Abstract
Phenotypic plasticity is predicted to evolve when subsequent generations are likely to experience alternating selection pressures; e.g., piscine predation on mosquitoes (Culex pipiens) varies strongly depending on habitat type. A prey-choice experiment (exp. 1) detected a predilection of common mosquito predators (sticklebacks, Gasterosteus aculeatus) for large-bodied mosquito larvae, suggesting that larvae could benefit from suppressing growth under predation risk, and experiment 2 confirmed reduced pupa size and weight when we exposed larvae to stickleback kairomones. In experiment 3, we measured adult (imago) size instead to test if altered larval growth-patterns affect adult life-history traits. We further asked how specific life-history responses are, and thus, also used kairomones from introduced Eastern mosquitofish (Gambusia holbrooki), and from algivorous, non-native catfish (Ancistrus sp.). Adult body mass was equally reduced in all three kairomone treatments, suggesting that a non-specific anti-predator response (e.g., reduced activity) results in reduced food uptake. However, imagines were distinctly smaller only in the stickleback treatment, pointing towards a specific, adaptive life-history shift in response to the presence of a coevolved predator: mosquito larvae appear to suppress growth when exposed to their native predator, which presumably reduces predation risk, but also affects body size after pupation. Our study suggests that (1) not all antipredator responses are necessarily predator-specific, and (2) fluctuation in the cost-benefit ratio of suppressing larval growth has selected for phenotypic plasticity in C. pipiens larval life histories. This implies costs associated with suppressed growth, for example, in the form of lower lifetime reproductive success.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Predation is a major selective force driving phenotypic diversification including adaptive variation in prey species’ life histories (Reznick and Endler 1982; Crowl and Covich 1990; Riesch et al. 2013), morphology (Walker 1997; Langerhans et al. 2004), and behavior (Dixon and Baker 1988; Lima and Dill 1990). Characters that provide protection from predation often show heritable (evolved) differences in mean trait expression between species or populations that are exposed to different predation regimes (Reznick 1982; Langerhans et al. 2004). However, building defensive traits can be associated with considerable costs (e.g., Stevens et al. 1999), and so inducible trait expression (i.e., phenotypic plasticity) ought to be favored under certain conditions (Hebert and Grewe 1985; Dodson 1989; Agrawal 2001; Benard 2004); but see, e.g., DeWitt et al. (1998) and Auld et al. (2009) for costs of phenotypic plasticity. Classic examples of inducible morphological responses to predation risk come from studies on water flees (genus Daphnia), where juveniles produce neck-teeth, helmet-like structures, or elongated spines on the dorsal surface of the carapace in response to predator presence (Krueger and Dodson 1981; Hebert and Grewe 1985; Dodson 1989). One condition that favors the evolution of inducible predator-defense traits is provided when individuals can move between habitats that differ in predation risk across generations. For example, insects with complex life cycles can experience starkly divergent predatory regimes during their (aquatic) larval stages (Wilbur 1980; Palmer and Poff 1997), but since imagines can move freely between water bodies for oviposition, successive generations often experience different predatory regimes, and so plastic responses to predators in larval life histories, morphology and behavior are to be expected (reviewed in Benard 2004).
Predation is often size-selective, and predators tend to consume large individuals to optimize their net energy uptake (Brooks and Dodson 1965; Reznick 1982; Wellborn 1994; Plath et al. 2003) unless gape limitation or problems of handling large prey items lead to different patterns (Werner 1974; Nilsson and Brönmark 2000). In holometabolous insects, life history responses to predation on larval stages ought to be governed by early emergence to evade predation, which can result in reduced body size at metamorphosis (Peckarsky et al. 2001; Benard 2004; Beketov and Liess 2007), as somatic growth is largely restricted to the larval stage (Nijhout and Wheeler 1996). However, even if accelerating larval development is impossible, larvae could still suppress growth to evade size-specific predation. On the other hand, previous studies on culicid larvae reported on delayed developmental times when exposed to kairomones, probably because larvae reduced activity and fed less, resulting in delayed development (Beketov and Liess 2007; van Uitregt et al. 2012). Some studies, however, found no developmental response to predation risk at all (e.g., Caudill and Peckarsky 2003 for larvae of the mayfly Callibaetis ferrugineus hageni), and so holometabolous insects seem to react to predator cues along a continuum of reaction norms.
Accelerated emergence times and/or suppressed larval growth ought to be balanced by trade-offs (sensu Wilbur 1980; Stearns 1989) involving the competitiveness of larvae and ultimately, adults’ realized reproductive potential (Bradshaw and Holzapfel 1992). Such a trade-off was demonstrated for the mosquito Aedes notoscriptus: when larvae of this Australian species were exposed to chemical cues of the native piscine predator Hypseleotris galii they reduced activity and, therefore, were better at avoiding predation than larvae that were not previously exposed to predator cues. However, predator-exposed larvae had reduced larval growth and development, were smaller at metamorphosis and less resistant to starvation than non-exposed ones (van Uitregt et al. 2012). Larvae of our study species, the common house mosquito Culex pipiens molestus (Forskal), inhabit stagnant or slow-flowing waters, ranging from small temporary puddles to large oxbow lakes (Vinogradova 2000). While transient water bodies are mostly predator-free environments, larvae in permanent (and mostly larger) water bodies are heavily preyed upon by multiple predators, including fishes, notonectid backswimmers, dytiscid beetles, dragonfly larvae, and others (Vinogradova 2000). Like several other aquatic invertebrates, Culex larvae detect predators through kairomones—natural chemicals released by their potential predators (Dodson et al. 1994; Ferrari et al. 2010). Little is known about the chemical structure of kairomones; an ongoing debate considers the question of whether (and to what extent) the predator’s diet (Huryn and Chivers 1999; Beketov and Liess 2007), its cutaneous mucus (Forward and Rittschof 2000; Alvarez et al. 2014) or its bacterial flora (Beklioglu et al. 2006) affect the chemical composition of kairomones. However, there is evidence that kairomones differ widely even among similar taxa (Lass and Spaak 2003; Relyea 2003; Ferrari et al. 2010). When exposed to kairomones originating from aquatic heteropterans or beetles, mosquito larvae (Culex spp. and Aedes spp.) reduce their activity (Sih 1986; Ohba et al. 2012), and imagines appear to use kairomones of larval predators as a cue to avoid high-predation environments when searching for oviposition sites (Spencer et al. 2002; Ohba et al. 2012; Afify and Galizia 2015).
