Abstract
Fungal infections are an increasing threat to global public health. There are more than six million fungal species worldwide, but less than 1% are known to infect humans. Most of these fungal infections are superficial, affecting the hair, skin and nails, but some species are capable of causing life-threatening diseases. The most common of these include Cryptococcus neoformans, Aspergillus fumigatus and Candida albicans. These fungi are typically innocuous and even constitute a part of the human microbiome, but if these pathogens disseminate throughout the body, they can cause fatal infections which account for more than one million deaths worldwide each year. Thus, systemic dissemination of fungi is a critical step in the development of these deadly infections. In this review, we discuss our current understanding of how fungi disseminate from the initial infection sites to the bloodstream, how immune cells eliminate fungi from circulation and how fungi leave the blood and enter distant organs, highlighting some recent advances and offering some perspectives on future directions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prevalence of fungal infections has been steadily increasing over the last three decades due to the increased use of immunosuppressants, as well as the increased number of patients with HIV/AIDS [1,2,3]. It is estimated that there are more than 6 million fungal species worldwide, but only a small fraction (less than 600) are capable of causing diseases in humans [3, 4]. While the majority of these fungal infections are superficial, some fungal species are capable of causing life-threatening infections [3]. The most common of these include Cryptococcus neoformans, Aspergillus fumigatus and Candida albicans [5].
C. neoformans and A. fumigatus are found throughout the environment and are inhaled into the lungs, where they initially cause pulmonary infections [1, 6]. C. albicans on the other hand is a human commensal and commonly colonizes mucosal tissues but can also be acquired in healthcare settings [1, 3, 7]. When these opportunistic pathogens disseminate from sites of initial infection, they can cause serious diseases including meningoencephalitis, invasive aspergillosis and invasive candidiasis, respectively. Together, these and other fungal infections affect over one billion people each year and kill more than 1.5 million annually [8]. In the USA alone, fungal diseases are estimated to cost upwards of $7.2 billion each year, a trend that is expected to continue to rise as advances in medical care for immunocompromised patients continue to be made [9]. As such, fungal infections impose a considerable economic burden on healthcare systems worldwide and represent an increasing threat to global public health [9]. Furthermore, current antifungal treatment options remain limited and are threatened by the continued emergence of resistant fungal strains [10, 11]. For these reasons, it is critical that we understand the pathogenesis of fungal diseases, specifically the ways by which mycopathogens disseminate, in order to develop alternative antifungal therapies. In this review, we discuss how C. neoformans, A. fumigatus and C. albicans disseminate from initial sites of infection to distant organs.
Dissemination of C. neoformans
C. neoformans is an encapsulated yeast fungus found globally in soil and avian excrement [6]. Infectious propagules in the form of desiccated yeast or spores are inhaled into the respiratory tract on a daily basis [12]. Due to their small size, these organisms are able to avoid cough and mucociliary clearance and penetrate deep into alveolar spaces [1]. Here, C. neoformans encounters lung resident immune cells. In healthy, or immunocompetent, individuals, these fungi are either successfully cleared or establish long-term, latent infections [1]. Alternatively, in the case of immunocompromised individuals, including AIDS patients, organ transplant recipients and patients treated with immunosuppressive therapies, C. neoformans can establish symptomatic pulmonary infections resulting in pneumonia, acute respiratory distress syndrome, and subsequent extrapulmonary dissemination [13]. Disseminated C. neoformans enters the bloodstream and can infect distant organs, but preferentially targets the central nervous system (CNS) [12]. If these fungal cells cross the blood–brain barrier (BBB) and enter the brain parenchyma, they can cause meningoencephalitis [14]. The global incidence of cryptococcal meningoencephalitis is estimated to be more than 220,000 cases annually and results in approximately 180,000 deaths each year [15].
Extrapulmonary dissemination
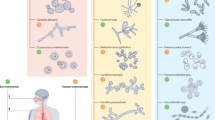
There are a number of proposed routes by which C. neoformans is thought to escape the lungs. These include an intracellular route within phagocytes known as the “Trojan horse” mechanism, transcellular crossing through epithelial cells, paracellular crossing between epithelial cells and an extracellular route in which free fungi escape through damaged epithelial barriers [12] (Fig. 1).
Mechanisms mediating extrapulmonary dissemination of C. neoformans a Trojan horse: following phagocytosis, C. neoformans (green) is able to survive within alveolar macrophages (purple) and is transported across the epithelium inside these migrating phagocytes where they can access the interstitium, lymph system and/or bloodstream. C. neoformans can exit macrophages through vomocytosis and disseminate throughout the host as free fungi or be taken up by peripheral monocytes in the bloodstream and transported to the vasculature of various organs including the brain. b Transcytosis: C. neoformans adheres to epithelial cells through interactions between glucuronoxylomannan (GXM) and CD14 as well as palmitic acid (PA) and surfactant protein-D (SP-D) receptors. Following adherence, epithelial cells endocytose yeast, transporting them across the epithelium. c Paracellular crossing and loss of barrier integrity: C. neoformans induces the production of IL-33 (peach), which along with urease (purple) disrupts tight junctions and allows fungal cells to pass between epithelial cells. C. neoformans also secretes Plb1 (green), urease (purple) and proteases (yellow) which damage epithelial barriers, allowing fungi to cross the epithelium and access the bloodstream
Trojan horse
Following pulmonary infection, one of the first immune cells C. neoformans encounters are lung resident alveolar macrophages. These professional phagocytes rapidly ingest cryptococcal cells for degradation [16, 17]. However, C. neoformans is a facultative intracellular pathogen and is equipped to survive and replicate within these cells [16]. This enables viable yeast to be transported out of the lungs within migrating phagocytes, a process known as the Trojan horse mechanism of dissemination [12].
Early studies provided indirect evidence supporting this mechanism, by demonstrating that the inability to survive within macrophages correlates with decreased extrapulmonary dissemination [18,19,20], while adoptive transfer studies confirmed that macrophages could indeed facilitate hematogenous dissemination to the brain [18, 21]. In addition, depletion of macrophages using clodronate liposomes significantly diminished dissemination to the CNS, suggesting that these cells may contribute to the extrapulmonary spread of C. neoformans [21,22,23]. It was later determined that CD11c+ lung resident cells, primarily alveolar macrophages and to a lesser extent dendritic cells, are responsible for internalizing and transporting cryptococci to the lymph system following intranasal instillation [24]. This event occurs early during infection, as fungal burdens were detected in mediastinal lymph nodes in as little as 24 h post-infection [24], and indicates that transport of C. neoformans may be an unintended consequence of the antigen-presenting function of these phagocytes. Depletion of these CD11c+ populations in transgenic mice resulted in a complete loss of dissemination [24], further supporting the role of these cells in Trojan horse transport.
In order to utilize phagocytes as vehicles for dissemination, C. neoformans must successfully survive within and exit from these cells following internalization. This requires that C. neoformans defends against acidic, oxidative and nitrosative stresses commonly encountered within macrophages [12]. Unlike A. fumigatus and C. albicans, which inhibit phagolysosomal maturation and must escape to the cytoplasm in order to establish an intracellular niche, C. neoformans can survive and replicate within phagosomes [25, 26]. The ability to grow in those acidic pHs found within mature phagolysosomes is dependent on C. neoformans expression of inositol phosphosphingolipid–phospholipase C 1 (Isc1) [20, 27]. Protection against oxidative and nitrosative stresses on the other hand is conferred by the cryptococcal capsule (a complex polysaccharide structure composed primarily of glucuronoxylomannan (GXM) and glucuronoxylomannogalactan (GXMGal)) [28,29,30,31,32,33,34], the iron oxidase laccase [35, 36] and the antioxidant melanin [19, 35,36,37,38]. In addition, phospholipase B1 (Plb1) is thought to alter macrophage activation through the production of immune regulatory eicosanoids in order to further promote intracellular survival and replication [39,40,41].
Once phagocytosed, C. neoformans can exit macrophages following intracellular replication. Cryptococcal replication within phagocytes is accompanied by the formation of a leaky phagosome and the accumulation of polysaccharide-containing vesicles in the cytoplasm and eventually culminates in the lysis of host cells [42]. This event triggers local inflammation and leaves extracellular C. neoformans exposed to host immune responses [43]. Conversely, C. neoformans can exit macrophages via non-lytic exocytosis, or vomocytosis. Vomocytosis is mediated by phagosomal extrusion: a process in which mature phagosomes containing cryptococcal cells fuse with the plasma membrane and release fungi into the extracellular milieu [43,44,45,46]. The cellular and molecular mechanisms governing vomocytosis remain poorly understood, but are reported to involve both host and pathogen factors [45, 47, 48]. After the expulsion event, both phagocytes and fungi remain viable, allowing C. neoformans to stealthily exit macrophages without triggering cell death-associated inflammation [43]. C. neoformans promotes vomocytosis through its expression of urease [49]. Urease catalyzes the hydrolysis of urea to yield the weak base ammonia [50, 51], which acts as a buffer and raises the pH within phagosomes [49]. For other pathogens, this activity can inhibit acid-induced damage; however, in the case of C. neoformans, this increase in pH results in yeast entering into a quiescent state, in which intracellular replication is delayed [25, 49]. This prolongs intracellular residence of C. neoformans and increases the likelihood that fungal cells will be transported outside of the lung prior to the non-lytic expulsion of dormant yeast.
Transcytosis
In addition to intracellular means of escape, C. neoformans can exit the lungs as free fungi via a number of extracellular routes. One such pathway is transcellular crossing, also known as transcytosis. This mechanism involves exploitation of host cell endocytic pathways to facilitate fungal traversal of epithelial barriers and grants C. neoformans access to the lung interstitium, vasculature or lymph system [12].
Transcytosis is preceded by fungal cell adherence to the epithelium. To date, a limited number of adhesins have been identified as mediating cryptococcal–epithelial interactions. Initial findings demonstrated that both encapsulated and acapsular strains of C. neoformans adhere to A549 lung epithelial cells in vitro [52, 53]. In the case of encapsulated C. neoformans, the major capsule component GXM is responsible for mediating adherence by binding to CD14 on epithelial cells [54, 55]. As for acapsular strains, the mannoprotein MP84 was determined to bind A549 cells through interactions with a yet identified host cell receptor [56]. In addition, a temperature-sensitive adhesin was reported to facilitate adherence independently of GXM and was determined to be cryptococcal Plb1 [52, 54, 57]. Plb1 possesses multiple enzymatic activities that allow it to break down phospholipids found in host cell membranes and lung surfactant [40, 58, 59]. Degradation of dipalmitoylphosphatidylcholine (DPPC), the main component of lung surfactant, results in the release of palmitic acid (PA) which is thought to mediate adherence by binding to surfactant protein D (SP-D) receptors on A549 cells [41, 57]. This is supported by the dose-dependent increase in adherence that occurs when PA is added to co-cultures of A549 cells and a Plb1-deficient strain of C. neoformans [53, 57].
Adherence to epithelial cells results in the internalization of C. neoformans as well as eventual epithelial cell lysis [52, 54]. While little is known about the mechanisms involved in this process, it is likely actin-dependent, similar to those facilitating C. neoformans invasion of brain endothelial cells [60, 61].
Paracellular crossing and loss of barrier integrity
In addition to traveling through epithelial cells, C. neoformans can pass between epithelial cells as the result of mechanical or biochemical disruption of tight junctions, a process known as paracellular crossing or paracytosis [12]. During pulmonary infection, C. neoformans induces the production of interleukin (IL)-33 by alveolar type 2 epithelial cells [62]. IL-33 promotes type 2 innate immune responses and suppresses the expression of the tight junction protein E-cadherin which may allow fungal cells to pass through weakened tight junctions. Similarly, urease activity is thought to disrupt the integrity of the pulmonary epithelium. While the effects of cryptococcal urease on epithelial tissues have yet to be fully examined, its activity has been extensively studied in the context of the bacterial pathogen Helicobacter pylori and was reported to increase phosphorylation of myosin regulatory light chains (MLCs) in epithelial cells, which can in turn disrupt tight junctions and result in a loss of barrier function [63]. In this way, C. neoformans may disrupt tight junctions allowing for free fungi to pass between epithelial cells.
Extracellular C. neoformans can also pass freely through weakened or damaged epithelial barriers [12]. This model is supported by the observation that prolonged incubation of C. neoformans with A549 cells results in significant amounts of host cell death [54] and the fact that C. neoformans produces a number of enzymes capable of disrupting host tissues [64]. Of these, urease, phospholipases and proteases have been implicated in contributing to fungal dissemination [65].
In addition to disrupting epithelial tight junctions, urease activity was found to be toxic to host cells [50, 51]. Ammonia derived from H. pylori urease was reported to cause local tissue damage and induce host cell death [50, 51]. Urease has also been implicated in causing immune-mediated injury to host tissues, as it can act as a chemotactic factor and cause local inflammation as well as result in the production of inducible nitric oxide synthase (iNOS) which may also have cytotoxic effects [50, 51]. It is possible that cryptococcal urease may function similarly during pulmonary infection and could damage the epithelium, thereby promoting fungal dispersal and subsequent dissemination [65, 66].
Plb1 activity is also thought to contribute to the disruption of epithelial barriers. Once C. neoformans is in proximity to the epithelium, secreted or cell wall-associated Plb1 can access and degrade epithelial cell membranes [47, 67]. Disruption of these membranes can result in the lysis of host cells and lead to a weakened barrier through which extracellular yeast can pass [18, 65].
Finally, C. neoformans produces one or more unnamed serine proteases [68, 69]. These enzymes are capable of degrading extracellular matrix (ECM) and basement membrane elements [68]. Together, these proteolytic activities can assist C. neoformans in tissue invasion and dissemination by degrading structural components of the epithelium [68]. Supporting their role in the breakdown of host barrier tissues is the report that cryptococcal serine proteases mediate disruption of the BBB [69]. It is possible that within the lungs, these proteases could act similarly to disrupt epithelial barriers.
Intravascular clearance
Once outside of the lungs, cryptococcal cells can enter into the bloodstream resulting in blood infections known as fungemia [18, 19]. Early studies have demonstrated that brain infections occur following fungemia and that there is a direct correlation between the severity of the infection and the magnitude of fungemia [70,71,72]. As such, intravascular recognition and clearance of C. neoformans represent an important step in preventing dissemination to the central nervous system (Fig. 2).
