skidmore AK, Toxopeus AG (2014) Where is positional uncertainty a problem for species distribution modelling? Ecography 37(2):191–203. https://doi.org/10.1111/j.1600-0587.2013.00205.x
Nakamura F, Frederick JS, Wondzell SM (2000) Disturbance regimes of stream and riparian systems-disturbance cascade perspective. Hydrol Process 14:2849–2860
Northington RM, Webster JR, Benfield EF, Cheever BM, Niederlehner BR (2013) Ecosystem function in Appalachian headwater streams during an active invasion by the hemlock woolly adelgid. PloS One 8:4 e61171. https://doi.org/10.1371/journal.pone.0061171
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Szoecs E, Wagner H (2017) Vegan: community ecology package. R package version 2.4-2. https://CRAN.R-project.org/package=vegan
Orwig DA, Foster DR (1998) Forest response to the introduced hemlock woolly adelgid in southern New England, USA. J Torrey Bot Soc 125:60–73. https://doi.org/10.2307/2997232
Orwig DA, Foster DR, Mausel DL (2002) Landscape patterns of hemlock decline in New England due to the introduced hemlock woolly adelgid. J Biogeogr 29(10–11):1475–1487. https://doi.org/10.1046/j.1365-2699.2002.00765.x
Paradis A, Elkinton J, Hayhoe K, Buonaccorsi J (2008) Role of winter temperature and climate change on the survival and future range expansion of the hemlock woolly adelgid (Adelges tsugae) in eastern North America. Mitig Adapt Strat Glob 13(5–6):541–554. https://doi.org/10.1007/s11027-007-9127-0
Pickett ST (1989) Space-for-time substitution as an alternative to long-term studies. In: Likens GE (ed) Long-term studies in ecology. Springer-Verlag, New York, pp 110–135
Pielou EC (1966) The measurement of diversity in different types of biological collections. J Theor Biol 13:131–144
Poff NL, Olden JD, Vieira NK, Finn DS, Simmons MP, Kondratieff BC (2006) Functional trait niches of North American lotic insects: traits-based ecological applications in light of phylogenetic relationships. J N Am Benthol Soc 25(4):730–755. https://doi.org/10.1899/0887-3593(2006)025%5B0730:FTNONA%5D2.0.CO;2
Polis GA, Anderson WB, Holt RD (1997) Toward an integration of landscape and food web ecology: the dynamics of spatially subsidized food webs. Annu Rev Ecol Syst 28:289–316. https://doi.org/10.1146/annurev.ecolsys.28.1.289
Quinn JM, Hickey CW (1990) Magnitude of effects of substrate particle size, recent flooding, and catchment development on benthic invertebrates in 88 New Zealand rivers. NZ J Mar Freshwat Res 24(3):411–427. https://doi.org/10.1080/00288330.1990.9516433
Rahel FJ (2002) Homogenization of freshwater faunas. Annu Rev Ecol Syst 33:291–315. https://doi.org/10.1146/annurev.ecolsys.33.010802.150429
Roberts SW, Tankersley R, Orvis KH (2009) Assessing the potential impacts to riparian ecosystems resulting from hemlock mortality in Great Smoky Mountains National Park. Environ Manage 44(2):335–345. https://doi.org/10.1007/s00267-009-9317-5
Rogers RS (1978) Forests dominated by hemlock (Tsuga canadensis): distribution as related to site and postsettlement history. Can J Bot 56(7):843–854. https://doi.org/10.1139/b78-096
Rowell TJ, Sobczak WV (2008) Will stream periphyton respond to increases in light following forecasted regional hemlock mortality? J Freshw Ecol 23:33–40. https://doi.org/10.1080/02705060.2008.9664555
Sabo JL, Sponseller R, Dixon M, Grade K, Harms T, Heffernan J et al (2005) Riparian zones increase regional species richness by harboring different not more species. Ecology 86:56–62. https://doi.org/10.1890/04-0668
Schindler DE, Knapp RA, Leavitt PR (2001) Alteration of nutrient cycles and algal production resulting from fish introductions into mountain lakes. Ecosystems 4(4):308–321. https://doi.org/10.1007/s10021-001-0013-4
Shannon CE, Weaver W (1949) The mathematical theory of communication. University of Illinois Press, Urbana
Siderhurst LA, Griscom HP, Hudy M, Bortolot ZJ (2010) Changes in light levels and stream temperatures with loss of eastern hemlock (Tsuga canadensis) at a southern Appalachian stream: implications for brook trout. For Ecol Manag 260(10):1677–1688. https://doi.org/10.1016/j.foreco.2010.08.007
Simberloff D, Martin JL, Genovesi P, Maris V, Wardle DA, Aronson J et al (2013) Impacts of biological invasions: what’s what and the way forward. Trends Ecol Evol 28:58–66. https://doi.org/10.1016/j.tree.2012.07.013