A number of studies focused on predator–prey interactions between culicid mosquito larvae (including the genus Culex) and non-indigenous predators that have received attention as potential biological control agents against these vectors of human diseases (e.g., Krumholz 1948; Rosenheim et al. 1995; Kumar and Hwang 2006). For example, Offill and Walton (1999) compared a common native predator of Culex spp., the three-spined stickleback (Gasterosteus aculeatus Linnaeus) and Gambusia affinis (Baird and Girard), an introduced predator that is often used in mosquito control programs (Krumholz 1948), in terms of their efficiency of larval predation and found higher predation rates in G. affinis. However, predator–prey interactions between the widely distributed common house mosquito and its common native predator in Europe, G. aculeatus (see Medlock and Snow 2008), have received little attention in empirical research. Therefore, our first question was whether stickleback predation on C. pipiens is size-selective, and we conducted a prey choice experiment in which individual sticklebacks were offered two different size classes of mosquito larvae to answer this question. Our first experiment corroborated an increased predation risk of large-bodied larvae (see “Results”), and so we conducted another experiment to investigate potential predator-induced larval life history responses by raising larvae in water containing kairomones from sticklebacks, or water without kairomones in the control treatment. Specifically, we asked whether (size-specific) predation risk results in an accelerated developmental time and/or reduced larval body size, as measured by the size at pupation. Because we detected several life history responses when larvae were exposed to stickleback kairomones, we expanded our study and asked if mosquito larvae respond specifically to the presence of naturally co-occurring insectivorous fishes, or if any fish species elicits those responses. This question is of particular interest because of the increasing impact of invasive species—including non-native teleost fishes—in freshwaters worldwide (Mack et al. 2000; Sakai et al. 2001). Native, predator-naive prey species may be more vulnerable to predation by introduced, unfamiliar predators due to their inability to recognize novel predators and to show adaptive antipredator responses (Salo et al. 2007). In our third experiment we therefore compared life histories of native mosquito larvae exposed to no kairomones (control), kairomones from a non-native algivorous fish (Ancistrus sp.), sticklebacks, and from Eastern mosquitofish (Gambusia holbrooki Girard). The latter species is a severe predator of mosquitoes that has been introduced to southern Europe from the USA for malaria prophylaxis in the 1920s (Vidal et al. 2010), but does not presently co-occur with mosquitoes in Germany, where our study population of C. pipiens originated. We predicted the strongest shift in life histories to occur in response to chemical cues of sticklebacks, but a weaker or no response at all when exposed to kairomones from Ancistrus and mosquitofish. In this experiment, we focused on size and weight measurements of imagines, which allowed determination of whether and how larval life history shifts (as seen in our second experiment) translate into an altered adult body size. Taken together, our present study is the first of its kind to not only demonstrate altered larval development in response to predator cues, but to demonstrate how developmental plasticity relates to size-specific predation risk, and to what extent co-evolved and invasive alien (i.e., not co-evolved) predators elicit the same or different responses in essential Culex life-history traits.
Materials and methods
Study organisms and their maintenance
A randomly outbred laboratory strain of Culex pipiens molestus (Culicidae), founded from wild-caught animals collected near Regensburg, Germany, was obtained from Biogents AG (Regensburg). The subspecies C. p. molestus is autogenous, stenogamous, remains active throughout the year, and mainly feeds on mammalian, especially human blood (Harbach et al. 1984). Females produce at least one egg-raft after emergence, for which they do not obligatorily require a blood meal (Twohy and Rozeboom 1957). Mosquitoes were maintained as randomly outbred stocks consisting of several hundred imagines at 22 °C in two cages (60 cm × 60 cm × 60 cm gauze-covered frames), which were equipped with a water-filled Petri dish. Adult females were fed on saturated grape-sugar solution, which we offered ad libitum in the form of sugar-water soaked paper towels. Egg-rafts were removed from the culture and transferred to 10-L aquaria with equal amounts of deionized and tap water for hatching. Larvae were fed weekly on commercially available fine-ground fish food (Tetra Min®).
All fishes used to produce kairomones for the tests were maintained in aerated and filtered ≥80-L tanks at 21 °C and fed ad libitum with flake food twice a day. Three-spined stickleback (Gasterosteus aculeatus; Gasterosteidae) are widely distributed throughout Europe; they feed mainly on crustaceans and aquatic insects (Hynes 1950). Sticklebacks were collected in a small creek in Niederursel, Germany, and were kept in the laboratory for 2–3 weeks before experimental use. In our third experiment we also included a laboratory strain of Eastern mosquitofish (Gambusia holbrooki; Poeciliidae), presumably of Floridian origin. Mosquitofish were actively released in southern Europe during the 20th century for mosquito prophylaxis, and are nowadays present in nearly all southern European water bodies (Vidal et al. 2010; pers. obs. for Italian, Spanish and southern French streams), but are currently not known to have established permanent populations in Germany. Our third experiment included a domestic form of the algivorous South American armored catfish (Ancistrus sp. Loricariidae), which we obtained from a commercial aquarium breeder.
