Abstract
It is well known that adipose tissue has a critical role in the development of obesity and metabolic diseases and that adipose tissue acts as an endocrine organ to regulate lipid and glucose metabolism. Accumulating in the adipose tissue, fatty acids serve as a primary source of essential nutrients and act on intracellular and cell surface receptors to regulate biological events. G protein-coupled receptor 120 (GPR120) represents a promising target for the treatment of obesity-related metabolic disorders for its involvement in the regulation of adipogenesis, inflammation, glucose uptake, and insulin resistance. In this review, we summarize recent studies and advances regarding the systemic role of GPR120 in adipose tissue, including both white and brown adipocytes. We offer a new perspective by comparing the different roles in a variety of homeostatic processes from adipogenic development to adipocyte metabolism, and we also discuss the effects of natural and synthetic agonists that may be potential agents for the treatment of metabolic diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The global incidence of obesity and type 2 diabetes has increased over the last three decades. It is well confirmed that adipose tissues which consist of the white adipose tissues and the brown adipocytes greatly contribute to obesity-associated disease. As the main reservoir to store fatty acids contain lipid droplets as a type of energy, the white adipose tissues are to protect and insulate the body at the physical level [1]. Brown adipocytes, on the other hand, show high thermogenic capacity and dissipate the stored energy in the form of heat [2, 3]. Being a remarkably complex organ with significant effects on physiology, adipose tissue also plays a pivotal role as an endocrine organ in glucose metabolism and immune function through secretion of a vast range of regulatory factors [4, 5]. Studies on the developmental and functional roles of adipose tissue have widely expanded after the 1990s [3]. This paper examines the functions and regulatory mechanisms of adipose tissue in the hope of helping to find an efficient way to decrease the rate of obesity and metabolic diseases.
Free-fatty acids are the basic components of essential nutrients and serve as signalling molecules that modulate energy homeostasis [6]. In particular, beneficial effects of n-3 polyunsaturated fatty acids (n-3 PUFAs) are clearly observed in anti-inflammatory processes, lipid metabolism, and glucose homeostasis, as well as in insulin-sensitizing effects [7, 8]. Notably, the n-3 PUFAs act as ligands of G protein-coupled receptor 120 (GPR120) [9]. A number of studies done in the last decade have shown that GPR120 is implicated in crucial homeostatic processes, including adipogenesis, anti-inflammatory processes, glucose uptake, and insulin sensitivity, as well as the secretion of hormones, prompting recent studies to thrust GPR120 into the realm of drug discovery [10–12]. Many excellent recent reviews have provided insights into the biological and physiological roles of GPR120 [13–16]. However, the detailed role of GPR120 in adipose tissue has not been well demonstrated yet.
Hence, the primary goal of this review is to summarize the most recent and seminal studies that have contributed to our understanding of the function and regulation of GPR120, especially in adipose tissue. We first describe the biological characteristics of GPR120. We then expound on the inconsistencies and certainties that have been presented due to advances in our knowledge of the function of adipose tissue. Finally, we discuss the natural and synthetic agonists that are known to regulate the activity of GPR120 to resist obesity and diabetes.
Biological characteristics of GPR120
The novel fatty acid receptor GPR120, also known as free-fatty acid receptor 4 (FFAR4), is a seven transmembrane receptor and was deorphanised in 2005 when long chain fatty acids were shown to respond well to it [9, 17]. Numerous studies have reported the characteristics of GPR120 from specific species [18–20]. The human GPR120 exists as two splice variants, and the short variant has a structure similar to that of the rodent and monkey receptor. Our previous work reported the three alternatively spliced transcripts of pigs, and the wild-type variant is well matched with the human short isoform [21]. As a GPCR, GPR120 shares the ability to activate heterotrimeric G proteins and induces downstream second messenger pathways in the context of ligand treatment. Hirasawa et al. reported that the activation of GPR120 by fatty acids augment intracellular calcium (Ca2+) and extracellular signal-regulated kinase 1/2 (ERK1/2) without affecting the level of cyclic AMP [17]. Furthermore, Oh et al. showed that GPR120 can effectively respond to n-3 fatty acids by activating Gαq/11 in adipocytes and by recruiting cytosolic β-arrestin 2 to the cell membrane in macrophages [11]. Hence, we infer that the various events induced by the two pathways may involve one of the two coupled proteins, Gαq/11 and β-arrestin 2. However, other biological roles of GPR120 remain to be explored.