Clearance of intravascaular C. neoformans a Neutrophils (dark purple) are recruited to intravascular C. neoformans (green) in a C5a-dependent manner. Fungal cells are phagocytosed by neutrophils via iC3b–CR3 interactions and are killed through oxidative and nonoxidative mechanisms. b Liver-resident Kupffer cells (light purple) recognize and bind C3b/iC3b on circulating C. neoformans through CRIg receptors. Following phagocytosis, Kupffer cells can inhibit cryptococcal growth and limit fungal dissemination to target organs
Neutrophils
Neutrophils are the most abundant phagocytes in the bloodstream and are typically the first immune cells to be recruited to sites of infection [73]. To date, their role in defense against C. neoformans remains controversial. In vitro, neutrophils were found to effectively kill cryptococci [74,75,76,77,78,79,80,81], although it was also reported that C. neoformans can negatively regulate the extracellular killing activity of these cells [82, 83]. In vivo, augmentation of neutrophil activity enhanced anticryptococcal activity [84,85,86], while impaired neutrophil activity significantly compromised survival during cryptococcal infection [87, 88]. These suggest that neutrophils may be protective. By contrast, depletion of neutrophils markedly reduced pulmonary fungal burdens following infection with C. neoformans [89], and in AIDS patients with cryptococcosis, enhanced blood neutrophil counts are associated with mortality [90], indicating instead that neutrophils may play a detrimental role during cryptococcal infection. Nevertheless, neutrophils have been shown to contribute to the intravascular clearance of disseminating C. neoformans through the efficient phagocytosis and removal of fungal cells from the microvasculature of organs as demonstrated by intravital imaging in both a mouse and zebrafish model [91, 92].
As C. neoformans circulates throughout the host, they can become mechanically trapped in the microvasculature of organs with closed capillary networks, including the brain [93]. Here, cryptococci activate the complement system [93,94,95,96,97] which induces neutrophil migration to arrested fungal cells [98,99,100]. Neutrophils then bind and ingest opsonized C. neoformans [91, 98] and, in turn, can secrete leukotriene B4 (LTB4), which attracts additional neutrophils to sites of fungal infection [101, 102]. Following phagocytosis, neutrophils can kill yeast through oxidative [74, 77,78,79,80] and nonoxidative mechanisms [81]. In this way, neutrophils help to limit dissemination by directly killing and/or removing fungal cells from the endothelium [91, 92, 98, 100].
This ability for neutrophils to clear intravascular cryptococci is less efficient in the brain as compared to other organs such as the lungs [100]. This is likely due to the fact that brain vasculature structure is different and lacks complement, which may explain the lower rates of neutrophil recruitment to the brain and the increased susceptibility of the CNS to infections by C. neoformans [99, 103]. Indeed, enhancing the recruitment of neutrophils to the brain vasculature significantly improves intravascular clearance of C. neoformans in the brain [100], further supporting the role of neutrophils in the clearance of disseminating C. neoformans in the blood.
Kupffer cells
In addition to neutrophils, liver-resident macrophages, known as Kupffer cells (KCs), have been shown to play a role in clearing intravascular C. neoformans. The liver is the largest internal organ, receiving 30% of the total volume of blood in the body each minute, and contains approximately 90% of the total tissue macrophages in the body [104, 105]. Here, KCs scan passing blood and remove potentially harmful substances [104,105,106,107,108,109] and have been identified as playing an important role in maintaining blood sterility through the capture and removal of intravascular bacteria and parasites [107,108,109,110]. Although the liver is not the target organ of C. neoformans, evidence suggests that liver disease is a risk factor for cryptococcosis [111,112,113,114]. In this context, recent results revealed that the liver plays a prominent role in filtering disseminating C. neoformans and C. albicans out of circulation through KCs [115, 116].
Intravital imaging showed that disseminating C. neoformans is captured in the liver sinusoids [116]. This capture is mediated by KCs through the recognition of complement C3 on fungal cells by complement receptor CRIg (complement receptor of the immunoglobulin superfamily). As such, depletion of KCs significantly reduces the fungal burden in the liver, leading to enhanced fungemia in the blood and increased deposition of fungi in other organs [116]. Following capture, KCs phagocytose C. neoformans and can inhibit fungal growth in a manner independent of IFN-γR signaling [116]. This demonstrates an important role for KCs in reducing fungal dissemination to other organs by directly removing organisms from circulation [116] and likely explains the association between liver disease and increased susceptibility to fungal infections [112,113,114].
Brain invasion
If C. neoformans avoids intravascular clearance, circulating fungal cells are preferentially deposited in the brain vasculature and eventually invade the brain [117]. Once inside the brain, C. neoformans begins to proliferate and causes fatal meningoencephalitis. Thus, although cryptococcal infection starts in the lungs, the most devastating event occurs when the fungus crosses the BBB and migrates to the brain parenchyma [117, 118]. The mechanism by which C. neoformans invades the brain remains to be completely understood. To date, there are a number of competing hypotheses that have been proposed for brain invasion by C. neoformans and include the Trojan horse mechanism, transcytosis, paracellular crossing and free entry through damaged endothelial barriers [118, 119] (Fig. 3).
Mechanisms mediating brain invasion by C. neoformans a Trojan horse: peripheral monocytes (dark pink) ingest intravascular C. neoformans (green) and transport them to the brain vasculature. Monocytes can carry yeast across the BBB through transendothelial pores (transcellular transmigration), or can paracellularly transmigrate between endothelial cells. In addition, monocytes can transfer C. neoformans directly to endothelial cells (red) (lateral transfer), facilitating transmigration of cryptococci across the BBB. b Transcytosis: hyaluronic acid (HA)–CD44 interactions along with Mpr1 promote C. neoformans adherence to brain endothelial cells. Engagement of CD44 activates EphA2, while cryptococcal Plb1 activates host cell Rac1 which in turn activates PKCα. Activation of these pathways induces endocytosis of C. neoformans. Following internalization, C. neoformans engages host AnxA2 via Mpr1 which facilitates exit of endothelial cells and successful crossing of the BBB. c Paracellular crossing and loss of barrier integrity: C. neoformans disrupts the BBB through the utilization of host plasmin (pink) and secretion of urease (purple) and other proteases (yellow) and migrates across the endothelium into the brain parenchyma
Trojan horse
It has been well reported that phagocytes can transmigrate from the blood to the brain parenchyma across the BBB [73], and as described, C. neoformans can survive within phagocytes [16]. This raised the possibility that, following phagocytosis of fungal cells, phagocytes can escort C. neoformans to the brain parenchyma through the so-called Trojan horse mechanism.
To directly visualize the dynamics of brain invasion by C. neoformans using Trojan horse crossing, live cell imaging was performed in vitro using human endothelial cell lines [120, 121]. The results confirmed that both C. neoformans and C. gattii can use the Trojan horse mechanism to cross endothelial cell layers [120]. Phagocytes derived from human monocytic cell lines containing C. neoformans were also seen to cross endothelial cell layers in real time [121]. Interestingly, transcellular transmigration (through endothelial cells) of monocytes harboring C. neoformans is the major pathway for Trojan horse crossing [121]. In contrast, paracellular transmigration (between endothelial cells) likely represents a small percentage of Trojan horse crossing as transendothelial electrical resistance (TEER) values remained stable during in vitro assays, indicating little to no disruption of endothelial tight junctions [121]. In vivo, monocytes containing C. neoformans were observed in the perivascular space of cortical post-capillary venules in the brains of infected mice [71, 122]. This is thought to be the major site of Trojan horse entry [122]. Once here, C. neoformans can escape the phagocyte and access the brain parenchyma and cerebrospinal fluid (CSF) [123], further confirming a role for these cells in dissemination to the CNS.
In addition to carrying yeast across the BBB, it has been proposed that phagocytes transport C. neoformans to the brain vasculature where they instead pass them directly to endothelial cells [124]. This cell-to-cell spread, known as lateral transfer or dragotcytosis, is an actin-dependent process that requires donor and acceptor cells to be in physical contact [124,125,126]. Originally, lateral transfer was observed between two macrophages [124, 125], but was later reported to occur between monocytes and endothelial cells in vitro [121]. Alternatively, phagocytes may carry fungal cells to the brain, where they then escape and cross the BBB alone through an extracellular mechanism [121].
Blood-derived monocytes have been implicated in the transport of C. neoformans across the BBB. In humans, these monocytes exist in two major populations: CD14hiCD16− and CD14lowCD16hi monocytes [127]. The corresponding populations in mice are CCR2+Ly6Chi and CX3CR1+Ly6Clow monocytes, respectively [128]. During intravenous infection with C. neoformans, CX3CR1+Ly6Clow monocytes are recruited to the brain vasculature starting at 12 h post-infection [129]. Through the use of intravital microscopy, these cells were observed to engulf C. neoformans in brain vasculature and carry the yeast as they crawled on and adhered to the luminal wall of brain vasculature and migrated to the brain parenchyma [129], suggesting the involvement of these monocytes in cryptococcal crossing of the BBB. CCR2+Ly6Chi monocytes on the other hand were observed to accumulate in the brain starting 14 days after intravenous infection, indicating that these cells are not involved in the early transport of C. neoformans to the CNS [130]. Instead, CCR2+Ly6Chi monocytes promote brain inflammation, leading to lethal immune pathology in the brain in mice as well as in humans [131, 132].
Transcytosis
In addition to Trojan horse crossing, extracellular C. neoformans cells can directly transmigrate across the BBB and invade the brain via transcytosis. Indirect evidence supporting this comes from a mouse model of C. neoformans infection, in which yeasts were detected in the cytoplasm of endothelial cells of small capillaries, suggesting that endothelial cells internalize C. neoformans in vivo [71]. Direct evidence on the other hand is derived from in vitro studies using human brain microvascular endothelial cells (HBMECs) [60, 133, 134]. In this model, free C. neoformans cells were observed to have crossed endothelial monolayers via a transcellular pathway that did not affect monolayer integrity [133].
Similar to the lungs, transcytosis in the brain involves exploitation of host cell endocytic pathways in order to traverse the BBB [119] and is dependent on cryptococcal adherence to and internalization by brain endothelial cells [133, 135]. Adherence of C. neoformans to endothelial cells occurs independently of capsule expression and is mediated by hyaluronic acid (HA) [136,137,138,139]. HA is the product of an HA synthase encoded by the gene CPS1 and binds primarily to CD44 receptors on brain endothelial cells, although the RHAMM receptor has also been reported to play a minor role in binding HA [140]. Interestingly, in response to the high levels of inositol found in mammalian brains, C. neoformans upregulates its expression of CPS1, leading to an increased production of HA and an enhanced association of cryptococcal cells and HBMECs [141].
Upon binding, C. neoformans is internalized by endothelial cells in an actin-dependent manner [60, 61, 142, 143]. Cryptococcal Plb1 activates host cell Rac1 (Ras-related C3 botulinum toxin substrate 1) which in turn activates the actin reorganizing protein, protein kinase C-α (PKCα) [138, 144, 145]. The mechanism by which this occurs is not well understood, though it has been suggested that Plb1 activity might generate lipid mediators required to facilitate Rac1 activation [145, 146]. In addition, engagement of CD44 by C. neoformans activates ephrin type-A receptor 2 (EphA2). This receptor is involved in various signaling pathways that regulate cytoskeleton remodeling [147] and has been reported to be utilized by a number of pathogens in order to invade host cells suggesting that C. neoformans could also use this pathway to enter endothelial cells [147].
Lipid rafts also play a role in the internalization of C. neoformans by endothelial cells. Supporting this is the report that the HA receptor CD44 co-localizes with the lipid raft marker ganglioside GM1 on the plasma membrane of endothelial cells and that C. neoformans adheres to host cells in areas where GM1 is enriched [143]. CD44 was also found to co-localize with and activate the caveolae membrane marker caveolin-1 (Cav1) upon engagement with C. neoformans [148]. These demonstrate that transcytosis of C. neoformans across the BBB occurs through a lipid raft/caveolae-dependent endocytotic process [143, 148].
C. neoformans can further promote internalization by endothelial cells, through secretion of C. neoformans-derived extracellular microvesicles (CnMVs). These vesicular compartments, referred to as “virulence bags,” contain polysaccharides, lipids and cytoplasmic proteins and can fuse with host cells independently of CD44 [149, 150]. This interaction increases lipid raft activity and promotes CD44 migration to membrane rafts [149]. In this way, CnMVs may enhance fungal adherence and endocytosis.
Transcytosis was also found to occur independently of CD44. C. neoformans further promotes its adherence to endothelial cells through expression of the metalloprotease, Mpr1 [151, 152]. Once bound, Mpr1 engages host annexin A2 (AnxA2), an important signaling protein involved in endocytosis that was found to be essential for the transmigration of cryptococcal cells across the endothelium [152, 153]. While the absence of AnxA2 in HBMECs had no effect on adherence or internalization of cryptococci, yeast cells were unable to exit endothelial cells and enter into the parenchyma following endocytosis, indicating that AnxA2-Mpr1-mediated interactions promote successful crossing of the BBB [152].
Paracellular crossing and loss of barrier integrity
Additional mechanisms of brain invasion by extracellular C. neoformans are paracellular crossing between brain endothelial cells as well as migration across damaged endothelial barriers [119]. These pathways involve the entry of free yeast through a damaged or weakened BBB and are supported by reports that accumulation of C. neoformans in the brain results in severe damage to endothelial cells as well as tight junction alterations [60, 121, 136]. In this context, C. neoformans produces several degradative enzymes capable of disrupting the BBB.
Cryptococcal urease has been identified as a major virulence factor during brain infection [154]. Initial studies reported that urease promotes cryptococcal sequestration within brain microcapillaries [155]. Later reports suggest a more active role for urease in cryptococcal penetration of the BBB [93]. While the mechanism remains unknown, it is possible that urease-derived ammonia may have toxic effects on endothelial cells which weakens the integrity of the BBB and promotes opening of tight junctions leading to transmigration of C. neoformans [93, 154,155,156]. This is supported by the fact that urease-positive cryptococcal strains cause a reduction in levels of the tight junction protein ZO1 in HBMECs, while urease-deficient strains have no effect on monolayer integrity [156].