To prepare water containing specific fish kairomones, groups of fish were transferred into aerated and filtered 10-L aquaria that were maintained at 21 °C. In an attempt to standardize kairomone concentrations, we combined small groups of stimulus fish such that their cumulative body size (standard length, SL) would equal about 160 mm [4 individuals per aquarium for Ancistrus (mean ± SE, SL = 39.3 ± 1.0 mm), 4 sticklebacks (39.6 ± 2.4 mm) and 5–6 mosquitofish (26.8 ± 1.5 mm)]. Fishes were daily fed ad libitum with Tetra Min® fish food. Water was refilled every day after water had been removed for the experiments described below (Experiments 2 and 3).
Experiment 1: prey choice of three-spined sticklebacks
Prey choice tests with sticklebacks were performed in 12-L aquaria. Test tanks were aerated between trials, but the air-stone was removed before mosquito larvae were introduced. Before each trial, we collected 14 larvae from our stock culture, i.e., seven from each of two visibly different size classes. Two individuals per size class were randomly taken from this sample and fixed in 70 % ethanol for subsequent size determination [large size class, mean (±SE) length from the cephalothorax to the tip of abdominal segment VIII: 5.2 ± 0.1 mm; small size class: 2.8 ± 0.1 mm). Since our aim was to detect general patterns of size-selective predation in sticklebacks, we chose mosquito larvae that clearly differed in size, which allowed visual differentiation of size classes by the observer. To initiate a trial, an individual stickleback (SL: 39.2 ± 1.4 mm) was introduced into the test tank and allowed to acclimate for 10 min. Afterwards, the remaining 10 larvae were gently introduced into the test tank. We observed the behavior of the focal fish from approximately 1 m distance and terminated a trial (i.e., removed the focal fish from the test tank) after five larvae had been eaten. All surplus larvae were retrieved from the test tank and their size class noted. In total, we conducted n = 31 independent trials.
Experiment 2: life history responses to stickleback kairomones (pupae size)
To determine larval responses to stickleback kairomones, larvae were reared in 100 mL vessels filled with 60 mL water (equal amounts of deionized and tap water). Each vessel was covered with fine nylon gauze. We introduced eight newly hatched (L1) larvae per vessel and thus tested 10 replicates (80 L1 larvae) in both treatments (control and stickleback kairomones; i.e., n = 160 larvae altogether). Larvae were fed on ground fish food until pupation. The consumption of food per larvae increased stepwise as follows: 0.5 mg (hatching day), 0.5 mg (day 2), 0.5 mg (day 4), 0.5 mg (day 5), and 1 mg per day from day 7 onwards. All experiments were conducted in a climate chamber at 21 °C, with 60 % humidity and a 12:12 h LD photoperiod. We exchanged 50 % of the water in the test vessels every day and replaced it with kairomone-water from the respective fish tanks or untreated water (according to the treatment). We used water from two replicate stimulus tanks containing sticklebacks (cumulative SL = 166 ± 3 mm) and two equal-sized and similarly equipped tanks without fish (control treatment).
Twice a day all mosquito test vessels were checked for mortality, and all dead larvae were immediately removed. Simultaneously, we checked for pupae, which were fixed in 70 % ethanol. We determined the sex of pupae based on the gonocoxopodites, which are large and partially bilobed in males but small and spiculate in females (cp. Harbach et al. 1984; personal observation). The same parameters as described in Müller et al. (2013) were measured under a stereo microscope: abdominal length (AL) from the third to the eighth segment, abdominal width (AW) at the fifth segment, and the area of the cephalothorax (CT) in lateral view.
We asked if any observable life history responses of the mosquito larvae can be ascribed to the presence of fish kairomones or whether it represents a response to metabolic waste products of fish and their related degradation products. We therefore analyzed nitrate and phosphate concentrations in randomly selected experimental vessels, the fish tanks and in the tanks containing control water using colorimetric tests (Merck KGaA, Darmstadt, Germany). Two nitrate and phosphate measurements were conducted per treatment. Sensitivity of the colorimetric tests is low, however, all measurements for nitrate and phosphate were uniformly high in the experimental mosquito vessels (~10 mg l−1 NO3 −; >0.43 mg l−1 H3PO4) and considerably lower in the fish and control tanks (~5 mg l−1 NO3 −; ~0.3 mg l−1 H3PO4). Therefore, waste products of the larvae themselves seem to have affected nitrate and phosphate concentrations in the test vessels, but the addition of fish (or control) water had no obvious effect on this.
Experiment 3: life history responses to different predator types (imago size)
In our third experiment, we used a similar approach as described before, but collected imagines directly after hatching. We set up three replicate stimulus tanks for each of the following fish species and three (empty) control tanks (i.e., 12 stimulus tanks in total): Ancistrus sp. (cumulative SL = 157 ± 4 mm), G. aculeatus (158 ± 3 mm) and G. holbrooki (152 ± 1 mm). We conducted 15 replicates (using 120 larvae) per treatment, amounting to a total sample size of n = 480 larvae.
Emerged imagines were frozen in individual 1.5 mL Eppendorf tubes at −80 °C until further processing. Mosquitoes were then dried at 60 °C for a minimum of 24 h, after which their dry weight was recorded using a Sartorius 4503 microbalance (accuracy: 1 µg). Wing length (as a proxy for body size) was determined to the nearest 0.1 mm as the distance from the axial incision to the R1 vein (Kreß et al. 2014) using a dissecting microscope (Nikon AZ100 Multizoom, Nikon Instruments Europe, Amsterdam, Netherlands) connected to a digital camera (Nikon DS-Fi1) with an image-analyzing system (NIS Elements BR, version 3.22.11, Laboratory Imaging). To determine fat content mosquitos were rinsed four times for at least 1.5 h with petroleum ether to extract nonpolar, non-structural lipids, then dried again and reweighed (Heulett et al. 1995; Riesch et al. 2010).