GPR120 has been shown in recent studies to be implicated in diverse physiological homeostasis processes, including release of gut peptides, insulin sensitization, anti-inflammatory processes, and regulation of appetite [11, 17, 22]. Consistent with the pleiotropic functions in biological events, stronger expression of GPR120 is observed in tissues, such as the small intestine, spleen, adipose tissue, and taste buds. Despite the differing expression pattern from varied species, a similarly high level of GPR120 is found to be present in the same tissues, such as the small intestine, which may reflect the concordant functions, for example, glucagon-like peptide 1 (GLP-1) secretion [17]. It is worth noting that GPR120 is found to be expressed endogenously both in adipocytes and in adipose tissue but not in preadipocytes [12], implying that the role of GPR120 may closely be related to the development and metabolism of adipose tissue. This special distribution might reflect the crucial functions of GPR120.
Physiological functions of GPR120 in adipose tissue
The role of GPR120 in adipose development
The expression of GPR120 in adipose tissue
A growing number of evidences point to the key role of GPR120 in adipose development, including both white and brown adipocytes. As mentioned above, GPR120 cannot be detected in preadipocytes, showing abundant expression in mature 3T3-L1 cells (a recognized cell line for white adipocyte research [23]) and human white adipocytes. The expression of GPR120 increases with MDI treatment (the differentiation medium containing isobutylmethylxanthine, dexamethasone, and insulin), a typical adipogenic induction cocktail for white adipocytes in vitro [23], indicating that this receptor is probably implicated in adipogenic differentiation. Using siRNA, Gotoh and his colleagues reported that GPR120 knockdown inhibited adipogenesis [12]. Moreover, the adipogenesis process is suppressed in GPR120 −/− mouse embryonic fibroblast-derived (MEF) adipocytes [10]. Our study also showed that the adipogenic ability was significantly inhibited in lentivirus-mediated shGPR120 transfected 3T3-L1 cells [24]. Therefore, we assume that GPR120 might act as an adipogenic receptor [25].
An earlier study demonstrated that high expression of GPR120 was detected in four types of adipose tissues, including subcutaneous, perinephric, mesenteric, and epididymis tissue collected from high-fat diet-fed mice [12]. Experiments in humans showed a similar pattern revealing a significantly higher expression of GPR120 in obese individuals than in lean individuals [10]. Another report, likewise, exhibited a high expression of GPR120 in the brown adipose tissue (BAT) and the inducement of protein level in the BAT after cold exposure. It has also been reported that the activation of GPR120 can promote the browning of white fat in mice [26]. Thus, it is safe to speculate that the expression of the receptor may be induced by high lipid intake in rodents and humans. Although the exhaustive role of GPR120 is still unclear, its indispensable function in adipogenesis is fairly certain.
The effects of GPR120 agonists in adipogenesis
As is well known, with the activation of ligand, the GPCR couples with G proteins and β-arrestins to transduce a signal through a downstream pathway, regulating physiological events. GPR120 showed a strong response to medium and long chain polyunsaturated fatty acids (LCPUFA), especially n-3 PUFAs. Our previous studies showed that α-linolenic acids (ALA), rather than docosahexaenoic acid (DHA), improved adipogenesis in a GPR120-dependent pathway in 3T3L1 cells (Fig. 1) [24]. However, when using a luciferase reporter assay, we found that both DHA and ALA can effectively activate GPR120 [21]. Despite the finding that LCPUFA act as natural ligands of GPR120, the effects of LCPUFA on modulation of 3T3-L1 adipogenic differentiation are inconclusive.
Schematic diagram of adipogenesis mediated by GPR120 activation in 3T3L1 cells. With the treatment of n-3 PUFAs or GPR120 synthetic agonist, TUG-891, GPR120 promotes adipogenesis by activating PPARγ and elevating the expression of key adipogenic gene via [Ca2+]i and ERK1/2 signal pathway in 3T3L1 cells
DHA, similar to other fatty acids, has multifunctional roles: it may accelerate, inhibit, or do not influence adipogenic processes. However, ALA was found to be more sensitive to this receptor in adipogenesis as well as other events [17]. Therefore, we chose the selective synthetic agonist TUG-891 to clarify the downstream signalling mediated by GPR120 and, to some extent, avoiding potential GPR120-independent mechanisms [27]. Our previous study showed that pig GPR120 was well activated by TUG-891, and the stromal vascular fraction cells from porcine adipose tissue, as well as 3T3-L1 preadipocytes, were promoted through adipogenesis [24]. Gao et al. reported that TUG-891, at a low concentration range, functions as a positive enhancer in the adipogenic process, and higher concentrations induce the opposite developmental direction, osteogenesis [28]. The different cell types and characteristics and the distinct expression of GPR120 in the two cells may be the cause of the diverse mechanisms. On the other hand, GPR120 which can be definitely stimulated by GW9508, another selective agonist for GPR120 may induce BAT activity as well as the n-3 PUFAs [26, 29]. Both the eicosapentaenoic acid (EPA) and GW9508 were found to have accelerated the adipogenesis of brown and beige adipocyte and upregulated the expression pf key thermogenic genes (Fig. 2). On the whole, GPR120 activation may induce adipogenesis in two different types of adipocytes, which implies the distinct roles of this receptor in the two biological events.