C. neoformans also secretes a number of proteases including serine proteases, which were found to degrade key components of ECM and the basement membrane [64, 68, 69, 157, 158]. This activity was reported to increase BBB permeability during infection with C. neoformans both in vitro and in vivo [68, 69]. In addition to its own proteases, C. neoformans is capable of utilizing host proteases. Cryptococcal cells can bind and activate host plasminogen, a plasma protein and central component of the fibrinolytic system. This interaction promotes the conversion of plasminogen to the serine protease plasmin via the urokinase-type plasminogen activator (uPA) [159,160,161]. Plasmin digests components of the BBB, as well as activates matrix metalloproteinases, which are capable of damaging tight junction components [159].
In addition to those factors expressed by C. neoformans, it is possible that host behaviors can also lead to the disruption of endothelial barriers and promote cryptococcal neuroinvasion. For example, abuse of methamphetamines was reported to enhance dissemination of C. neoformans to the CNS [162] and alter BBB integrity through the modified expression of tight junction and adhesion molecules [163]. This pharmacological disruption to the endothelium promotes transmigration of yeast into the brain parenchyma, demonstrating that external factors can also aid in dissemination of C. neoformans to the brain [163]. Regardless of entry mechanism, once inside the brain, C. neoformans can rapidly proliferate and cause life-threatening cases of meningoencephalitis.
Dissemination of A. fumigatus
A. fumigatus is a ubiquitously distributed saprophytic fungus that produces small spores known as conidia. On average, individuals inhale upwards of 200 conidia per day [164, 165]. By virtue of their size, conidia are able to avoid cough and mucociliary clearance and enter deeper into the lungs. In healthy individuals, these conidia are rapidly cleared, but in the case of immunocompromised patients, conidia can germinate and develop filamentous hyphae [1, 166]. This fungal growth can result in invasive aspergillosis: a severe and aggressive fungal disease characterized by tissue damage, necrosis and hypoxia [8, 164, 167]. Although the target organ of A. fumigatus is the lungs, Aspergillus is known to be angiotropic and has an affinity for host vasculature. As growing hyphae penetrate pulmonary tissues, they can invade the endothelial lining of nearby blood vessels and break off into circulation, causing sepsis and dissemination accompanied by thrombosis, hemorrhagic infarction and invasion of distant organs [8, 164, 167]. It is estimated that there are approximately 250,000 cases of invasive aspergillosis worldwide each year, with an associated mortality rate ranging from 30 to 80% [8].
The primary route by which A. fumigatus is thought to traverse the pulmonary epithelium is transcytosis. As with C. neoformans, A. fumigatus adheres to and invades epithelial cells [164, 168,169,170]. A. fumigatus adherence to the epithelium is mediated by several fungal factors, including sialic acid (SA), galactosaminogalactan (GAG) and β-1,3-glucan binding to Dectin-1 [171,172,173]. Following adherence, A. fumigatus is endocytosed in an actin-dependent manner [174,175,176,177,178]. The fungal invasin calcineurin A (calA) promotes this internalization through interactions with integrin α5β1 on host cells [179]. In addition, gliotoxin, a major mycotoxin of A. fumigatus, as well as the cell wall component β-1,3-glucan, activates actin cytoskeleton rearranging proteins in host epithelial cells, including cofilin-1 and phospholipase D (PLD) [175, 177, 180, 181], further promoting internalization. Inside these cells, a portion of engulfed conidia are killed, while approximately 3% remain viable [174, 176]. Of those, one-third of surviving conidia germinate and form extracellular hyphae without lysing host cells [170, 174, 176]. These organisms can continue to grow and invade pulmonary tissues and nearby blood vessels.
A. fumigatus also encodes an array of degradative enzymes which may facilitate migration across the epithelium [182,183,184]. Serine and cysteine proteases, including the serine protease AF-ALF, disrupt host epithelial tissues [182,183,184]. The enzyme elastase is required for the development of invasive disease suggesting a role in dissemination [164, 185, 186]. In addition, a number of secondary metabolites, including gliotoxin, fumagillin, helvolic acid, and verruculogen, have been implicated in modifying the epithelium [164, 187, 188]. This combination of proteolytic activity may contribute to the disruption of host tissues and enable A. fumigatus to penetrate the epithelium and enter the bloodstream directly.
As for the Trojan horse mechanism, there is evidence suggesting that A. fumigatus may be able to utilize host phagocytes in order to escape the lung. Firstly, A. fumigatus can survive within phagocytes [189, 190]. Secondly, there are reports that monocyte-derived CD11b+ lung dendritic cells internalize and transport A. fumigatus to mediastinal lymph nodes [128]. This event is reminiscent of the transport of C. neoformans to the lymph system by alveolar macrophages. Once inside of the lymph node, Aspergillus could escape from dendritic cells and disseminate via the bloodstream. As A. fumigatus circulates throughout the host, it can become stopped in host microvasculature and can invade various organs including the liver, kidneys, spleen and brain [167]. To date, the mechanism by which A. fumigatus penetrates the BBB is not well understood, though studies have shown that gliotoxin (GTX) is able to damage the endothelium which may facilitate transmigration [191].
Dissemination of C. albicans
C. albicans is a polymorphic fungus that can transition from a yeast phase to a hyphal phase and is commonly found as a commensal in the human body, asymptomatically colonizing the gastrointestinal, respiratory and reproductive tracts, as well as skin of approximately 30–80% of people [1, 3, 7]. While a considerable proportion of C. albicans infections arise from indwelling medical devices [192], studies have identified the intestinal population of C. albicans as the main source of endogenous infection, especially following immunosuppression [193]. These organisms can cause lethal bloodstream infections (candidemia) and lead to invasive disease when they manage to cross the intestinal epithelium [193]. Approximately 700,000 cases of invasive candidiasis are diagnosed worldwide each year, with mortality rates of up to 40% [8, 194].
As with other fungi, invasion of epithelial barriers by C. albicans is preceded first by adherence to the intestinal epithelium. C. albicans expresses multiple surface moieties that mediate this adherence. These adhesins exhibit differential expression in yeast and hyphal forms and promote binding by different mechanisms. In the case of yeast, adherence is due to passive forces such as hydrophobic and/or electrostatic interactions as well as agglutinin-like sequence (Als) 5 [2, 195]. In addition, yeast β-glucan is also recognized by the nonclassical pattern recognition receptor EphA2 [195]. These epithelial–yeast interactions stimulate germination, exposing several hyphal-associated adhesins that further promote adherence. These include a number of proteins from the Als family, particularly Als3, as well as hyphal wall protein 1 (Hwp1) [2, 195,196,197,198].
Following adherence, C. albicans invades the epithelium through induced endocytosis or active penetration. Induced endocytosis involves interactions between fungal invasins and host cell proteins. C. albicans invasins include Als3 and the heat shock protein Ssa1 [195, 199, 200]. These invasins bind epithelial cell E-cadherin and initiate endocytosis through an actin-dependent mechanism that requires clathrin [199, 201,202,203,204,205]. Once internalized, C. albicans prevents endolysosomal maturation and continues to grow. Intracellular hyphal extension is dependent on the expression of EED1 (epithelial escape and dissemination 1) [206], and continued growth of hyphae results in the piercing of epithelial cells and subsequent dissemination.
Active penetration on the other hand is a separate mechanism that occurs at later time points than endocytosis. It requires viable fungi and results from hyphal extension and invasion in between or through epithelial cells. To date, the process of active penetration remains poorly understood, and it is unclear which fungal components are involved, although secreted aspartic proteinases (Saps) (especially Sap3), lipases and phospholipases are thought to play a role through degradation of the epithelial tight junction protein E-cadherin [2, 198, 199, 207].
In addition to Saps, C. albicans secretes a number of other proteases and phospholipases which may contribute to fungal-induced epithelial damage and enable passage across the intestinal epithelium [198]. Similar to cryptococcal Plb1, candidal Plb1 promotes penetration of the epithelium by breaking down host cell membranes [208]. The cytolytic peptide toxin candidalysin was also found to be essential for C. albicans to damage host enterocytes [193]. In addition, a recent report indicates that C. albicans is also able to utilize phagocytes and the Trojan horse mechanism to disseminate throughout the host [209].
Once the intestinal epithelium has been breached, C. albicans can invade local tissues and nearby blood vessels and disseminate throughout the host. The primary target organs of disseminated C. albicans are the kidneys and brain [210,211,212,213]. Approximately 50% of patients with disseminated candidiasis have CNS fungal invasion, which can cause meningoencephalitis and has an associated mortality rate of up to 90% [213]. Kidneys on the other hand are the most heavily colonized organ following Candida infection [210, 214]. These fungal burdens correlate with mortality, suggesting that the kidney is the critical target organ during candidiasis [215]. Inside the kidneys, candidal infection can result in tissue damage and eventual organ failure [216].
Invasion into these distant organs requires fungal cells first adhere to endothelial cells prior to migrating into tissues [217]. C. albicans binding to the endothelium is not well understood, but similar to binding of the epithelium, endothelial binding involves adhesins and invasins that are differentially expressed during yeast and hyphal phases. Some such molecules include integrin-like proteins, members of the Als family and fungal cell wall components [217]. For example, invasins Als3 and Ssa1 bind to the heat shock protein gp96 and an unknown endothelial receptor respectively, and induce endocytosis into brain endothelial cells [211, 218]. Additionally, Als3 has also been reported to promote fungal cell internalization into endothelial cells by binding N-cadherin [201, 217, 219, 220].
Alternative methods of transmigration across the endothelium include Trojan horse transport and paracellular crossing between adjacent endothelial cells [217]. Lastly, fungal cell migration across damaged endothelial barriers also occurs and is facilitated by the host protease plasmin. Similar to C. neoformans, C. albicans binds and activates host plasminogen to plasmin via a number of cell wall proteins, including enolase [221, 222]. Surface-bound plasmin can degrade components of the endothelium and enhance the ability of C. albicans to invade tissues [222].
Conclusion
The most medically important fungal diseases are cryptococcosis, aspergillosis and candidiasis. Fungal infections are usually limited to initial sites of infection in immunocompetent individuals. However, if fungi disseminate from initial infection sites to the bloodstream, they can invade virtually any organ and cause fatal infections which account for more than one million deaths worldwide each year. Thus, fungal dissemination is a critical step in the development of invasive fungal diseases. Although the mechanism(s) involved in fungal dissemination remains incompletely understood, increasing evidence suggests that fungi can hijack phagocytes from initial sites of infection to enter the bloodstream, a process called Trojan horse crossing. Alternatively, free fungal cells can also directly enter into circulation through transcytosis, paracellular crossing or across damaged barrier tissues. During fungal dissemination, neutrophils and Kupffer cells have been shown to play a role in filtering circulating fungi out of the vasculature. Interestingly, fungi in the blood can also use similar strategies to those they use to escape from the site of initial infection to invade distant organs. The mechanism(s) involved in fungal dissemination reflects complicated interactions between the host and the pathogen, and an understanding of this mechanism(s) is fundamental for the development of therapeutic strategies (Table 1).
Future directions
In vitro studies utilizing HBMECs monolayers as a BBB model have been extensively used to study the migration of C. neoformans and other pathogens across the BBB. These studies have made great contributions to our understanding of those mechanisms governing the transcytosis [133, 138, 141, 145, 151, 223] and Trojan horse pathways [120, 121]. However, these BBB models cannot recapitulate some important in vivo features in the brain microvasculature, such as shear stress, cell–ECM interactions and cylindrical geometry characteristics. In addition, the BBB is composed of not only endothelial cells, but also pericytes and astrocytes. Therefore, what occurs in vivo in terms of fungal dissemination could be different to what we have observed in those in vitro models [224]. To overcome this weakness and to achieve physiological barrier function, three-dimensional self-organized microvascular models of the BBB containing endothelial cells, pericytes and astrocytes have been recently developed [225,226,227,228]. It is expected that these three-dimensional models of the BBB would provide us a better platform to study fungal dissemination in a way more closely related to what happens in vivo.
In vivo models of mouse infection with C. neoformans have also been used to study fungal dissemination. In line with in vitro findings, histological studies support both the Trojan horse mechanism [71, 122] and transcytosis crossing [71, 133]. However, fungal dissemination is a dynamic process, and histological studies are not an optimal approach to address dynamic events. In this regard, intravital microscopy has been recently used to study fungal dissemination through the imaging of dynamic interactions between fungal cells and endothelial cells as well as phagocytes [17, 91,92,93, 116, 129, 229]. However, the limitation of intravital microscopy is that only a small area can be visualized and that a limited number of fungal cells can be analyzed [230]. As such, it is unknown whether the observations reflect most of the fungal cells. To complement histology and intravital microscopy, colony-forming unit (CFU) determination has been used to study fungal dissemination in vivo [139, 141, 145, 151]. However, CFU analysis fails to distinguish between fungal cells residing within the vasculature bed and those in the brain parenchyma after crossing the BBB. More recently, flow cytometry has been used to determine the invasion of brain endothelial cells by the protozoan parasite Toxoplasma gondii [231]. This technique is likely a powerful tool that can be used for future studies on fungal dissemination by quantifying the frequency of endothelial cells containing fungal cells.
Lastly, fungal cells can disseminate using numerous different mechanisms, namely Trojan horse crossing, transcytosis, paracellular crossing and passage through damaged tissues [232]. Although there is evidence to support all of these mechanisms, an important aspect of fungal dissemination left unanswered is the relative frequency of each mechanism during infection. Determining the major pathway of fungal dissemination is fundamental for developing therapeutic strategies for invasive fungal diseases. It is a challenge to determine which pathway is the predominant route for fungal dissemination. To address this issue, an in vivo animal model of fungal infection is likely required, and when one mechanism is analyzed the other two mechanisms must be properly blocked simultaneously. With the emergence of new technologies and scientific approaches, we believe that we will have a better understanding of fungal dissemination in the future.