Statistical analysis
All statistical analyses were conducted using SPSS 22 (SPSS Inc., Chicago, IL). Numbers of large and small larvae consumed in experiment 1 were compared using a Wilcoxon signed rank test. We used multivariate General Linear Models (GLM) to compare life history traits between treatments in experiments 2 and 3; dependent data were z-transformed to standardize units. In experiment 2, ‘developmental time until pupation’ (days), ‘dry weight’ (mg), ‘cephalotorax area’ (mm2) ‘abdominal width’ (mm) and ‘abdominal length’ (mm) were treated as the dependent variables, and in experiment 3 ‘developmental time until emergence’ (days), ‘dry weight’ (mg), ‘wing length’ (mm) and arcsine(square root)-transformed ‘fat-content’ (%). ‘Sex’, ‘treatment’ and their interaction were used as fixed factors. Since the interaction terms had no significant effects (experiment 2: F 5,127 = 1.37, P = 0.24; experiment 3: F 12,784 = 0.99, P = 0.46), they were removed from the final models. To identify the source of variation in case of significant treatment effects we used univariate GLMs on all four variables separately (using non-transformed data) and employed LSD tests for post hoc pairwise comparisons between treatments. We illustrated significant effects using estimated marginal means from the respective analytical models. Mortality data were analyzed using survival analysis: a log-rank test was performed to check if larval mortality rates varied among treatments.
Results
Experiment 1: prey choice of three-spined sticklebacks
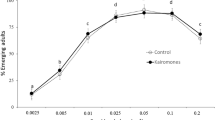
Sticklebacks consumed more large than small larvae in 29 of 31 trials, and a Wilcoxon signed rank test confirmed that overall more large larvae were consumed (i.e., 72.9 ± 3.2 % of consumed larvae; z = −4.63, P < 0.01, n = 31; Fig. 1).
Experiment 2: life history responses to stickleback kairomones (pupae size)
A multivariate GLM uncovered significant treatment (F 5,128 = 7.15, P < 0.001) and sex effects (F 5,128 = 179.25, P < 0.001) on the four investigated life history traits. Univariate GLMs—as a touch-down approach to uncover the source of variation—revealed that developmental time was not affected by exposure to stickleback kairomones, while cephalothorax area, abdominal width, abdominal length, and dry weight were significantly reduced in the stickleback treatment (Table 1; Fig. 2). Differences between sexes are reported in supplementary Table S1.
Effects of kairomones on life history traits of Culex pipiens pupae reared as larvae in absence (wave symbol, left) or presence (stickleback drawing, right) of chemical cues from Gasterosteus aculeatus. a Pupae of C. pipiens with additional illustration of the measured distances: c cephalothorax area, d abdominal width and e abdominal length. b–f Dependent variables assessed in experiment 2 are depicted as back-transformed estimated marginal means (±SE) from a GLM. Shown are b the time until pupation, c cephalothorax area, d abdominal width, e abdominal length and f dry weight of pupae
Survival analysis revealed that the mortality of larvae did not differ between treatments (log-rank test, χ 2 = 3.59, P = 0.06). While the marginally non-significant effect suggests a treatment effect, larvae exposed to kairomones actually had slightly increased (not decreased) survival (Fig. 3a). Mortality in the control treatment was 20.7 %, which meets the criteria of acceptable experimental baseline mortality for ecotoxicological tests in the non-biting midge Chironomus riparius (Chironomidae) provided in OECD guideline no. 219 (i.e., 30 %; OECD 2004).
Experiment 3: life history responses to different predator types (imago size)
A multivariate GLM detected significant treatment (F 12,791 = 2.86, P = 0.001) and sex effects (F 4,299 = 342.61, P < 0.001) on the four investigated life history traits. Univariate GLMs found significant differences in dry weight (Fig. 4b) and body size (Fig. 4c), while developmental duration (Fig. 4a) and fat content (Fig. 4d) did not differ between treatments (Table 2). Post-hoc tests revealed that average dry weight was significantly lower in larvae exposed to any of the three fish treatments compared to the control treatment (LSD tests: P < 0.01 in all cases; Fig. 4b). Body size (wing length) was significantly smaller in the stickleback treatment than in the control (P = 0.011) and Ancistrus treatments (P = 0.004; Fig. 4c). Body size of mosquitoes from the G. holbrooki treatment was also slightly reduced (1.03 %), but was not significantly different from the other treatments (P > 0.05 in all cases). In addition, we found pronounced differences between sexes, with males emerging faster than females, having a lower dry weight, smaller wing length and a higher fat content than females (Table 2; for descriptive statistics see supplementary Table 2).
Effects of kairomones on life history traits of Culex pipiens imagines when larvae were reared in absence (control) or presence of chemical cues from non-predatory, algivorous Ancistrus sp., the native predator Gasterosteus aculeatus and the non-native predator Gambusia holbrooki. Shown are back-transformed estimated marginal means (±SE) from a GLM, for a developmental time until emergence, b dry weight, c body size and d fat content of emerged imagines. Letters above the bars represent the results of LSD tests for pairwise post hoc comparisons, where different letters indicate significant differences between treatments (P < 0.05)
The survival of C. pipiens larvae did not significantly differ between treatments (log-rank test, χ 2 = 3.50, P = 0.32); mortality in control treatment was 16.6 % (Fig. 3b).
Discussion
We found sticklebacks to exert size-selective predation upon C. pipiens larvae (“Experiment 1”). In case of newly hatched (L1) larvae exposed to stickleback kairomones, pupae became significantly smaller and lighter compared to the control treatment without kairomones (“Experiment 2”). These observations were corroborated by investigating imagines, where we found a strong reduction in body size after exposure to stickleback kairomones (“Experiment 3”); however, body weight was reduced in all fish kairomone treatments irrespective of fish identity.