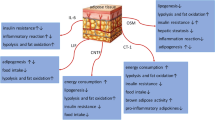
Schematic diagram of the role of GPR120 activation in adipose tissues related to the metabolic homeostasis. With the treatment of n-3 PUFAs or GPR120 synthetic agonists, GPR120 promotes adipogenesis in both white and brown preadipocytes. In white adipose tissue, GPR120 mediates anti-inflammation and insulin sensitization effects and GPR120 activation also induces the browning of white adipocytes. The pleiotropic functions of GPR120 in adipose tissue will contribute to the whole-body metabolic homeostasis
What are the cellular effectors of GPR120 in adipogenesis
During differentiation of 3T3-L1 cells, mRNA expression of GPR120 closely coincides with the master adipogenic regulator, peroxisome proliferator-activated receptor γ (PPARγ). Interestingly, GPR120 knockdown was found to reduce the mRNA level of PPARγ and the adipogenic marker gene, fatty acid binding protein 4 (FABP4) [12]. Moreover, adipogenesis-related genes, including PPARγ and FABP4, are decreased in adipocytes induced from GPR120−/− MEF cells [10]. On the other hand, it has been demonstrated that the PPARγ agonist troglitazone increased the mRNA expression of GPR120 [12]. These results may imply that PPARγ is regulated by GPR120 or that GPR120 can interact with PPARγ during adipocyte differentiation. How is the signal transduced from the membrane receptor to the nuclear receptor? It should be noted that both GPR120 and PPARγ have similar ligand binding pockets and both bind with DHA [30–32]. DHA and ALA are shown as PPARγ ligands that activate the receptor. In addition, they can also combine with GPR120 [24]. Therefore, a selective agonist is necessary for activation of PPARγ. Although TUG-891 is a potent agonist for pig and mouse GPR120, less evidence is available regarding the function of TUG-891 on PPARγ. Using a luciferase reporter system, Song et al. reported that TUG-891 could activate PPARγ in a GPR120-dependent manner [24]. This illustrates that with TUG-891 treatment, activated GPR120 may transduce the intracellular signal into the nucleus to activate PPARγ. We speculate that the GPR120-PPARγ pathway may be one of the main regulation mechanisms to modulate adipogenesis in 3T3L1 cells (as shown is Fig. 1). However, using GW9662 (an inhibitor of PPARγ) in differentiating brown adipocyte did not dramatically alter the effects of EPA or GW9508 on the phenotype of differentiation, indicating that PPARγ may be not essential for the EPA- or GW9508-induced brown adipocyte differentiation in some extent, and other key downstream regulators, such as uncoupling protein-1 (UCP1), may play a role consistent with GPR120 in this event [26, 29]. All these suggest that the targets of the white and brown fat adipogenesis induced by GPR120 are quite different and need to be well examined further.