References
Shoham S, Levitz SM (2005) The immune response to fungal infections. Br J Haematol 129:569–582. https://doi.org/10.1111/j.1365-2141.2005.05397.x
Naglik JR, Moyes DL, Wächtler B, Hube B (2011) Candida albicans interactions with epithelial cells and mucosal immunity. Microbes Infect 13:963–976. https://doi.org/10.1016/j.micinf.2011.06.009
Naglik JR (2014) Candida immunity. New J Sci. https://doi.org/10.1155/2014/390241 (Review)
Taylor DL, Hollingsworth TN, McFarland JW, Lennon NJ, Nusbaum C, Ruess RW (2014) A first comprehensive census of fungi in soil reveals both hyperdiversity and fine-scale niche partitioning. Ecol Monogr 84:3–20. https://doi.org/10.1890/12-1693.1
Köhler JR, Casadevall A, Perfect J (2015) The spectrum of fungi that infects humans. Cold Spring Harb Perspect Med 5:1–22. https://doi.org/10.1101/cshperspect.a019273
Buchanan KL, Murphy JW (1998) What makes Cryptococcus neoformans a pathogen? Emerg Infect Dis 4:71–83. https://doi.org/10.3201/eid0401.980109
Vautier S, Drummond RA, Chen K, Murray GI, Kadosh D, Brown AJP, Gow NAR, Maccallum DM, Kolls JK, Brown GD (2015) Candida albicans colonization and dissemination from the murine gastrointestinal tract: The influence of morphology and Th17 immunity. Cell Microbiol 17:445–450. https://doi.org/10.1111/cmi.12388
Bongomin F, Gago S, Oladele RO, Denning DW (2017) Global and multi-national prevalence of fungal diseases—estimate precision. J Fungi 3:57. https://doi.org/10.3390/jof3040057
Benedict K, Jackson BR, Chiller T, Beer KD (2019) Estimation of direct healthcare costs of fungal diseases in the United States. Clin Infect Dis 68:1791–1797. https://doi.org/10.1093/cid/ciy776
Templeton SP, Rivera A, Hube B, Jacobsen ID (2018) Editorial: Immunity to human fungal pathogens: mechanisms of host recognition, protection, pathology, and fungal interference. Front Immunol 9:1–4. https://doi.org/10.3389/fimmu.2018.02337
Salazar F, Brown GD (2018) Antifungal innate immunity: a perspective from the last 10 years. J Innate Immun 10:373–397. https://doi.org/10.1159/000488539
Denham ST, Brown JCS (2018) Mechanisms of pulmonary escape and dissemination by Cryptococcus neoformans. J Fungi 4:25. https://doi.org/10.3390/jof4010025
Voelz K, May RC (2010) Cryptococcal interactions with the host immune system. Eukaryot Cell 9:835–846. https://doi.org/10.1128/EC.00039-10
Liu T, Perlin DS, Xue C (2012) Molecular mechanisms of cryptococcal meningitis. Virulence 3:173–181. https://doi.org/10.4161/viru.18685
Rajasingham R, Smith RM, Park BJ, Jarvis JN, Govender NP, Chiller TM, Denning DW, Loyse A, Boulware DR (2017) Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis 17:873–881. https://doi.org/10.1016/S1473-3099(17)30243-8
Feldmesser M, Kress Y, Novikoff P, Casadevall A (2000) Cryptococcus neoformans is a facultative intracellular pathogen in murine pulmonary infection. Infect Immun 68:4225–4237. https://doi.org/10.1128/IAI.68.7.4225-4237.2000
Bojarczuk A, Miller KA, Hotham R, Lewis A, Ogryzko NV, Kamuyango AA, Frost H, Gibson RH, Stillman E, May RC, Renshaw SA, Johnston SA (2016) Cryptococcus neoformans intracellular proliferation and capsule size determines early macrophage control of infection. Sci Rep 6:1–15. https://doi.org/10.1038/srep21489
Santangelo R, Zoellner H, Sorrell T, Wilson C, Donald C, Djordjevic J, Shounan Y, Wright L (2004) Role of extracellular phospholipases and mononuclear phagocytes in dissemination of cryptococcosis in a murine model. Infect Immun 72:2229–2239. https://doi.org/10.1128/IAI.72.4.2229-2239.2004
Noverr MC, Williamson PR, Fajardo RS, Huffnagle GB (2004) CNLAC1 is required for extrapulmonary dissemination of Cryptococcus neoformans but not pulmonary persistence. Society 72:1693–1699. https://doi.org/10.1128/IAI.72.3.1693
Shea JM, Kechichian TB, Luberto C, Del Poeta M (2006) The cryptococcal enzyme inositol phosphosphingolipid-phospholipase C confers resistance to the antifungal effects of macrophages and promotes fungal dissemination to the central nervous system. Infect Immun 74:5977–5988. https://doi.org/10.1128/IAI.00768-06
Charlier C, Nielsen K, Daou S, Brigitte M, Chretien F, Dromer F (2009) Evidence of a role for monocytes in dissemination and brain invasion by Cryptococcus neoformans. Infect Immun 77:120–127. https://doi.org/10.1128/IAI.01065-08
Shao X, Mednick A, Alvarez M, van Rooijen N, Casadevall A, Goldman DL (2005) An innate immune system cell is a major determinant of species-related susceptibility differences to fungal pneumonia. J Immunol 175:3244–3251. https://doi.org/10.4049/jimmunol.175.5.3244
Kechichian TB, Shea J, Del Poeta M (2007) Depletion of alveolar macrophages decreases the dissemination of a glucosylceramide-deficient mutant of Cryptococcus neoformans in immunodeficient mice. Infect Immun 75:4792–4798. https://doi.org/10.1128/IAI.00587-07
Walsh NM, Botts MR, McDermott AJ, Ortiz SC, Wüthrich M, Klein B, Hull CM (2019) Infectious particle identity determines dissemination and disease outcome for the inhaled human fungal pathogen Cryptococcus. PLoS Pathog 15:1–30. https://doi.org/10.1371/journal.ppat.1007777
Levitz SM, Harrison TS, Tabuni A, Liu X (1997) Chloroquine induces human mononuclear phagocytes to inhibit and kill Cryptococcus neoformans by a mechanism independent of iron deprivation. J Clin Invest 100:1640–1646. https://doi.org/10.1172/JCI119688
Levitz SM, Nong SH, Seetoo KF, Harrison TS, Speizer RA, Simons ER (1999) Cryptococcus neoformans resides in an acidic phagolysosome of human macrophages. Infect Immun 67:885–890. https://doi.org/10.1128/iai.67.2.885-890.1999
Farnoud AM, Mor V, Singh A, Del Poeta M (2014) Inositol phosphosphingolipid phospholipase C1 regulates plasma membrane ATPase (Pma1) stability in Cryptococcus neoformans. FEBS Lett 588:3932–3938. https://doi.org/10.1016/j.febslet.2014.09.005
Feldmesser M, Kress Y, Casadevall A (2001) Dynamic changes in the morphology of Cryptococcus neoformans during murine pulmonary infection. Microbiology 147:2355–2365. https://doi.org/10.1099/00221287-147-8-2355
Hommel B, Mukaremera L, Cordero RJB, Coelho C, Desjardins CA, Sturny-Leclère A, Janbon G, Perfect JR, Fraser JA, Casadevall A, Cuomo CA, Dromer F, Nielsen K, Alanio A (2018) Titan cells formation in Cryptococcus neoformans is finely tuned by environmental conditions and modulated by positive and negative genetic regulators. PLoS Pathog 14:1–38. https://doi.org/10.1371/journal.ppat.1006982
Zaragoza O, Rocío GR, Nosanchuk JD, Cuenca-Estrella M, Rodríguez-Tudela JL, Casadevall A (2010) Fungal cell gigantism during mammalian infection. PLoS Pathog 6:e1000945. https://doi.org/10.1371/journal.ppat.1000945
García-Barbazán I, Trevijano-Contador N, Rueda C, de Andrés B, Pérez-Tavárez R, Herrero-Fernández I, Gaspar ML, Zaragoza O (2016) The formation of titan cells in Cryptococcus neoformans depends on the mouse strain and correlates with induction of Th2-type responses. Cell Microbiol 18:111–124. https://doi.org/10.1111/cmi.12488
Crabtree JN, Okagaki LH, Wiesner DL, Strain AK, Nielsen JN, Nielsen K (2012) Titan cell production enhances the virulence of Cryptococcus neoformans. Infect Immun 80:3776–3785. https://doi.org/10.1128/IAI.00507-12
Zaragoza O, Chrisman CJ, Castelli MV, Frases S, Cuenca-Estrella M, Rodríguez-Tudela JL, Casadevall A (2008) Capsule enlargement in Cryptococcus neoformans confers resistance to oxidative stress suggesting a mechanism for intracellular survival. Cell Microbiol 10:2043–2057. https://doi.org/10.1111/j.1462-5822.2008.01186.x
Zaragoza O, Rodrigues ML, De Jesus M, Frases S, Dadachova E, Casadevall A (2009) Chapter 4: The capsule of the fungal pathogen Cryptococcus neoformans. Adv Appl Microbiol 68:133–216. https://doi.org/10.1016/S0065-2164(09)01204-0
Liu L, Tewari RP, Williamson PR (1999) Laccase protects Cryptococcus neoformans from antifungal activity of alveolar macrophages. Infect Immun 67:6034–6039. https://doi.org/10.1128/iai.67.11.6034-6039.1999
Zhu X, Gibbons J, Garcia-Rivera J, Casadevall A, Williamson PR (2001) Laccase of Cryptococcus neoformans is a cell wall-associated virulence factor. Infect Immun 69:5589–5596. https://doi.org/10.1128/IAI.69.9.5589-5596.2001
Wang Y, Casadevall A (1994) Susceptibility of melanized and nonmelanized Cryptococcus neoformans to nitrogen- and oxygen-derived oxidants. Infect Immun 62:3004–3007. https://doi.org/10.1128/iai.62.7.3004-3007.1994
Wang Y, Aisen P, Casadevall A (1995) Cryptococcus neoformans melanin and virulence: mechanism of action. Infect Immun 63:3131–3136. https://doi.org/10.1128/iai.63.8.3131-3136.1995
Noverr MC, Phare SM, Toews GB, Coffey MJ, Huffnagle GB (2001) Pathogenic yeasts Cryptococcus neoformans and Candida albicans produce immunomodulatory prostaglandins. Infect Immun 69:2957–2963. https://doi.org/10.1128/IAI.69.5.2957
Noverr MC, Cox GM, Perfect JR, Huffnagle GB (2003) Role of PLB1 in pulmonary inflammation and cryptococcal eicosanoid production. Infect Immun 71:1538–1547. https://doi.org/10.1128/IAI.71.3.1538-1547.2003
Wright LC, Santangelo RM, Ganendren R, Payne J, Djordjevic JT, Sorrell TC (2007) Cryptococcal lipid metabolism: phospholipase B1 is implicated in transcellular metabolism of macrophage-derived lipids. Eukaryot Cell 6:37–47. https://doi.org/10.1128/EC.00262-06
Tucker SC, Casadevall A (2002) Replication of Cryptococcus neoformans in macrophages is accompanied by phagosomal permeabilization and accumulation of vesicles containing polysaccharide in the cytoplasm. Proc Natl Acad Sci U S A 99:3165–3170. https://doi.org/10.1073/pnas.052702799
Ma H, Croudace JE, Lammas DA, May RC (2006) Expulsion of live pathogenic yeast by macrophages. Curr Biol 16:2156–2160. https://doi.org/10.1016/j.cub.2006.09.032
Alvarez M, Casadevall A (2006) Phagosome extrusion and host-cell survival after Cryptococcus neoformans phagocytosis by macrophages. Curr Biol 16:2161–2165. https://doi.org/10.1016/j.cub.2006.09.061
Gilbert AS, Seoane PI, Sephton-Clark P, Bojarczuk A, Hotham R, Giurisato E, Sarhan AR, Hillen A, Vande VG, Gray NS, Alessi DR, Cunningham DL, Tournier C, Johnston SA, May RC (2017) Vomocytosis of live pathogens from macrophages is regulated by the atypical MAP kinase ERK5. Sci Adv 3:1–8. https://doi.org/10.1126/sciadv.1700898
Johnston SA, May RC (2010) The human fungal pathogen Cryptococcus neoformans escapes macrophages by a phagosome emptying mechanism that is inhibited by Arp2/3 complex-mediated actin polymerisation. PLoS Pathog 6:27–28. https://doi.org/10.1371/journal.ppat.1001041
Chayakulkeeree M, Johnston SA, Oei JB, Lev S, Williamson PR, Wilson CF, Zuo X, Leal AL, Vainstein MH, Meyer W, Sorrell TC, May RC, Djordjevic JT (2011) SEC14 is a specific requirement for secretion of phospholipase B1 and pathogenicity of Cryptococcus neoformans. Mol Microbiol 80:1088–1101. https://doi.org/10.1111/j.1365-2958.2011.07632.x
Stukes S, Coelho C, Rivera J, Jedlicka AE, Hajjar KA, Casadevall A (2016) The membrane phospholipid binding protein annexin A2 promotes phagocytosis and nonlytic exocytosis of Cryptococcus neoformans and impacts survival in fungal infection. J Immunol 197:1252–1261. https://doi.org/10.4049/jimmunol.1501855
Fu MS, Coelho C, De Leon-Rodriguez CM, Rossi DCP, Camacho E, Jung EH, Kulkarni M, Casadevall A (2018) Cryptococcus neoformans urease affects the outcome of intracellular pathogenesis by modulating phagolysosomal pH. PLoS Pathog 14:1–31. https://doi.org/10.1371/journal.ppat.1007144
Konieczna I, Zarnowiec P, Kwinkowski M, Kolesinska B, Fraczyk J, Kaminski Z, Kaca W (2013) Bacterial urease and its role in long-lasting human diseases. Curr Protein Pept Sci 13:789–806. https://doi.org/10.2174/138920312804871094
Dunn BE, Phadnis SH (1998) Structure, function and localization of Helicobacter pylori urease. Yale J Biol Med 71:63–73