Size selective predation on aquatic arthropods by piscine predators is well documented for other teleost fishes; e.g., bluegill sunfish (Lepomis macrochirus) prefer large amphipod prey (Wellborn 1994), pumpkinseed sunfish (L. gibbosus) prefer large dragonfly larvae (Dixon and Baker 1988), and brook trout (Salvelinus fontinalis) exhibit a preference for large mayfly larvae (Allan 1978). We argue that lower predation risk of small-sized C. pipiens larvae by their common piscine predator in European freshwaters, the three-spined stickleback (G. aculeatus), provides them with a relative advantage over larger larvae in sites with high levels of predation, and so we asked if mosquitoes respond to predation risk with altered larval life histories, especially with suppressed growth, which was confirmed in experiment 2.
We further asked how specific this response is, and whether mosquito larvae differ in their responses to a co-evolved piscine predator, a not co-evolved (invasive) predator, and a non-insectivorous fish. Investigating the response of native prey organisms to novel (invasive) predators is of particular interest in light of the steady increase of biological invasions worldwide (Mack et al. 2000; Sakai et al. 2001). Different predators usually release predator-specific chemical profiles (Relyea 2001; Iyengar and Harvell 2002; Relyea 2003), but it remains to be investigated if prey species can respond to novel predator types with which they have not coevolved. Moreover, our second experiment as well as several previous studies reporting suppressed growth of mosquito larvae exposed to predator kairomones (e.g., van Uitregt et al. 2012 for A. notoscriptus; Ohba et al. 2012 for C. tritaeniorhynchus) did not answer the question of whether suppressed larval body growth is merely an indirect consequence of reduced activity (and thus, reduced feeding), or if mosquito larvae actively alter larval growth patterns as an adaptive life history response to evade predation—experiment 3 in our present study provides answers to both questions.
Our prediction for an adaptive response to predation risk was that larvae might accelerate larval development to evade predation (see “Introduction”), but we found no support for such a pattern. Neither did we find delayed developmental times in our present study (an effect found in previous studies, e.g., Beketov and Liess 2007; van Uitregt et al. 2012), and so it seems that developmental times show rather narrow reaction norms in our study population. Still, the results of experiment 3 suggest that mosquito larvae exhibit an unspecific stress response to kairomones from different fish species, as it seems plausible to explain reduced body weight as a consequence of reduced activity and thus, reduced food uptake (compare Beketov and Liess 2007; van Uitregt et al. 2012). Generally, time spent foraging correlates positively with the likelihood of being detected by visual predators or encountering ambush predators, and so individuals typically decrease foraging under predation risk (Lima and Dill 1990; Benard 2004; Stoks et al. 2005). Also C. pipiens larvae were found to move less when exposed to kairomones from a heteropteran predator, the backswimmer Notonecta undulata (Sih 1986), and reduced foraging under the influence of predator kairomones was also reported from other culicid larvae (Kesavaraju and Juliano 2004; Ohba et al. 2012).
Moreover, we detected another effect—reduced body size—that was strongest in the treatment with stickleback kairomones, considerably weaker when kairomones of insectivorous, but not co-evolved, Gambusia were present, while no effect at all was seen in the Ancistrus treatment. Since we did not measure activity in our study, a link between reduced foraging and reduced body size cannot be excluded. However, if this explanation was true, and if our interpretation is correct that equally reduced body weight in all three fish kairomone treatments is the result of reduced activity (see above), then we would have expected at least slightly reduced body size also in the treatment using Ancistrus kairomones, but no such pattern was uncovered (Fig. 4c). We calculated the ratio between body weight and body size. The non-specific body weight reduction in all kairomone treatments was reflected by a high body mass-to-body size ratio in the control treatment (0.270; calculated from EMMs), but the highest ratio among the fish kairomone treatments was detected when Gasterosteus kairomones were presented (Ancistrus: 0.254, Gasterosteus: 0.261, Gambusia: 0.254), and so it appears as if larvae are indeed actively suppressing growth and thus, become ‘denser’ (i.e., have a higher weight-to-body size ratio compared to the Ancistrus and Gambusia treatments) to reduce predation risk in the presence of their common predator. This suggests that, at least in C. pipiens, the evolutionary history of this predator–prey interaction seems to be more important than the relative risk of predation posed by the predator, and some component of sticklebacks’ chemical cues may allow a specific recognition of the coevolved predator. The significance of evolutionary history in predator–prey recognition was also observed by Alvarez et al. (2014), who investigated predator avoidance behavior of mayfly larvae (Baetis spp.) to chemical cues (cutaneous mucus). The authors showed that larvae exhibited no behavioral response to novel predator species, while larvae did respond to five co-occurring freshwater fishes. However, the response to co-occurring fishes was not predator-specific even though fish species differed in their strength of predation on mayfly larvae. Given that all fish species in our study obtained the same diet, species-specific kairomones likely stemmed from a combination of cutaneous mucus and mucosa-associated bacteria (Beklioglu et al. 2006; Alvarez et al. 2014).
With regards to reduced larval body size, our results suggest that size reduction confers benefits under predation risk. However, since body size of female mosquitoes correlates positively with fecundity (Briegel 1990; Lyimo and Takken 1993; McCann et al. 2009), this reduction may come at a cost at the adult stage, essentially leading to a life-history trade-off. Furthermore, van Uitregt et al. (2012) found smaller mosquito imagines to be less resistant to starvation, while larger imagines can have longer reproductive life-spans (Neems et al. 1990), have an increased ability to disperse (Kaufmann et al. 2013), and tend to be superior in mate competition (Wellborn and Bartholf 2005). This trade-off between costs and benefits of suppressed larval growth likely governs the evolution of the remarkable plasticity of larval life histories in C. pipiens we describe here, as subsequent larval generations can experience starkly different piscine predation pressures.