The downstream signals mediated by GPR120 in adipogenesis
Moreover, the precise signalling mediated by GPR120 is also worth studying. Numerous studies have shown that GPR120 coupled with Gαq/11 induces an increase in intracellular calcium concentration [Ca2+]i and phosphorylation of the ERK1/2 cascade [17, 27]. Several studies have reported that both ERK1/2 and [Ca2+]i play a critical role in adipogenesis. ERK1/2 facilitates the early stage of adipogenesis, while this signal needs to be closed at later stages, which suggests a multifunctional role for ERK1/2 [33, 34]. In addition, there is evidence suggesting that the [Ca2+]i signal manifests to exert a biphasic function in adipocyte differentiation both in mice and human preadipocytes [35, 36]. In the first 2 days of adipogenesis, increasing [Ca2+]i levels suppress this progress but accelerate the later maturation stage of adipogenic differentiation [35, 36]. The intracellular signal in adipogenesis mediated by GPR120 remains largely unknown. As shown in Fig. 1, [Ca2+]i and ERK1/2 may provide new insights to determine the mechanism of GPR120 in adipogenesis. In 3T3-L1 preadipocytes, TUG-891 activates both of the signals. In addition, combined treatment with TUG-891 and BAPTA-AM (a Ca2+ chelator) or U0126 (ERK1/2 inhibitor) abolished the adipogenesis process. Furthermore, PPARγ mRNA and protein levels are also influenced by blocking the two signals. This demonstrates that the [Ca2+]i-ERK1/2 pathway is involved in GPR120-induced processes and ultimately targets PPARγ to affect adipogenesis [24]. This may shed light on one of the possible intracellular pathways. In BMMSCs, Gao et al. reported that 3 days of treatment with a low concentration of TUG-891 (0.5 µM) promoted adipogenesis, shown both by the adipogenic phenotype and the expression of PPARγ [28]. However, the ERK1/2 signal is blocked in these events. Therefore, the precise underlying mechanism may be different depending on the cell types and treatment methods. Others have shown that phosphorylation of AKT (p-AKT) cannot be detected in white adipose tissue separated from GPR120−/− mice [10]. Perhaps p-AKT is also related to GPR120-mediated adipogenesis, but this association has not been identified. Overall, the question is how these mentioned signals can be transduced into nuclear signals to activate PPARγ, and the answer still needs to be clarified. Given the relationship between GPR120 and PPARγ in adipogenesis, it will be interesting to demonstrate whether the GPR120-PPARγ pathway functions in white adipose metabolism and even in other tissues. In comparison, the down signaling modulated by GPR120 is somewhat complicated in the brown and beige adipocytes. It seems that GPR120 activation upregulates the microRNA 30b/microRNA 378 and the cyclic AMP (cAMP), in turn, and both microRNAs and cAMP can affect the level of GPR120 [26, 29].
Considering the especially high expression of GPR120 in adipocytes and adipose tissue, the precise mechanism of GPR120 in both WAT and BAT is being explored. It is reasonable to say that the role of GPR120 in adipogenesis is clear, while the important functions in brown/beige adipocytes adipogenesis still need to be clarified.
The metabolic regulation of GPR120 in adipose tissue
Anti-inflammatory effects
Obesity gives rise to chronic low-grade inflammation, which can trigger insulin resistance and type 2 diabetes [37]. White adipose tissue acts as an important endocrine organ and releases numerous adipokines that can contribute to pro- or anti-inflammation states [38]. Adipose tissue inflammation is a process characterized by an inflammatory response mediated by adipose tissue macrophages [39]. There is increasing interest focused on the role of adipose tissue macrophages (ATMs) in obesity and in ATM functions in the inflammatory pathways activated in adipose tissue, which change the characteristics of obesity in obese individuals [40, 41]. Moreover, ATMs are highly responsible for the expression of tumour necrosis factor α (TNFα) and interleukin-6 (IL-6), which can block normal activity in adipocytes [42, 43]. Thus, ATMs provide a potential and pivotal function in the metabolic role of adipose tissue.
Oh et al. analysed the ATMs from wild-type (WT) and GPR120-deficient mice in vivo. The HFD induced the total number of F4/80 marked M1 macrophages separated from adipose tissues [11]. In context of n-3 PUFA treatment, the number of M1 macrophages decreased, while the number of macrophage galactose-type C-type lectin 1 (MGL1) marked M2 macrophages strikingly increased in the WT mice but not in the GPR120-deficient mice [11]. Moreover, GPR120 also accelerated the M2 macrophage polarization, which facilitated the formation of an anti-inflammatory status in adipose tissue [11]. In addition, adding n-3 PUFA to the normal diet resulted in decreased macrophage chemotaxis capacity in a GPR120-dependent manner. Furthermore, using the synthetic GPR120 agonist cpdA, the positive effect of GPR120 in the ATMs was confirmed [44]. In view of the above studies, GPR120 functions as an n-3 PUFA sensor in adipose tissue that triggers beneficial anti-inflammatory effects. In an in vitro model, the mechanism of the anti-inflammatory role has also been elucidated in RAW 264.7 cells (a type of mouse macrophage). Ligand-activated GPR120 binds to β-arrestin2, and the complex interacts with transforming growth factor-β-activated kinase binding protein 1 (TAB1), in turn, blocking downstream key pro-inflammatory signalling molecules, including nuclear factor-κB (NFκB) and c-Jun N-terminal kinase (JNK), and inhibiting inflammation [11]. Other studies have shown different GPR120-dependent pathways that are involved with cyclooxygenase 2 (COX-2) expression and prostaglandin E2 (PGE2) synthesis [45, 46]. These data are referred in another excellent review [13].