Merkel GJ, Scofield BA (1997) The in vitro interaction of Cryptococcus neoformans with human lung epithelial cells. FEMS Immunol Med Microbiol 19:203–213. https://doi.org/10.1016/S0928-8244(97)00085-0
Taylor-Smith LM (2017) Cryptococcus–epithelial interactions. J Fungi 3:53. https://doi.org/10.3390/jof3040053
Barbosa FM, Fonseca FL, Holandino C, Alviano CS, Nimrichter L, Rodrigues ML (2006) Glucuronoxylomannan-mediated interaction of Cryptococcus neoformans with human alveolar cells results in fungal internalization and host cell damage. Microbes Infect 8:493–502. https://doi.org/10.1016/j.micinf.2005.07.027
Barbosa FM, Fonseca FL, Figueiredo RT, Bozza MT, Casadevall A, Nimrichter L, Rodrigues ML (2007) Binding of glucuronoxylomannan to the CD14 receptor in human A549 alveolar cells induces interleukin-8 production. Clin Vaccine Immunol 14:94–98. https://doi.org/10.1128/CVI.00296-06
Teixeira PAC, Penha LL, Mendonça-Previato L, Previato JO (2014) Mannoprotein MP84 mediates the adhesion of Cryptococcus neoformans to epithelial lung cells. Front Cell Infect Microbiol 4:1–9. https://doi.org/10.3389/fcimb.2014.00106
Ganendren R, Carter E, Sorrell T, Widmer F, Wright L (2006) Phospholipase B activity enhances adhesion of Cryptococcus neoformans to a human lung epithelial cell line. Microbes Infect 8:1006–1015. https://doi.org/10.1016/j.micinf.2005.10.018
Chen SCA, Wright LC, Santangelo RT, Muller M, Moran VR, Kuchel PW, Sorrell TC (1997) Identification of extracellular phospholipase B, lysophospholipase, and acyltransferase produced by Cryptococcus neoformans. Infect Immun 65:405–411. https://doi.org/10.1128/iai.65.2.405-411.1997
Djordjevic JT (2010) Role of phospholipases in fungal fitness, pathogenicity, and drug development—lessons from Cryptococcus neoformans. Front Microbiol 1:1–13. https://doi.org/10.3389/fmicb.2010.00125
Chen SHM, Stins MF, Huang SH, Chen YH, Kwon-Chung KJ, Chang Y, Kim KS, Suzuki K, Jong AY (2003) Cryptococcus neoformans induces alterations in the cytoskeleton of human brain microvascular endothelial cells. J Med Microbiol 52:961–970. https://doi.org/10.1099/jmm.0.05230-0
Vu K, Eigenheer RA, Phinney BS, Gelli A (2013) Cryptococcus neoformans promotes its transmigration into the central nervous system by inducing molecular and cellular changes in brain endothelial cells. Infect Immun 81:3139–3147. https://doi.org/10.1128/IAI.00554-13
Heyen L, Müller U, Siegemund S, Schulze B, Protschka M, Alber G, Piehler D (2016) Lung epithelium is the major source of IL-33 and is regulated by IL-33-dependent and IL-33-independent mechanisms in pulmonary cryptococcosis. Pathog Dis 74:1–11. https://doi.org/10.1093/femspd/ftw086
Wroblewski LE, Shen L, Ogden S, Romero-Gallo J, Lapierre LA, Israel DA, Turner JR, Peek RM (2009) Helicobacter pylori dysregulation of gastric epithelial tight junctions by urease-mediated myosin II activation. Gastroenterology 136:236–246. https://doi.org/10.1053/j.gastro.2008.10.011
Chen LC, Blank ES, Casadevall A (1996) Extracellular proteinase activity of Cryptococcus neoformans. Clin Diagn Lab Immunol 3:570–574. https://doi.org/10.1128/cdli.3.5.570-574.1996
Casadevall A, Coelho C, Alanio A (2018) Mechanisms of Cryptococcus neoformans-mediated host damage. Front Immunol 9:1–8. https://doi.org/10.3389/fimmu.2018.00855
Rutherford JC (2014) The emerging role of urease as a general microbial virulence factor. PLoS Pathog 10:1–3. https://doi.org/10.1371/journal.ppat.1004062
Siafakas AR, Sorrell TC, Wright LC, Wilson C, Larsen M, Boadle R, Williamson PR, Djordjevic JT (2007) Cell wall-linked cryptococcal phospholipase B1 is a source of secreted enzyme and a determinant of cell wall integrity. J Biol Chem 282:37508–37514. https://doi.org/10.1074/jbc.M707913200
Rodrigues ML, Dos Reis FCG, Puccia R, Travassos LR, Alviano CS (2003) Cleavage of human fibronectin and other basement membrane-associated proteins by a Cryptococcus neoformans serine proteinase. Microb Pathog 34:65–71. https://doi.org/10.1016/S0882-4010(02)00195-X
Xu CY, Zhu HM, Wu JH, Wen H, Liu CJ (2014) Increased permeability of blood-brain barrier is mediated by serine protease during Cryptococcus meningitis. J Int Med Res 42:85–92. https://doi.org/10.1177/0300060513504365
Lortholary O, Improvisi L, Nicolas M, Provost F, Dupont B, Dromer F (1999) Fungemia during murine cryptococcosis sheds some light on pathophysiology. Med Mycol 37:169–174. https://doi.org/10.1046/j.1365-280X.1999.00215.x
Chrétien F, Lortholary O, Kansau I, Neuville S, Gray F, Dromer F (2002) Pathogenesis of cerebral Cryptococcus neoformans infection after fungemia. J Infect Dis 186:522–530. https://doi.org/10.1086/341564
Dromer F, Mathoulin-Pélissier S, Launay O, Lortholary O, Achard J, Chabasse D, Bland S, Bru JP, Pulik M, Leturdu F, Lepeu X, Lefrand H, Ferrand M, Larrouy M, Bentata M, Bouges-Michel C, Camuset J, Guillevin L, Jarrousse B, Lortholary O, Robineau M, Rousset JJ, Couprie B, Dupon M, Dutronc H, Lacut JY, Pellegrin JL, Ragnaud JM, Viallard JF, Weil FX, Bougnoux ME, Montreal X, Morelon S, Rouveix E, Granier P, De Montclos H, Desveaux A, Gavignet M, Labussiere AS, Mornet M, De Saint-Martin L, Moalic E, Roucoules J, Loriferne JF, Otterbein G, Desson JF, Leporrier M, Duhamel C, Korach JM, Salles B, Sire C, Herve V, Souleau B, Beytout J, Cambon M, Boussougant Y, Dreyfuss D, Michon X, Vinceneux P, Belkacem-Belkaki G, Bretagne S, Chousterman M, Grimberg P, Lascaux AS, Schaeffer A, Sobel A, Bacri JL, Berthelot G, Bonnin A, Duong M, Lopez J, Portier H, Gauthier M, Salmon O, Bizet J, Gaillard JL, Perronne C, Desailly MA, Maisonneuve H, Bedos JP, Doll J, Eloy O, Ghnassia JC, Roussin-Bretagne S, Brocard C, Guiffault P, Layet A, Morel A, Botterel F, Bouree P, Delfraissy JF, Kertaimont Y, Lozeron P, Rérat K, Saïd G, Cricks X, Darde ML, Jaccard A, Bouhour D, Dannaoui E, Mallet X, Peyramond D, Piens MA, Trepo C, Berardi L, Tremolieres F, Berland Y, Blancard A, Collet L, Delmont J, Gallais H, Gamby X, Michel Nguyen A, Moreau J, Petit N, Sainty JM, Sampol-Roubicek J, Bietrix M, Nezri M, Fiacre A, Levy S, Chandesris C, La Torre X, Andres P, Billaud E, Boiffin F, Hamidou M, Morin O, Planchon B, Poirier P, Raffi F, Villers D, Clevenbergh PH, De Salvador F, Dellamonica P, Durand X, Gari-Toussaint M, Romaru A, Texereau M, Bret L, Prazuk T, Bernard X, Pacheco Y, Becq-Giraudon B, Kauffmann-Lacroix C, Meurice JC, Pasdeloup T, Deville J, Toubas D, Arvieux C, Cartier F, Chevrier S, Degeilh B, Frouget T, Guiguen C, Le Cavorzin P, Michelet C, Noyon V, Abboud P, Brasseur P, Leroy J, Muir JF, Babinet P, Fraisse F, Godineau N, Hamane S, Margent P, Mechali D, Thuong M, Soler C, Hery B, Leberre JY, Gregory A, Prevot O, Christmann D, Waller J, Bletry O, Cahen P, Zucman D, Fortier B, Aubert X, Chadapaud S, Delbeck X, Lafeuillade A, Raoult X, Bonnet E, Cassin S, Gadroy A, Linas MD, Magnaval JF, Massip P, Prudhomme L, Sailler L, Baclet V, Coignard C, Mouton Y, Ravaux I, Eloy C, Fur A, Rezzouk L, Fontier C, Mazards E, Biava MF, Canton P, Kures L, Rabaud C, Vittecocq D, Dellion S, Patey O, Bedos JP, Benveniste O, Bouchard C, Belaich S, Carbon C, Chochillon C, Coulaud JP, Descamps V, Duval X, Leport C, Lheriteau F, Longuet P, Mouas H, Vachon F, Vilde JL, Yeni P, Lavarde V, Piketty C, Christoforov B, Dupouy-Camet J, Luton JP, Desplaces N, Raguin G, Chevalier P, Kazatchkine M, Lavarde V, Meyrier A, Bernadou A, Cornet M, Marie JP, Oudart S, Gayraud M, Pean Y, Aznar C, Dupont B, Poncelet H, Berche P, Dupont B, Mathé V, Baril L, Bossi P, Bricaire F, Carrière J, Datry A, Herson S, Jouan M, Levy-Soussan M, Mouquet C, Orcel B, Thiebaut MM, Frottier J, Guiard-Schmidt JB, Lebeau B, Meynard JL, Meyohas MC, Poirot JL, Roux P, Urban X, Daniel F, Gilquin J, Timsit JF, Brouet JC, Decazes JM, Derouin F, Eurin B, Legall JR, Legendre C, Neuville S, Escande JP, Delzant G, Kac G, Trivalle C (2007) Determinants of disease presentation and outcome during cryptococcosis: the CryptoA/D study. PLoS Med 4:0297–0308. https://doi.org/10.1371/journal.pmed.0040021
Kolaczkowska E, Kubes P (2013) Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol 13:159–175. https://doi.org/10.1038/nri3399
Diamond RD, Root RK, Bennett JE (1972) Factors influencing killing of Cryptococcus neoformans by human leukocytes in vitro. J Infect Dis 125:367–376. https://doi.org/10.1093/infdis/125.4.367
Kozel TR, Highison B, Stratton CJ (1984) Localization on encapsulated Cryptococcus neoformans of serum components opsonic for phagocytosis by macrophages and neutrophils. Infect Immun 43:574–579. https://doi.org/10.1128/iai.43.2.574-579.1984
Qureshi A, Subathra M, Grey A, Schey K, Del Poeta M, Luberto C (2010) Role of sphingomyelin synthase in controlling the antimicrobial activity of neutrophils against Cryptococcus neoformans. PLoS ONE 5:e15587. https://doi.org/10.1371/journal.pone.0015587
Miller MF, Mitchell TG (1991) Killing of Cryptococcus neoformans strains by human neutrophils and monocytes. Infect Immun 59:24–28. https://doi.org/10.1128/iai.59.1.24-28.1991
Miller GPG, Kohl S (1983) Antibody-dependent leukocyte killing of Cryptococcus neoformans. J Immunol 131:1455–1459
Chaturvedi V, Wong B, Newman SL (1996) Oxidative killing of Cryptococcus neoformans by human neutrophils. Evidence that fungal mannitol protects by scavenging reactive oxygen intermediates. J Immunol 156:3836–3840
Tacker JR, Farhi F, Bulmer GS (1972) Intracellular fate of Cryptococcus neoformans. Infect Immun 6:162–167. https://doi.org/10.1128/iai.6.2.162-167.1972
Mambula SS, Simons ER, Hastey R, Selsted ME, Levitz SM (2000) Human neutrophil-mediated nonoxidative antifungal activity against Cryptococcus neoformans. Infect Immun 68:6257–6264. https://doi.org/10.1128/IAI.68.11.6257-6264.2000
Rocha JDB, Nascimento MTC, Decote-Ricardo D, Côrte-Real S, Morrot A, Heise N, Nunes MP, Previato JO, Mendonça-Previato L, Dosreis GA, Saraiva EM, Freire-De-Lima CG (2015) Capsular polysaccharides from Cryptococcus neoformans modulate production of neutrophil extracellular traps (NETs) by human neutrophils. Sci Rep 5:8008. https://doi.org/10.1038/srep08008
Qureshi A, Grey A, Rose KL, Schey KL, Del Poeta M (2011) Cryptococcus neoformans modulates extracellular killing by neutrophils. Front Microbiol 2:1–11. https://doi.org/10.3389/fmicb.2011.00193
Graybill JR, Bocanegra R, Lambros C, Luther MF (1997) Granulocyte colony stimulating factor therapy of experimental cryptococcal meningitis. J Med Vet Mycol 35:243–247. https://doi.org/10.1080/02681219780001221
Coffey MJ, Phare SM, George S, Peters-Golden M, Kazanjian PH (1998) Granulocyte colony-stimulating factor administration to HIV-infected subjects augments reduced leukotriene synthesis and anticryptococcal activity in neutrophils. J Clin Invest 102:663–670. https://doi.org/10.1172/JCI2117
Vecchiarelli A, Monari C, Baldelli F, Pietrella D, Retini C, Tascini C, Francisci D, Bistoni F (1995) Beneficial effect of recombinant human granulocyte colony-stimulating factor on fungicidal activity of polymorphonuclear leukocytes from patients with AIDS. J Infect Dis 171:1448–1454. https://doi.org/10.1093/infdis/171.6.1448
Aratani Y, Kura F, Watanabe H, Akagawa H, Takano Y, Ishida-Okawara A, Suzuki K, Maeda N, Koyama H (2006) Contribution of the myeloperoxidase-dependent oxidative system to host defence against Cryptococcus neoformans. J Med Microbiol 55:1291–1299. https://doi.org/10.1099/jmm.0.46620-0
Klebanoff SJ (2005) Myeloperoxidase: friend and foe. J Leukoc Biol 77:598–625. https://doi.org/10.1189/jlb.1204697.1
Mednick AJ, Feldmesser M, Rivera J, Casadevall A (2003) Neutropenia alters lung cytokine production in mice and reduces their susceptibility to pulmonary cryptococcosis. Eur J Immunol 33:1744–1753. https://doi.org/10.1002/eji.200323626