Several studies have shown how alternative phenotypes can be induced through alterations of hormone release and enzymatic activity, as well as altered gene expression mediated by DNA methylation and transcription factor activation (Gilbert and Epel 2009; Miyakawa et al. 2010; Snell-Rood et al. 2010; Beldade et al. 2011; Sommer and Ogawa 2011; Schneider et al. 2014). These mechanisms are highly interactive: environmental cues can affect the dynamics of hormone production and thus, trigger changes in hormone titers, while hormones can affect gene expression (Gilbert and Epel 2009; Beldade et al. 2011). Future studies will need to address the molecular mechanisms underlying adaptive life-history shifts of mosquito larvae exposed to predator kairomones. This may also answer the question of how specific kairomones of the investigated fish species are and how these affect the entailing cascades. The reduced body weight in all fish treatments (Fig. 4b) implies that this particular response is triggered by chemical cues shared by a broad array of teleost species, whereas the reduction in body size was much more specific: the co-evolved predator triggered the strongest response, but a weak (albeit not significant) response was also triggered by Gambusia. This may be explained by the greater phylogenetic proximity between sticklebacks and Gambusia (Betancur-R et al. 2013), resulting in somewhat similar chemical profiles. Furthermore, it remains to be tested if mosquitoes will evolve predator recognition in regions where Gambusia is invasive.
Finally, the picture becomes even more complex when other studies on adaptive responses to invasive alien species are evaluated. Some studies found no behavioral anti-predator response of native species to an unknown predator (e.g., Kesavaraju and Juliano 2004; Kesavaraju et al. 2007), whereas others found morphological and behavioral adaptations to invasive predators (e.g., Flecker 1992; Pease and Wayne 2014). Thus, our study demonstrates that—with regards to invertebrate prey presented with chemical cues of potential predators—the lack of a specific response does not always equate to an inability of the prey species to discriminate. Rather, the antipredator response can be finely nuanced so that only certain traits exhibit a predator-specific response, while other traits show similar responses across a range of potential (co-evolved native or invasive alien) predators.
Author contribution statement
JJ, RM and MP conceived and designed the study. JJ and JB conducted the experiments. JJ, RR, RM and MP analyzed data. RM and MP provided laboratory, rearing and infrastructural possibilities. All authors wrote, read and approved the manuscript.
References
Afify A, Galizia CG (2015) Chemosensory cues for mosquito oviposition site selection. J Med Entomol. doi:10.1093/jme/tju024
Agrawal AA (2001) Phenotypic plasticity in the interactions and evolution of species. Science 294:321–326
Allan JD (1978) Trout predation and the size composition of stream drift. Limnol Oceanogr 23:1231–1237
Alvarez M, Landeira-Dabarca A, Peckarsky B (2014) Origin and specificity of predatory fish cues detected by Baetis larvae (Ephemeroptera; Insecta). Anim Behav 96:141–149
Auld JR, Agrawal AA, Relyea RA (2009) Re-evaluating the costs and limits of adaptive phenotypic plasticity. Proc R Soc Lond B Biol Sci 277:503–511
Beketov MA, Liess M (2007) Predation risk perception and food scarcity induce alterations of life-cycle traits of the mosquito Culex pipiens. Ecol Entomol 32:405–410
Beklioglu M, Telli M, Gozen AG (2006) Fish and mucus-dwelling bacteria interact to produce a kairomone that induces diel vertical migration in Daphnia. Freshw Biol 51:2200–2206
Beldade P, Mateus ARA, Keller RA (2011) Evolution and molecular mechanisms of adaptive developmental plasticity. Mol Ecol 20:1347–1363
Benard MF (2004) Predator-induced phenotypic plasticity in organisms with complex life histories. Annu Rev Ecol Syst 35:651–673
Betancur-R R et al (2013) The tree of life and a new classification of bony fishes. PLoS Curr 2013:5. doi:10.1371/currents.tol.53ba26640df0ccaee75bb165c8c26288
Bradshaw WE, Holzapfel CM (1992) Reproductive consequences of density-dependent size variation in the pitcherplant mosquito, Wyeomyia smithii (Diptera: Culicidae). Ann Entomol Soc Am 85:274–281
Briegel H (1990) Metabolic relationship between female body size, reserves, and fecundity of Aedes aegypti. J Insect Physiol 36:165–172
Brooks JL, Dodson SI (1965) Predation, body size, and composition of plankton. Science 150:28–35
Caudill CC, Peckarsky BL (2003) Lack of appropriate behavioral or developmental responses by mayfly larvae to trout predators. Ecology 84:2133–2144
Crowl TA, Covich AP (1990) Predator-induced life-history shifts in a freshwater snail. Science 247:949–951
DeWitt TJ, Sih A, Wilson DS (1998) Costs and limits of phenotypic plasticity. Trends Ecol Evol 13:77–81
Dixon S, Baker R (1988) Effects of size on predation risk, behavioural response to fish, and cost of reduced feeding in larval Ischnura verticalis (Coenagrionidae: Odonata). Oecologia 76:200–205
Dodson S (1989) Predator-induced reaction norms. Bioscience 39:447–452
Dodson SI, Crowl TA, Peckarsky BL, Kats LB, Covich AP, Culp JM (1994) Non-visual communication in freshwater benthos: an overview. J North Am Benthol Soc 13:268–282. doi:10.2307/1467245
Ferrari MC, Wisenden BD, Chivers DP (2010) Chemical ecology of predator-prey interactions in aquatic ecosystems: a review and prospectus. Can J Zool 88:698–724