Simpson EH (1949) Measurement of diversity. Nature 163(4148):688. https://doi.org/10.1038/163688a0
Smith B, Wilson JB (1996) A consumer’s guide to evenness indices. Oikos 76:70–82. https://doi.org/10.2307/3545749
Snyder CD, Young J, Smith D, Lemarie D, Ross R, Bennett R (1999) Influence of eastern hemlock on aquatic biodiversity in Delaware Water Gap National Recreation Area. Kearneysville, West Virginia
Snyder CD, Young JA, Lemarié DP, Smith DR (2002) Influence of eastern hemlock (Tsuga canadensis) forests on aquatic invertebrate assemblages in headwater streams. Can J Fish Aquat Sci 59(2):262–275. https://doi.org/10.1139/F02-003
Suberkropp K, Gulis V, Rosemond AD, Benstead JP (2010) Ecosystem and physiological scales of microbial responses to nutrients in a detritus-based stream: results of a 5-year continuous enrichment. Limno Oceanogr 55:149–160. https://doi.org/10.4319/lo.2010.55.1.0149
Sullivan SMP, Watzin MC, Hession WC (2004) Understanding stream geomorphic state in relation to ecological integrity: evidence using habitat assessments and macroinvertebrates. Environ Manage 34(5):669–683. https://doi.org/10.1007/s00267-004-4032-8
Suren AM (1992) Enhancement of invertebrate food resources by bryophytes in New Zealand alpine headwater streams. New Zeal J Mar Freshw 26(2):229–239. https://doi.org/10.1080/00288330.1992.9516518
Thompson R, Townsend C (2003) Impacts on stream food webs of native and exotic forest: an intercontinental comparison. Ecology 84:145–161. https://doi.org/10.1890/0012-9658(2003)084%5B0145:IOSFWO%5D2.0.CO;2
Vieira NKM, Poff NL, Carlisle DM, Moulton IISR, Koski ML, Kondratieff BC (2006) A database of lotic invertebrate traits for North America, Data Series 187. US Geological Survey, Reston
Vilà M, Espinar JL, Hejda M, Hulme PE, Jarošík V, Maron JL et al (2011) Ecological impacts of invasive alien plants: a meta-analysis of their effects on species, communities and ecosystems. Ecol Lett 14(7):702–708. https://doi.org/10.1111/j.1461-0248.2011.01628.x
Walker LR, Wardle DA, Bardgett RD, Clarkson BD (2010) The use of chronosequences in studies of ecological succession and soil development. J Ecol 98(4):725–736. https://doi.org/10.1111/j.1365-2745.2010.01664.x
Wallace JB, Eggert SL (2009) Benthic invertebrate fauna, small streams. Encycl Inland Waters 2:173–190
Wallace JB, Webster JR (1996) The role of macroinvertebrates in stream ecosystem function. Annu Rev Entomol 41:115–139. https://doi.org/10.1146/annurev.en.41.010196.000555
Ward JS, Montgomery ME, Cheah CAS, Onken BP, Cowles RS (2004) Eastern hemlock forests: Guidelines to minimize the impacts of hemlock woolly adelgid (NA-TP-03-04) Morgantown, WV
Webster J, Morkeski K, Wojculewski C, Niederlehner B, Benfield E, Elliott K (2012) Effects of hemlock mortality on streams in the southern Appalachian Mountains. Am Midl Nat 168:112–131. https://doi.org/10.1674/0003-0031-168.1.112
Willacker JJ Jr, Sobczak WV, Colburn EA (2009) Stream macroinvertebrate communities in paired hemlock and deciduous watersheds. Northeast Nat 16:101–112. https://doi.org/10.1656/045.016.0108
Acknowledgements
Research support was provided by state and federal funds appropriated to The Ohio State University, Ohio Agricultural Research and Development Center (OARDC) through the OARDC Research Enhancement Competitive Grants Program (SMPS). We also wish to thank members of the Stream and River Ecology (STRIVE) Lab for their assistance in the lab and the field, as well as additional support from P. Soltesz, Dr. P.C. Goebel, and Dr. K. Jaeger.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
27_2018_607_MOESM1_ESM.xlsx
Online Resource 1 Raw data including macroinvertebrate taxa, sampling dates, microhabitat, and functional feeding group designation (XLSX 84 KB)
Rights and permissions
About this article
Cite this article
Diesburg, K.M., Sullivan, S.M.P. & Manning, D.W.P. Changes in benthic invertebrate communities of central Appalachian streams attributed to hemlock woody adelgid invasion. Aquat Sci 81, 11 (2019). https://doi.org/10.1007/s00027-018-0607-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00027-018-0607-y