Musubire AK, Meya DB, Rhein J, Meintjes G, Bohjanen PR, Nuwagira E, Muzoora C, Boulware DR, Hullsiek KH (2018) Blood neutrophil counts in HIV-infected patients with cryptococcal meningitis: association with mortality. PLoS ONE 13:1–15. https://doi.org/10.1371/journal.pone.0209337
Zhang M, Sun D, Liu G, Wu H, Zhou H, Shi M (2016) Real-time in vivo imaging reveals the ability of neutrophils to remove Cryptococcus neoformans directly from the brain vasculature. J Leukoc Biol 99:467–473. https://doi.org/10.1189/jlb.4ab0715-281r
Davis JM, Huang M, Botts MR, Hull CM, Huttenlocher A (2016) A zebrafish model of cryptococcal infection reveals roles for macrophages, endothelial cells, and neutrophils in the establishment and control of sustained fungemia. Infect Immun 84:3047–3062. https://doi.org/10.1128/IAI.00506-16
Shi M, Li SS, Zheng C, Jones GJ, Kim KS, Zhou H, Kubes P, Mody CH (2010) Real-time imaging of trapping and urease-dependent transmigration of Cryptococcus neoformans in mouse brain. J Clin Invest 120:1683–1693. https://doi.org/10.1172/JCI41963
Kozel TR, Wilson MA, Murphy JW (1991) Early events in initiation of alternative complement pathway activation by the capsule of Cryptococcus neoformans. Infect Immun 59:3101–3110. https://doi.org/10.1128/iai.59.9.3101-3110.1991
Mershon-Shiera KL, Vasuthasawata A, Takahashib K, Morrison SL, Beenhouwer DO (2012) In vitro C3 deposition on Cryptococcus capsule occurs via multiple complement activation pathways. Bone 23:1–7. https://doi.org/10.1038/jid.2014.371
Davies SF, Clifford DP, Hoidal JR, Repine JE (1982) Opsonic requirements for the uptake of Cryptococcus neoformans by human polymorphonuclear leukocytes and monocytes. J Infect Dis 145:870–874. https://doi.org/10.1093/infdis/145.6.870
Kozel TR, Pfrommer GST (1986) Activation of the complement system by Cryptococcus neoformans leads to binding of iC3b to the yeast. Infect Immun 52:1–5. https://doi.org/10.1128/iai.52.1.1-5.1986
Sun D, Zhang M, Liu G, Wu H, Zhu X, Zhou H, Shi M (2015) Real-time imaging of interactions of neutrophils with Cryptococcus neoformans demonstrates a crucial role of complement C5a–C5aR signaling. Infect Immun 84:216–229. https://doi.org/10.1128/IAI.01197-15
Lovchik JA, Lipscomb MF (1993) Role for C5 and neutrophils in the pulmonary intravascular clearance of circulating Cryptococcus neoformans. AmJRespirCell MolBiol 9:617–627. https://doi.org/10.1165/ajrcmb/9.6.617
Sun D, Zhang M, Liu G, Wu H, Li C, Zhou H, Zhang X, Shi M (2016) Intravascular clearance of disseminating Cryptococcus neoformans in the brain can be improved by enhancing neutrophil recruitment in mice. Eur J Immunol 46:1704–1714. https://doi.org/10.1002/eji.201546239
Sun D, Shi M (2016) Neutrophil swarming toward Cryptococcus neoformans is mediated by complement and leukotriene B4. Biochem Biophys Res Commun 477:945–951. https://doi.org/10.1016/j.bbrc.2016.07.005
Lee EKS, Gillrie MR, Li L, Arnason JW, Kim JH, Babes L, Lou Y, Sanati-Nezhad A, Kyei SK, Kelly MM, Mody CH, Ho M, Yipp BG (2018) Leukotriene B4-mediated neutrophil recruitment causes pulmonary capillaritis during lethal fungal sepsis. Cell Host Microbe 23:121-133.e4. https://doi.org/10.1016/j.chom.2017.11.009
Diamond RD, May JE, Kane MA, Frank MM, Bennett JE (1974) The role of the classical and alternate complement pathways in host defenses against Cryptococcus neoformans infection. J Immunol 112:2260–2270
Jenne CN, Kubes P (2013) Immune surveillance by the liver. Nat Immunol 14:996–1006. https://doi.org/10.1038/ni.2691
Hickey MJ, Kubes P (2009) Intravascular immunity: the host-pathogen encounter in blood vessels. Nat Rev Immunol 9:364–375. https://doi.org/10.1038/nri2532
Balmer ML, Slack E, De Gottardi A, Lawson MAE, Hapfelmeier S, Miele L, Grieco A, Van Vlierberghe H, Fahrner R, Patuto N, Bernsmeier C, Ronchi F, Wyss M, Stroka D, Dickgreber N, Heim MH, McCoy KD, Macpherson AJ (2014) The liver may act as a firewall mediating mutualism between the host and its gut commensal microbiota. Sci Transl Med 6:1–11. https://doi.org/10.1126/scitranslmed.3008618
Broadley SP, Plaumann A, Coletti R, Lehmann C, Wanisch A, Seidlmeier A, Esser K, Luo S, Rämer PC, Massberg S, Busch DH, van Lookeren CM, Verschoor A (2016) Dual-track clearance of circulating bacteria balances rapid restoration of blood sterility with induction of adaptive immunity. Cell Host Microbe 20:36–48. https://doi.org/10.1016/j.chom.2016.05.023
Zeng Z, Surewaard BGJ, Wong CHY, Geoghegan JA, Jenne CN, Kubes P (2016) CRIg functions as a macrophage pattern recognition receptor to directly bind and capture blood-borne gram-positive bacteria. Cell Host Microbe 20:99–106. https://doi.org/10.1016/j.chom.2016.06.002
Wong CHY, Jenne CN, Petri B, Chrobok NL (2016) Nucleation of platelets with bloodborne pathogens on Kupffer cell precedes other innate immunity and contributes to bacterial clearance. Nat Immunol 14:785–792. https://doi.org/10.1038/ni.2631.Nucleation
Liu G, Fu Y, Yosri M, Chen Y, Sun P, Xu J, Zhang M, Sun D, Strickland AB, Mackey ZB, Shi M (2019) CRIg plays an essential role in intravascular clearance of bloodborne parasites by interacting with complement. Proc Natl Acad Sci U S A 116:24214–24220. https://doi.org/10.1073/pnas.1913443116
Ting PS, Agarwalla A, Woreta TA (2019) A mimic of hepatic encephalopathy two cases of cryptococcal meningitis in North America. J Clin Transl Hepatol 7:1–3. https://doi.org/10.14218/jcth.2019.00005
Lin YY, Shiau S, Fang CT (2015) Risk factors for invasive Cryptococcus neoformans diseases: a case-control study. PLoS ONE 10:1–13. https://doi.org/10.1371/journal.pone.0119090
Spec A, Raval K, Powderly WG (2016) End-stage liver disease is a strong predictor of early mortality in cryptococcosis. Open Forum Infect Dis 3:1–5. https://doi.org/10.1093/ofid/ofv197
Singh N, Husain S, De Vera M, Gayowski T, Cacciarelli TV (2004) Cryptococcus neoformans infection in patients with cirrhosis, including liver transplant candidates. Medicine (Baltimore) 83:188–192. https://doi.org/10.1097/01.md.0000126760.45299.69
Coelho C, Drummond RA (2019) Kupffer cells mediate systemic antifungal immunity. Trends Immunol 40:1071–1073. https://doi.org/10.1016/j.it.2019.11.001
Sun D, Sun P, Li H, Zhang M, Liu G, Strickland AB, Chen Y, Fu Y, Xu J, Yosri M, Nan Y, Zhou H, Zhang X, Shi M (2019) Fungal dissemination is limited by liver macrophage filtration of the blood. Nat Commun 10:4566. https://doi.org/10.1038/s41467-019-12381-5
Gottfredsson M, Perfect JR (2014) Fungal meningitis. Infect Cent Nerv Syst Fourth Ed 20:307–322
Casadevall A (2010) Cryptococci at the brain gate: break and enter or use a Trojan horse? J Clin Invest 120:1389–1392. https://doi.org/10.1172/JCI42949
May RC, Stone NRH, Wiesner DL, Bicanic T, Nielsen K (2016) Cryptococcus: from environmental saprophyte to global pathogen. Nat Rev Microbiol 14:106–117. https://doi.org/10.1038/nrmicro.2015.6
Sorrell TC, Juillard PG, Djordjevic JT, Kaufman-Francis K, Dietmann A, Milonig A, Combes V, Grau GER (2016) Cryptococcal transmigration across a model brain blood-barrier: evidence of the Trojan horse mechanism and differences between Cryptococcus neoformans var. grubii strain H99 and Cryptococcus gattii strain R265. Microbes Infect 18:57–67. https://doi.org/10.1016/j.micinf.2015.08.017
Santiago-Tirado FH, Onken MD, Cooper JA, Klein RS, Doering TL (2017) Trojan horse transit contributes to blood-brain barrier crossing of a eukaryotic pathogen. MBio 8:1–16. https://doi.org/10.1128/mBio.02183-16
Kaufman-Francis K, Djordjevic JT, Juillard PG, Lev S, Desmarini D, Grau GER, Sorrell TC (2018) The early innate immune response to, and phagocyte-dependent entry of, Cryptococcus neoformans map to the perivascular space of cortical post-capillary venules in neurocryptococcosis. Am J Pathol 188:1653–1665. https://doi.org/10.1016/j.ajpath.2018.03.015
Thomas JH (2019) Fluid dynamics of cerebrospinal fluid flow in perivascular spaces. J R Soc Interface 16:20190572. https://doi.org/10.1098/rsif.2019.0572
Ma H, Croudace JE, Lammas DA, May RC (2007) Direct cell-to-cell spread of a pathogenic yeast. BMC Immunol 8:4–8. https://doi.org/10.1186/1471-2172-8-15
Alvarez M, Casadevall A (2007) Cell-to-cell spread and massive vacuole formation after Cryptococcus neoformans infection of murine macrophages. BMC Immunol 8:1–7. https://doi.org/10.1186/1471-2172-8-16
Dragotakes Q, Fu MS, Casadevall A (2019) Dragotcytosis: elucidation of the mechanism for Cryptococcus neoformans macrophage-to-macrophage transfer. J Immunol 202:2661–2670. https://doi.org/10.4049/jimmunol.1801118
Cros J, Cagnard N, Woollard K, Patey N, Zhang SY, Senechal B, Puel A, Biswas SK, Moshous D, Picard C, Jais JP, D’Cruz D, Casanova JL, Trouillet C, Geissmann F (2010) Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity 33:375–386. https://doi.org/10.1016/j.immuni.2010.08.012
Hohl TM, Rivera A, Lipuma L, Gallegos A, Shi C, Mack M, Pamer EG (2009) Inflammatory monocytes facilitate adaptive CD4 T cell responses during respiratory fungal infection. Cell Host Microbe 6:470–481. https://doi.org/10.1016/j.chom.2009.10.007
Sun D, Zhang M, Sun P, Liu G, Strickland AB, Chen Y, Fu Y, Yosri M, Shi M (2020) VCAM1/VLA4 interaction mediates Ly6Clow monocyte recruitment to the brain in a TNFR signaling dependent manner during fungal infection. PLoS Pathog 16:1–25. https://doi.org/10.1371/journal.ppat.1008361
Osterholzer JJ, Chen G-H, Olszewski MA, Curtis JL, Huffnagle GB, Toews GB (2009) Accumulation of CD11b + lung dendritic cells in response to fungal infection results from the CCR2-mediated recruitment and differentiation of Ly-6C high monocytes. J Immunol 183:8044–8053. https://doi.org/10.4049/jimmunol.0902823
Panackal AA, Wuest SC, Lin YC, Wu T, Zhang N, Kosa P, Komori M, Blake A, Browne SK, Rosen LB, Hagen F, Meis J, Levitz SM, Quezado M, Hammoud D, Bennett JE, Bielekova B, Williamson PR (2015) Paradoxical immune responses in Non-HIV Cryptococcal meningitis. PLoS Pathog 11:1–27. https://doi.org/10.1371/journal.ppat.1004884
Jarvis JN, Meintjes G, Bicanic T, Buffa V, Hogan L, Mo S, Tomlinson G, Kropf P, Noursadeghi M, Harrison TS (2015) Cerebrospinal fluid cytokine profiles predict risk of early mortality and immune reconstitution inflammatory syndrome in HIV-associated Cryptococcal meningitis. PLoS Pathog 11:1–17. https://doi.org/10.1371/journal.ppat.1004754
Chang YC, Stins MF, McCaffery MJ, Miller GF, Pare DR, Dam T, Paul-Satyaseela M, Kim KS, Kwon-Chung KJ (2004) Cryptococcal yeast cells invade the central nervous system via transcellular penetration of the blood-brain barrier. Infect Immun 72:6753. https://doi.org/10.1128/IAI.72.11.6753.2004
Vu K, Weksler B, Romero I, Couraud PO, Gelli A (2009) Immortalized human brain endothelial cell line HCMEC/D3 as a model of the blood-brain barrier facilitates in vitro studies of central nervous system infection by Cryptococcus neoformans. Eukaryot Cell 8:1803–1807. https://doi.org/10.1128/EC.00240-09
Sabiiti W, May RC (2012) Mechanisms of infection by the human fungal pathogen Cryptococcus neoformans. Future Microbiol 7:1297–1313. https://doi.org/10.2217/fmb.12.102
Ibrahim AS, Filler SG, Alcouloumre MS, Kozel TR, Edwards JE, Ghannoum MA (1995) Adherence to and damage of endothelial cells by Cryptococcus neoformans in vitro: role of the capsule. Infect Immun 63:4368–4374. https://doi.org/10.1128/iai.63.11.4368-4374.1995
Sabiiti W, May RC (2012) Capsule independent uptake of the fungal pathogen Cryptococcus neoformans into brain microvascular endothelial cells. PLoS ONE 7:e35455. https://doi.org/10.1371/journal.pone.0035455
Jong A, Wu CH, Shackleford GM, Kwon-Chung KJ, Chang YC, Chen HM, Ouyang Y, Huang SH (2008) Involvement of human CD44 during Cryptococcus neoformans infection of brain microvascular endothelial cells. Cell Microbiol 10:1313–1326. https://doi.org/10.1111/j.1462-5822.2008.01128.x