Flecker AS (1992) Fish predation and the evolution of invertebrate drift periodicity: evidence from neotropical streams. Ecology 73:438–448
Forward RB, Rittschof D (2000) Alteration of photoresponses involved in diel vertical migration of a crab larva by fish mucus and degradation products of mucopolysaccharides. J Exp Mar Biol Ecol 245:277–292
Gilbert SF, Epel D (2009) Ecological developmental biology: integrating epigenetics, medicine, and evolution. Sinauer Associates, Sunderland
Harbach RE, Harrison BA, Gad AM (1984) Culex (culex) molestus Forskal (Diptera: Culicidae): neotype designation, description, variation, and taxonomic status. Proc Entomol Soc Wash 86:521–542
Hebert PD, Grewe PM (1985) Chaoborus-induced shifts in the morphology of Daphnia ambigua. Limnol Oceanogr 30:1291–1297
Heulett ST, Weeks SC, Meffe GK (1995) Lipid dynamics and growth relative to resource level in juvenile eastern mosquitofish (Gambusia holbrooki: Poeciliidae). Copeia 1995:97–104
Huryn AD, Chivers DP (1999) Contrasting behavioral responses by detritivorous and predatory mayflies to chemicals released by injured conspecifics and their predators. J Chem Ecol 25:2729–2740
Hynes H (1950) The food of fresh-water sticklebacks (Gasterosteus aculeatus and Pygosteus pungitius), with a review of methods used in studies of the food of fishes. J Anim Ecol 19:36–58
Iyengar EV, Harvell CD (2002) Specificity of cues inducing defensive spines in the bryozoan Membranipora membranacea. Mar Ecol Prog Ser 225:205–218
Kaufmann C, Reim C, Blanckenhorn WU (2013) Size-dependent insect flight energetics at different sugar supplies. Biol J Linn Soc 108:565–578. doi:10.1111/j.1095-8312.2012.02042.x
Kesavaraju B, Juliano S (2004) Differential behavioral responses to water-borne cues to predation in two container-dwelling mosquitoes. Ann Entomol Soc Am 97:194
Kesavaraju B, Alto BW, Lounibos LP, Juliano SA (2007) Behavioural responses of larval container mosquitoes to a size-selective predator. Ecol Entomol 32:262–272
Kreß A, Oehlmann J, Kuch U, Müller R (2014) Impact of temperature and nutrition on the toxicity of the insecticide λ-cyhalothrin in fulllifecycle tests with the target mosquito species Aedes albopictus and Culex pipiens. J Pest Sci 87:739–750. doi:10.1007/s10340-014-0620-4
Krueger DA, Dodson SI (1981) Embryological induction and predation ecology in Daphnia pulex. Limnol Oceanogr 26:219–223
Krumholz LA (1948) Reproduction in the Western Mosquitofish, Gambusia affinis affinis (Baird & Girard) and its use in mosquito control. Ecol Monogr 18:1–43. doi:10.2307/1948627
Kumar R, Hwang JS (2006) Larvicidal efficiency of aquatic predators: a perspective for mosquito biocontrol. Zool Stud 45:447–466
Langerhans RB, Layman CA, Shokrollahi A, DeWitt TJ (2004) Predator-driven phenotypic diversification in Gambusia affinis. Evolution 58:2305–2318
Lass S, Spaak P (2003) Chemically induced anti-predator defences in plankton: a review. Hydrobiologia 491:221–239
Lima SL, Dill LM (1990) Behavioral decisions made under the risk of predation: a review and prospectus. Can J Zool 68:619–640
Lyimo EO, Takken W (1993) Effects of adult body size on fecundity and the pre-gravid rate of Anopheles gambiae females in Tanzania. Med Vet Entomol 7:328–332
Mack RN, Simberloff D, Lonsdale WM, Evans H, Clout M, Bazzaz FA (2000) Biotic invasions: causes, epidemiology, global consequences, and control. Ecol Appl 10:689–710. doi:10.2307/2641039
McCann S, Day JF, Allan S, Lord CC (2009) Age modifies the effect of body size on fecundity in Culex quinquefasciatus Say (Diptera: Culicidae). J Vector Ecol 34:174–181
Medlock J, Snow K (2008) Natural predators and parasites of British mosquitoes—a review. European Mosquito Bulletin 25:1–11
Miyakawa H et al (2010) Gene up-regulation in response to predator kairomones in the water flea, Daphnia pulex. BMC Dev Biol 10:45
Müller R, Knautz T, Völker J, Kreß A, Kuch U, Oehlmann J (2013) Appropriate larval food quality and quantity for Aedes albopictus (Diptera: Culicidae). J Med Entomol 50:668–673
Neems R, McLachlan A, Chambers R (1990) Body size and lifetime mating success of male midges (Diptera: Chironomidae). Anim Behav 40:648–652
Nijhout H, Wheeler D (1996) Growth models of complex allometries in holometabolous insects. Am Nat 148:40–56
Nilsson PA, Brönmark C (2000) Prey vulnerability to a gape-size limited predator: behavioural and morphological impacts on northern pike piscivory. Oikos 88:539–546
OECD (2004) OECD guideline for testing chemicals: Test No. 219: Sediment-water chironomid toxicity using 474 spiked water. Organization for Economic Cooperation and Development, Paris
Offill Y, Walton W (1999) Comparative efficacy of the threespine stickleback (Gasterosteus aculeatus) and the mosquitofish (Gambusia affinis) for mosquito control. J Am Mosq Control Assoc 15:380–390
Ohba S-Y, Ohtsuka M, Sunahara T, Sonoda Y, Kawashima E, Takagi M (2012) Differential responses to predator cues between two mosquito species breeding in different habitats. Ecol Entomol 37:410–418
Palmer MA, Poff NL (1997) The influence of environmental heterogeneity on patterns and processes in streams. J North Am Benthol Soc 16:169–173
Pease KM, Wayne RK (2014) Divergent responses of exposed and naive Pacific tree frog tadpoles to invasive predatory crayfish. Oecologia 174:241–252. doi:10.1007/s00442-013-2745-1
Peckarsky BL, Taylor BW, McIntosh AR, McPeek MA, Lytle DA (2001) Variation in mayfly size at metamorphosis as a developmental response to risk of predation. Ecology 82:740–757