Jong A, Wu CH, Gonzales-Gomez I, Kwon-Chung KJ, Chang YC, Tseng HK, Cho WL, Huang SH (2012) Hyaluronic acid receptor CD44 deficiency is associated with decreased Cryptococcus neoformans brain infection. J Biol Chem 287:15298–15306. https://doi.org/10.1074/jbc.M112.353375
Jong A, Wu CH, Chen HM, Luo F, Kwon-Chung KJ, Chang YC, LaMunyon CW, Plaas A, Huang SH (2007) Identification and characterization of CPS1 as a hyaluronic acid synthase contributing to the pathogenesis of Cryptococcus neoformans infection. Eukaryot Cell 6:1486–1496. https://doi.org/10.1128/EC.00120-07
Liu TB, Kim JC, Wang Y, Toffaletti DL, Eugenin E, Perfect JR, Kim KJ, Xue C (2013) Brain inositol is a novel stimulator for promoting Cryptococcus penetration of the blood-brain barrier. PLoS Pathog 9:e1003247. https://doi.org/10.1371/journal.ppat.1003247
Jong A, Wu CH, Prasadarao NV, Kwon-Chung KJ, Chang YC, Ouyang Y, Shackleford GM, Huang SH (2008) Invasion of Cryptococcus neoformans into human brain microvascular endothelial cells requires protein kinase C-α activation. Cell Microbiol 10:1854–1865. https://doi.org/10.1111/j.1462-5822.2008.01172.x
Huang SH, Long M, Wu CH, Kwon-Chung KJ, Chang YC, Chi F, Lee S, Jong A (2011) Invasion of Cryptococcus neoformans into human brain microvascular endothelial cells is mediated through the lipid rafts-endocytic pathway via the dual specificity tyrosine phosphorylation-regulated kinase 3 (DYRK3). J Biol Chem 286:34761–34769. https://doi.org/10.1074/jbc.M111.219378
Kim JC, Crary B, Chang YC, Kwon-Chung KJ, Kim KJ (2012) Cryptococcus neoformans activates RhoGTPase proteins followed by protein kinase C, focal adhesion kinase, and ezrin to promote traversal across the blood-brain barrier. J Biol Chem 287:36147–36157. https://doi.org/10.1074/jbc.M112.389676
Maruvada R, Zhu L, Pearce D, Zheng Y, Perfect J, Kwon-Chung KJ, Kim KS (2012) Cryptococcus neoformans phospholipase B1 activates host cell Rac1 for traversal across the blood-brain barrier. Cell Microbiol 14:1544–1553. https://doi.org/10.1111/j.1462-5822.2012.01819.x
Zhu L, Maruvada R, Sapirstein A, Peters-Golden M, Kim KS (2017) Cysteinyl leukotrienes as novel host factors facilitating Cryptococcus neoformans penetration into the brain. Cell Microbiol 19:e12661. https://doi.org/10.1111/cmi.12661
Aaron PA, Jamklang M, Uhrig JP, Gelli A (2018) The blood–brain barrier internalises Cryptococcus neoformans via the EphA2-tyrosine kinase receptor. Cell Microbiol 20:1–23. https://doi.org/10.1111/cmi.12811
Long M, Huang SH, Wu CH, Shackleford GM, Jong A (2012) Lipid raft/caveolae signaling is required for Cryptococcus neoformans invasion into human brain microvascular endothelial cells. J Biomed Sci 19:1–14. https://doi.org/10.1186/1423-0127-19-19
Huang SH, Wu CH, Chang YC, Kwon-Chung KJ, Brown RJ, Jong A (2012) Cryptococcus neoformans-derived microvesicles enhance the pathogenesis of fungal brain infection. PLoS ONE 7:e48570. https://doi.org/10.1371/journal.pone.0048570
Rodrigues ML, Nakayasu ES, Oliveira DL, Nimrichter L, Nosanchuk JD, Almeida IC, Casadevall A (2008) Extracellular vesicles produced by Cryptococcus neoformans contain protein components associated with virulence. Eukaryot Cell 7:58–67. https://doi.org/10.1128/EC.00370-07
Vu K, Tham R, Uhrig JP, Thompson GR, Na Pombejra S, Jamklang M, Bautos JM, Gelli A (2014) Invasion of the central nervous system by Cryptococcus neoformans requires a secreted fungal metalloprotease. MBio 5:1–13. https://doi.org/10.1128/mBio.01101-14
Na Pombejra S, Salemi M, Phinney BS, Gelli A (2017) The metalloprotease, Mpr1, engages AnnexinA2 to promote the transcytosis of fungal cells across the blood-brain barrier. Front Cell Infect Microbiol 7:1–16. https://doi.org/10.3389/fcimb.2017.00296
Fang W, Fa ZZ, Xie Q, Wang GZ, Yi J, Zhang C, Meng GX, Gu JL, Liao WQ (2017) Complex roles of annexin A2 in host blood-brain barrier invasion by Cryptococcus neoformans. CNS Neurosci Ther 23:291–300. https://doi.org/10.1111/cns.12673
Cox GM, Mukherjee J, Cole GT, Casadevall A, Perfect JR (2000) Urease as a virulence factor in experimental cryptococcosis. Infect Immun 68:443–448. https://doi.org/10.1128/IAI.68.2.443-448.2000
Olszewski MA, Noverr MC, Chen GH, Toews GB, Cox GM, Perfect JR, Huffnagle GB (2004) Urease expression by Cryptococcus neoformans promotes microvascular sequestration, thereby enhancing central nervous system invasion. Am J Pathol 164:1761–1771. https://doi.org/10.1016/S0002-9440(10)63734-0
Singh A, Panting RJ, Varma A, Saijo T, Waldron KJ, Jong A, Ngamskulrungroj P, Chang YC, Rutherford JC, Kwon-Chung KJ (2013) Factors required for activation of urease as a virulence determinant in Cryptococcus neoformans. MBio 4:1–11. https://doi.org/10.1128/mBio.00220-13
Aoki S, Ito-Kuwa S, Nakamura K, Kato J, Ninomiya K, Vidotto V (1994) Extracellular proteolytic activity of Cryptococcus neoformans. Mycopathologia 128:143–150. https://doi.org/10.1007/BF01138475
Pinti M, Orsi CF, Gibellini L, Esposito R, Cossarizza A, Blasi E, Peppoloni S, Mussini C (2007) Identification and characterization of an aspartyl protease from Cryptococcus neoformans. FEBS Lett 581:3882–3886. https://doi.org/10.1016/j.febslet.2007.07.006
Stie J, Bruni G, Fox D (2009) Surface-associated plasminogen binding of Cryptococcus neoformans promotes extracellular matrix invasion. PLoS ONE 4:e5780. https://doi.org/10.1371/journal.pone.0005780
Stie J, Fox D (2012) Induction of brain microvascular endothelial cell urokinase expression by Cryptococcus neoformans facilitates blood-brain barrier invasion. PLoS ONE 7:e49402. https://doi.org/10.1371/journal.pone.0049402
Stie J, Fox D (2012) Blood-brain barrier invasion by Cryptococcus neoformans is enhanced by functional interactions with plasmin. Microbiology 158:240–258. https://doi.org/10.1099/mic.0.051524-0
Patel D, Desai GM, Frases S, Cordero RJB, DeLeon-Rodriguez CM, Eugenin EA, Nosanchuk JD, Martinez LR (2013) Methamphetamine enhances Cryptococcus neoformans pulmonary Infection and Dissemination to the Brain. 4:1–10. https://doi.org/10.1128/mBio.00400-13
Eugenin EA, Greco JM, Frases S, Nosanchuk JD, Martinez LR (2013) Methamphetamine alters blood brain barrier protein expression in mice, facilitating central nervous system infection by neurotropic Cryptococcus neoformans. J Infect Dis 208:699–704. https://doi.org/10.1093/infdis/jit117
Dagenais TRT, Keller NP (2009) Pathogenesis of Aspergillus fumigatus in invasive aspergillosis. Clin Microbiol Rev 22:447–465. https://doi.org/10.1128/CMR.00055-08
Escobar N, Ordonez SR, Wösten HAB, Haas PJA, de Cock H, Haagsman HP (2016) Hide, keep quiet, and keep low: properties that make Aspergillus fumigatus a successful lung pathogen. Front Microbiol 7:1–13. https://doi.org/10.3389/fmicb.2016.00438
Xu S, Shinohara ML (2017) Tissue-resident macrophages in fungal infections. Front Immunol 8:1–7. https://doi.org/10.3389/fimmu.2017.01798
Latgé JP, Chamilos G (2020) Aspergillus fumigatus and aspergillosis in 2019. Clin Microbiol Rev 33:1–75. https://doi.org/10.1128/CMR.00140-18
Bromley IMJ, Donaldson K (1996) Binding of Aspergillus fumigatus spores to lung epithelial cells and basement membrane proteins: relevance to the asthmatic lung. Thorax 51:1203–1209. https://doi.org/10.1136/thx.51.12.1203
Paris S, Boisvieux-Ulrich E, Crestani B, Houcine O, Taramelli D, Lombardi L, Latge JP (1997) Internalization of Aspergillus fumigatus conidia by epithelial and endothelial cells. Infect Immun 65:1510–1514. https://doi.org/10.1128/iai.65.4.1510-1514.1997
DeHart DJ, Agwu DE, Julian NC, Washburn RG (1997) Binding and germination of Aspergillus fumigatus conidia on cultured A549 pneumocytes. J Infect Dis 175:146–150. https://doi.org/10.1093/infdis/175.1.146
Bouchara JP, Sanchez M, Chevailler A, Marot-Leblond A, Lissitzky JC, Tronchin G, Chabasse D (1997) Sialic acid-dependent recognition of laminin and fibrinogen by Aspergillus fumigatus conidia. Infect Immun 65:2717–2724. https://doi.org/10.1128/iai.65.7.2717-2724.1997
Gravelat FN, Beauvais A, Liu H, Lee MJ, Snarr BD, Chen D, Xu W, Kravtsov I, Hoareau CMQ, Vanier G, Urb M, Campoli P, Al Abdallah Q, Lehoux M, Chabot JC, Ouimet MC, Baptista SD, Fritz JH, Nierman WC, Latgé JP, Mitchell AP, Filler SG, Fontaine T, Sheppard DC (2013) Aspergillus galactosaminogalactan mediates adherence to host constituents and conceals hyphal β-glucan from the immune system. PLoS Pathog 9:e1003575. https://doi.org/10.1371/journal.ppat.1003575
Croft CA, Culibrk L, Moore MM, Tebbutt SJ (2016) Interactions of Aspergillus fumigatus conidia with airway epithelial cells: a critical review. Front Microbiol 7:1–15. https://doi.org/10.3389/fmicb.2016.00472
Wasylnka JA, Moore MM (2002) Uptake of Aspergillus fumigatus conidia by phagocytic and nonphagocytic cells in vitro: quantitation using strains expressing green fluorescent protein. Infect Immun 70:3156–3163. https://doi.org/10.1128/IAI.70.6.3156-3163.2002
Bao Z, Han X, Chen F, Jia X, Zhao J, Zhang C, Yong C, Tian S, Zhou X, Han L (2015) Evidence for the involvement of cofilin in Aspergillus fumigatus internalization into type II alveolar epithelial cells. BMC Microbiol 15:1–11. https://doi.org/10.1186/s12866-015-0500-y
Osherov N (2012) Interaction of the pathogenic mold Aspergillus fumigatus with lung epithelial cells. Front Microbiol 3:1–9. https://doi.org/10.3389/fmicb.2012.00346
Zhang C, Chen F, Liu X, Han X, Hu Y, Su X, Chen Y, Sun Y, Han L (2019) Gliotoxin induces cofilin phosphorylation to promote actin cytoskeleton dynamics and internalization of Aspergillus fumigatus into type II human pneumocyte cells. Front Microbiol 10:1–16. https://doi.org/10.3389/fmicb.2019.01345
Culibrk L, Croft CA, Toor A, Yang SJ, Singhera GK, Dorscheid DR, Moore MM, Tebbutt SJ (2019) Phagocytosis of Aspergillus fumigatus by human bronchial epithelial cells is mediated by the Arp2/3 complex and WIPF2. Front Cell Infect Microbiol 9:16. https://doi.org/10.3389/fcimb.2019.00016
Liu H, Lee MJ, Solis NV, Phan QT, Swidergall M, Ralph B, Ibrahim AS, Sheppard DC, Filler SG (2016) Aspergillus fumigatus CalA binds to Integrin α5β1 and mediates host cell invasion. Physiol Behav 176:139–148. https://doi.org/10.1016/j.physbeh.2017.03.040
Jia X, Chen F, Pan W, Yu R, Tian S, Han G, Fang H, Wang S, Zhao J, Li X, Zheng D, Tao S, Liao W, Han X, Han L (2014) Gliotoxin promotes Aspergillus fumigatus internalization into type II human pneumocyte A549 cells by inducing host phospholipase D activation. Microbes Infect 16:491–501. https://doi.org/10.1016/j.micinf.2014.03.001
Han X, Yu R, Zhen D, Tao S, Schmidt M, Han L (2011) β-1,3-Glucan-induced host phospholipase D activation is involved in Aspergillus fumigatus internalization into type II human pneumocyte A549 cells. PLoS ONE 6:e21468. https://doi.org/10.1371/journal.pone.0021468
Kogan TV, Jadoun J, Mittelman L, Hirschberg K, Osherov N (2004) Involvement of secreted Aspergillus fumigatus proteases in disruption of the actin fiber cytoskeleton and loss of focal adhesion sites in infected A549 lung pneumocytes. J Infect Dis 189:1965–1973. https://doi.org/10.1086/420850
Cody DT, McCaffrey TV, Roberts G, Kern EB (1997) Effects of Aspergillus fumigatus and Alternaria alternata on human ciliated epithelium in vitro. Laryngoscope 107:1511–1514. https://doi.org/10.1097/00005537-199711000-00016
Amitani R, Murayama T, Nawada R, Lee WJ, Niimi A, Suzuki K, Tanaka E, Kuze F (1995) Aspergillus culture filtrates and sputum sols from patients with pulmonary aspergillosis cause damage to human respiratory ciliated epithelium in vitro. Eur Respir J 8:1681–1687. https://doi.org/10.1183/09031936.95.08101681
Kothary MH, Chase T, MacMillan JD (1984) Correlation of elastase production by some strains of Aspergillus fumigatus with ability to cause pulmonary invasive aspergillosis in mice. Infect Immun 43:320–325. https://doi.org/10.1128/iai.43.1.320-325.1984
Blanco JL, Hontecillas R, Bouza E, Blanco I, Pelaez T, Muñoz P, Perez Molina J, Garcia ME (2002) Correlation between the elastase activity index and invasiveness of clinical isolates of Aspergillus fumigatus. J Clin Microbiol 40:1811–1813. https://doi.org/10.1128/JCM.40.5.1811-1813.2002
Amitani R, Taylor G, Elezis EN, Llewellyn-Jones C, Mitchell J, Kuze F, Cole PJ, Wilson R (1995) Purification and characterization of factors produced by Aspergillus fumigatus which affect human ciliated respiratory epithelium. Infect Immun 63:3266–3271. https://doi.org/10.1128/iai.63.9.3266-3271.1995
Guruceaga X, Ezpeleta G, Mayayo E, Sueiro-Olivares M, Abad-Diaz-De-Cerio A, Urízar JMA, Liu HG, Wiemann P, Bok JW, Filler SG, Keller NP, Hernando FL, Ramirez-Garcia A, Rementeria A (2018) A possible role for fumagillin in cellular damage during host infection by Aspergillus fumigatus. Virulence 9:1548–1561. https://doi.org/10.1080/21505594.2018.1526528
Heinekamp T, Schmidt H, Lapp K, Pähtz V, Shopova I, Köster-Eiserfunke N, Krüger T, Kniemeyer O, Brakhage AA (2015) Interference of Aspergillus fumigatus with the immune response. Semin Immunopathol 37:141–152. https://doi.org/10.1007/s00281-014-0465-1
Mitchell CG, Slight J, Donaldson K (1997) Diffusible component from the spore surface of the fungus Aspergillus fumigatus which inhibits the macrophage oxidative burst is distinct from gliotoxin and other hyphal toxins. Thorax 52:796–801. https://doi.org/10.1136/thx.52.9.796
Patel R, Hossain MA, German N, Al-Ahmad AJ (2018) Gliotoxin penetrates and impairs the integrity of the human blood-brain barrier in vitro. Mycotoxin Res 34:257–268. https://doi.org/10.1007/s12550-018-0320-7
Kojic EM, Darouiche RO (2004) Candida infections of medical devices. Clin Microbiol Rev 17:255–267. https://doi.org/10.1128/CMR.17.2.255-267.2004
Allert S, Förster TM, Svensson C-M, Richardson JP, Pawlik T, Hebecker B, Rudolphi S, Juraschitz M, Schaller M, Blagojevic M, Morschhäuser J, Figge MT, Jacobsen ID, Naglik JR, Kasper L, Mogavero S, Hube B, Figge T, Jacobsen ID, Naglik JR, Kasper L, Mogavero S (2018) Candida albicans-induced epithelial damage mediates translocation through intestinal barriers. MBio 9:1–20. https://doi.org/10.1128/mBio.00915-18
Mukaremera L, Lee KK, Mora-Montes HM, Gow NAR (2017) Candida albicans yeast, pseudohyphal, and hyphal morphogenesis differentially affects immune recognition. Front Immunol 8:1–12. https://doi.org/10.3389/fimmu.2017.00629
Nikou SA, Kichik N, Brown R, Ponde NO, Ho J, Naglik JR, Richardson JP (2019) Candida albicans interactions with mucosal surfaces during health and disease. Pathogens 8:1–23. https://doi.org/10.3390/pathogens8020053
Liu Y, Filler SG (2011) Candida albicans Als3, a multifunctional adhesin and invasin. Eukaryot Cell 10:168–173. https://doi.org/10.1128/EC.00279-10
Staab JF, Bradway SD, Fidel PL, Sundstrom P (1999) Adhesive and mammalian transglutaminase substrate properties of Candida albicans Hwp1. Science 283:1538–1538. https://doi.org/10.1126/science.283.5407.1538
Zhu W, Filler SG (2010) Interactions of Candida albicans with epithelial cells. Cell Microbiol 12:273–282. https://doi.org/10.1111/j.1462-5822.2009.01412.x
Yang W, Yan L, Wu C, Zhao X, Tang J (2014) Fungal invasion of epithelial cells. Microbiol Res 169:803–810. https://doi.org/10.1016/j.micres.2014.02.013
Basmaciyan L, Bon F, Paradis T, Lapaquette P, Dalle F (2019) Candida albicans interactions with the host: crossing the intestinal epithelial barrier. Tissue Barriers 7:1–31. https://doi.org/10.1080/21688370.2019.1612661
Phan QT, Myers CL, Fu Y, Sheppard DC, Yeaman MR, Welch WH, Ibrahim AS, Edwards JE, Filler SG (2007) Als3 is a Candida albicans invasin that binds to cadherins and induces endocytosis by host cells. PLoS Biol 5:0543–0557. https://doi.org/10.1371/journal.pbio.0050064
Sun JN, Solis NV, Phan QT, Bajwa JS, Kashleva H, Thompson A, Liu Y, Dongari-Bagtzoglou A, Edgerton M, Filler SG (2010) Host cell invasion and virulence mediated by Candida albicans Ssa1. PLoS Pathog 6:e1001181. https://doi.org/10.1371/journal.ppat.1001181
Filler SG, Swerdloff JN, Hobbs C, Luckett PM (1995) Penetration and damage of endothelial cells by Candida albicans. Infect Immun 63:976–983. https://doi.org/10.1128/iai.63.3.976-983.1995
Park H, Myers CL, Sheppard DC, Phan QT, Sanchez AA, Edwards JE, Filler SG (2005) Role of the fungal Ras-protein kinase A pathway in governing epithelial cell interactions during oropharyngeal candidiasis. Cell Microbiol 7:499–510. https://doi.org/10.1111/j.1462-5822.2004.00476.x
Moreno-Ruiz E, Galán-Díez M, Zhu W, Fernández-Ruiz E, D’Enfert C, Filler SG, Cossart P, Veiga E (2009) Candida albicans internalization by host cells is mediated by a clathrin-dependent mechanism. Cell Microbiol 11:1179–1189. https://doi.org/10.1111/j.1462-5822.2009.01319.x
Martin R, Moran GP, Jacobsen ID, Heyken A, Domey J, Sullivan DJ, Kurzai O, Hube B (2011) The Candida albicans-specific gene EED1 encodes a key regulator of hyphal extension. PLoS ONE 6:e18394. https://doi.org/10.1371/journal.pone.0018394
Naglik JR, Moyes D, Makwana J, Kanzaria P, Tsichlaki E, Weindl G, Tappuni AR, Rodgers CA, Woodman AJ, Challacombe SJ, Schaller M, Hube B (2008) Quantitative expression of the Candida albicans secreted aspartyl proteinase gene family in human oral and vaginal candidiasis. Microbiology 154:3266–3280. https://doi.org/10.1099/mic.0.2008/022293-0
Leidich SD, Ibrahim AS, Fu Y, Koul A, Jessup C, Vitullo J, Fonzi W, Mirbod F, Nakashima S, Nozawa Y, Ghannoum MA (1998) Cloning and disruption of caPLB1, a phospholipase B gene involved in the pathogenicity of Candida albicans. J Biol Chem 273:26078–26086. https://doi.org/10.1074/jbc.273.40.26078
Scherer AK, Blair BA, Park J, Seman BG, Kelley JB, Wheeler RT (2020) Redundant Trojan horse and endothelial-circulatory mechanisms for host-mediated spread of Candida albicans yeast. PLoS Pathog 16:1–36. https://doi.org/10.1371/journal.ppat.1008414
MacCallum DM (2009) Massive induction of innate immune response to Candida albicans in the kidney in a murine intravenous challenge model. FEMS Yeast Res 9:1111–1122. https://doi.org/10.1111/j.1567-1364.2009.00576.x
Jong AY, Stins MF, Huang S-H, Chen SHM, Kim KS (2001) Traversal of Candida albicans across human blood-brain barrier in vitro. Society 69:4536–4544. https://doi.org/10.1128/IAI.69.7.4536
Drummond RA, Lionakis MS (2018) Candidiasis of the central nervous system in neonates and children with primary immunodeficiencies. Curr Fungal Infect Rep 12:92–97. https://doi.org/10.1007/s12281-018-0316-y
Navarathna DH, Roberts DD, Munasinghe J, Lizak MJ (2016) Imaging Candida infections in the host. Methods Mol Biol 1356:69–78. https://doi.org/10.1007/978-1-4939-3052-4_6
Lionakis MS, Lim JK, Lee CCR, Murphy PM (2011) Organ-specific innate immune responses in a mouse model of invasive candidiasis. J Innate Immun 3:180–199. https://doi.org/10.1159/000321157
Ashman RB (1997) Genetic determination of susceptibility and resistance in the pathogenesis of Candida albicans infection. FEMS Immunol Med Microbiol 19:183–189
Jae-Chen S, Young-Joo J, Seon-Min P, Kang Seok S, Jung-Hyun S, Jung-Il C (2015) Mechanism underlying renal failure caused by pathogenic Candida albicans infection. Biomed Reports 3:179–182. https://doi.org/10.3892/br.2014.393
Grubb SEW, Murdoch C, Sudbery PE, Saville SP, Lopez-Ribot JL, Thornhill MH (2008) Candida albicans-endothelial cell interactions: a key step in the pathogenesis of systemic candidiasis. Infect Immun 76:4370–4377. https://doi.org/10.1128/IAI.00332-08
Liu Y, Mittal R, Solis NV, Prasadarao NV, Filler SG (2011) Mechanisms of Candida albicans trafficking to the brain. PLoS Pathog 7:e1002305. https://doi.org/10.1371/journal.ppat.1002305
Swidergall M (2019) Candida albicans at host barrier sites: pattern recognition receptors and beyond. Pathogens 8:1–14. https://doi.org/10.3390/pathogens8010040
Phan QT, Fratti RA, Prasadarao NV, Edwards JE, Filler SG (2005) N-cadherin mediates endocytosis of Candida albicans by endothelial cells. J Biol Chem 280:10455–10461. https://doi.org/10.1074/jbc.M412592200
Crowe JD, Sievwright IK, Auld GC, Moore NR, Gow NAR, Booth NA (2003) Candida albicans binds human plasminogen: identification of eight plasminogen-binding proteins. Mol Microbiol 47:1637–1651. https://doi.org/10.1046/j.1365-2958.2003.03390.x
Jong AY, Chen SHM, Stins MF, Kim KS, Tuan TL, Huang SH (2003) Binding of Candida albicans enolase to plasmin(ogen) results in enhanced invasion of human brain microvascular endothelial cells. J Med Microbiol 52:615–622. https://doi.org/10.1099/jmm.0.05060-0
Darling TK, Mimche PN, Bray C, Umaru B, Brady LM, Stone C, Moukoko CEE, Lane TE, Ayong LS, Lamb TJ (2020) EphA2 contributes to disruption of the blood-brain barrier in cerebral malaria. PLoS Pathog 16:1–33. https://doi.org/10.1371/journal.ppat.1008261
Shi M, Calaruso P, Mody CH (2012) Real-time in vivo imaging of fungal migration to the central nervous system. Cell Microbiol 14:1819–1827. https://doi.org/10.1111/cmi.12027
Campisi M, Shin Y, Osaki T, Hajal C, Chiono V, Kamm RD (2018) 3D self-organized microvascular model of the human blood-brain barrier with endothelial cells, pericytes and astrocytes. Biomaterials 180:117–129. https://doi.org/10.1016/j.biomaterials.2018.07.014
Faley SL, Neal EH, Wang JX, Bosworth AM, Weber CM, Balotin KM, Lippmann ES, Bellan LM (2019) iPSC-derived brain endothelium exhibits stable, long-term barrier function in perfused hydrogel scaffolds. Stem Cell Reports 12:474–487. https://doi.org/10.1016/j.stemcr.2019.01.009
Grifno GN, Farrell AM, Linville RM, Arevalo D, Kim JH, Gu L, Searson PC (2019) Tissue-engineered blood-brain barrier models via directed differentiation of human induced pluripotent stem cells. Sci Rep 9:1–13. https://doi.org/10.1038/s41598-019-50193-1
Linville RM, DeStefano JG, Sklar MB, Xu Z, Farrell AM, Bogorad MI, Chu C, Walczak P, Cheng L, Mahairaki V, Whartenby KA, Calabresi PA, Searson PC (2019) Human iPSC-derived blood-brain barrier microvessels: validation of barrier function and endothelial cell behavior. Biomaterials 190–191:24–37. https://doi.org/10.1016/j.biomaterials.2018.10.023
Tenor JL, Oehlers SH, Yang JL, Tobin DM, Perfect JR (2015) Live imaging of host-parasite interactions in a zebrafish infection model reveals cryptococcal determinants of virulence and central nervous system invasion. MBio 6:1–11. https://doi.org/10.1128/mBio.01425-15
Shi M, Mody CH (2016) Fungal infection in the brain: what we learned from intravital imaging. Front Immunol 7:1–7. https://doi.org/10.3389/fimmu.2016.00292
Konradt C, Ueno N, Christian DA, Delong JH, Pritchard GH, Herz J, Bzik DJ, Koshy AA, McGavern DB, Lodoen MB, Hunter CA (2016) Endothelial cells are a replicative niche for entry of Toxoplasma gondii to the central nervous system. Nat Microbiol 1:16001. https://doi.org/10.1038/nmicrobiol.2016.1
Zhang M, Sun D, Shi M (2015) Dancing cheek to cheek: Cryptococcus neoformans and phagocytes. Springerplus 4:410. https://doi.org/10.1186/s40064-015-1192-3
Acknowledgements
This work was supported by National Institutes of Health (NIH) Grants to M.S. (AI131219 and AI131905). Figures included in this review were created with BioRender.com.
Author information
Authors and Affiliations
Contributions
AS and MS wrote the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The experiments comply with the current laws of the country in which they were performed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Strickland, A.B., Shi, M. Mechanisms of fungal dissemination. Cell. Mol. Life Sci. 78, 3219–3238 (2021). https://doi.org/10.1007/s00018-020-03736-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-020-03736-z