Plath M, Parzefall J, Schlupp I (2003) The role of sexual harassment in cave and surface dwelling populations of the Atlantic molly, Poecilia mexicana (Poeciliidae, Teleostei). Behav Ecol Sociobiol 54:303–309
Relyea RA (2001) Morphological and behavioral plasticity of larval anurans in response to different predators. Ecology 82:523–540
Relyea RA (2003) How prey respond to combined predators: a review and an empirical test. Ecology 84:1827–1839
Reznick D (1982) The impact of predation on life history evolution in Trinidadian guppies: genetic basis of observed life history patterns. Evolution 36:1236–1250
Reznick D, Endler JA (1982) The impact of predation on life history evolution in Trinidadian guppies (Poecilia reticulata). Evolution 36:160–177. doi:10.2307/2407978
Riesch R, Plath M, Schlupp I (2010) Toxic hydrogen sulfide and dark caves: life-history adaptations in a livebearing fish (Poecilia mexicana, Poeciliidae). Ecology 91:1494–1505. doi:10.1890/09-1008.1
Riesch R, Martin RA, Langerhans RB (2013) Predation’s role in life-history evolution of a livebearing fish and a test of the Trexler-DeAngelis model of maternal provisioning. Am Nat 181:78–93
Rosenheim JA, Kaya H, Ehler L, Marois JJ, Jaffee B (1995) Intraguild predation among biological-control agents: theory and evidence. Biol Control 5:303–335
Sakai AK et al (2001) The population biology of invasive species. Annu Rev Ecol Syst 32:305–332. doi:10.1146/annurev.ecolsys.32.081501.114037
Salo P, Korpimäki E, Banks PB, Nordström M, Dickman CR (2007) Alien predators are more dangerous than native predators to prey populations. Proc R Soc Lond B Biol Sci 274:1237–1243
Schneider RF, Li Y, Meyer A, Gunter HM (2014) Regulatory gene networks that shape the development of adaptive phenotypic plasticity in a cichlid fish. Mol Ecol 23:4511–4526. doi:10.1111/mec.12851
Sih A (1986) Antipredator responses and the perception of danger by mosquito larvae. Ecology 67:434–441
Snell-Rood EC, Van Dyken JD, Cruickshank T, Wade MJ, Moczek AP (2010) Toward a population genetic framework of developmental evolution: the costs, limits, and consequences of phenotypic plasticity. BioEssays 32:71–81
Sommer RJ, Ogawa A (2011) Hormone signaling and phenotypic plasticity in nematode development and evolution. Curr Biol 21:R758–R766
Spencer M, Blaustein L, Cohen JE (2002) Oviposition habitat selection by mosquitoes (Culiseta longiareolata) and consequences for population size. Ecology 83:669–679
Stearns SC (1989) Trade-offs in life-history evolution. Funct Ecol 3:259–268
Stevens DJ, Hansell MH, Freel JA, Monaghan P (1999) Developmental trade–offs in caddis flies: increased investment in larval defence alters adult resource allocation. Proc R Soc Lond B Biol Sci 266:1049–1054
Stoks R, Block MD, Van De Meutter F, Johansson F (2005) Predation cost of rapid growth: behavioural coupling and physiological decoupling. J Anim Ecol 74:708–715. doi:10.1111/j.1365-2656.2005.00969.x
Twohy DW, Rozeboom LE (1957) A comparison of food reserves in autogenous and anautogenous Culex pipiens populations. Am J Epidemiol 65:316–324
van Uitregt VO, Hurst TP, Wilson RS (2012) Reduced size and starvation resistance in adult mosquitoes, Aedes notoscriptus, exposed to predation cues as larvae. J Anim Ecol 81:108–115. doi:10.1111/j.1365-2656.2011.01880.x
Vidal O, Garcia-Berthou E, Tedesco PA, Garcia-Marin J-L (2010) Origin and genetic diversity of mosquitofish (Gambusia holbrooki) introduced to Europe. Biol Invasions 12:841–851. doi:10.1007/s10530-009-9505-5
Vinogradova EB (2000) Culex pipiens pipiens mosquitoes: taxonomy, distribution, ecology, physiology, genetic, applied importance and control. Pensoft Publishers, Sofia, Bulgaria
Walker JA (1997) Ecological morphology of lacustrine threespine stickleback Gasterosteus aculeatus L. (Gasterosteidae) body shape. Biol J Linn Soc 61:3–50
Wellborn GA (1994) Size-biased predation and prey life histories: a comparative study of freshwater amphipod populations. Ecology 75:2104–2117
Wellborn GA, Bartholf SE (2005) Ecological context and the importance of body and gnathopod size for pairing success in two amphipod ecomorphs. Oecologia 143:308–316
Werner EE (1974) The fish size, prey size, handling time relation in several sunfishes and some implications. J Fish Res Bd Can 31:1531–1536
Wilbur HM (1980) Complex life cycles. Annu Rev Ecol Syst 11:67–93
Acknowledgments
We thank H. Geupel and E. Wörner, who kindly helped with animal care. We also thank J. Kirchgesser for help with data assessment. Artworks (drawings of C. pipiens larvae and pupae, as well as G. aculeatus) were provided by V. Achenbach (ink-theater.com). The present study was prepared at the Biodiversity and Climate Research Centre (BiK-F), Frankfurt am Main, and financially supported by the research funding program “LOEWE—Landes-Offensive zur Entwicklung Wissenschaftlich-ökonomischer Exzellenz” of the Hessian Ministry of Higher Education, Research, and the Arts. We further thank two anonymous reviewers for their valuable comments that helped to improve the manuscript. The authors do not have any conflict of interests to declare.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jourdan, J., Baier, J., Riesch, R. et al. Adaptive growth reduction in response to fish kairomones allows mosquito larvae (Culex pipiens) to reduce predation risk. Aquat Sci 78, 303–314 (2016). https://doi.org/10.1007/s00027-015-0432-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00027-015-0432-5