Some metabolins may influence the GPR120-dependent β-arrestin2 involved in anti-inflammation. It is worth mentioning that hyperhomocysteinaemia (HHcy) inhibits insulin sensitivity in adipose tissue and is considered a chronic inflammatory state by the epidemiological literature [47]. HHcy, characterized by an abnormally high level of homocysteine, acts as a pro-inflammatory factor to induce the expression and secretion of resistin [48]. Recently, it has been reported that in rat adipocytes, GPR120 activation by GW9508 can reverse HHcy-induced insulin resistance by inhibiting adipose inflammation rather than endoplasmic reticulum (ER) stress [49]. GPR120 prevents HHcy-induced JNK activation and inflammation. Meanwhile, the downstream signal β-arrestin2 mediated by GPR120 can be repressed by homocysteine [50]. Thus, it may be interesting to clarify the precise mechanism of the expression and activation of GPR120-dependent β-arrestin2 involved in anti-inflammation.
Moreover, it cannot be ignored that the WAT depots, in the form of triglycerides (TG), store fatty acids derived from diets, and endogenous synthesis [51]. The lipolysis of TG provides the serum with varying chains FFAs to influence the macrophage activity [52]. Several excellent reviews focus on the variety of FFAs which modify the macrophages and other inflammatory cells [52–54]. Recently, Rodriguez-Pacheco et al. have reported that FFAs have different effects on the inflammatory profile of the adipocytes from the visceral adipocytes tissue and the pro-inflammatory effects of different fatty acids in the GPR120 knockdown visceral adipocytes can be weakened [55]. This is because GPR120 act partially as an inflammatory mediator to regulate the inflammatory effects induced by the FFAs [55]. In addition, different fatty acids show the varying degrees of the promotion function on the expression of GPR120 in both nonobese and morbidly obese subjects [56]. Perhaps, GPR120 in adipocytes tissues from different location shows various roles which are based on the character of the native adipocytes [57]. The observation of Rodriguez-Pacheco et al. may need to be well dissected further, especially in vivo test [55].
In addition, during adipose tissue remodelling in obesity, another key factor that we focus on is the role of hypoxia. Although adipose tissue can recruit new blood vessels during expansion, even elevating the oxygen tension in the fat pads, hypoxia may develop [58]. Moreover, hypoxia-inducible factor 1 (HIF-1) becomes activated in obese adipocytes [59]. Vascular endothelial grow factor-A (VEGF-A) plays a critical role in promoting local vascular development in the growing adipose tissue [60]. Hansan et al. reported that EPA could increase the release of VEGF-A through GPR120-dependent and independent pathways in 3T3-L1 adipocytes [61]. This suggests that the activation of GPR120 could act in another role to partly promote angiogenesis, which may have an anti-inflammatory function in adipose tissue [60]. Indeed, given the functions in both adipocytes and macrophages in the adipose tissue, it seems possible that GPR120 can act in an anti-diabetic role through an anti-inflammatory effect in adipose tissue [11, 60]. Currently, several precise mechanisms regarding the anti-inflammation function of GPR120 in adipose tissue are being dissected, as well as the interactions among different cell types [3].
Glucose uptake and energy metabolism
Consistent with the basic function of fatty acids in energy metabolism in adipose tissue, the fatty acids receptor, GPR120, seems to have a pivotal role in regulating adipose metabolism. As described before, GPR120 is found to be highly expressed in adipocytes and adipose tissue. With a high-fat diet (HFD) treatment, GPR120-deficient mice show a notable decrease in adipogenic and lipogenic genes, while these genes increased in the liver [10]. Seminal work by Oh et al. has shown that GPR120 activated by n-3 PUFA and GW9508 leads to insulin sensitization in vivo and strongly alleviates glucose intolerance in HFD-induced obesity [11]. In both 3T3-L1 adipocytes and primary adipose tissue, activated GPR120 significantly enhances the activation of the PI3K/Akt pathway, triggering GLUT4 translocation to the cell membrane and increasing glucose uptake in a manner dependent on coupling to Gαq/11 not β-arrestin [11]. It is worth mentioning that Gαq/11 and IRS1 are independent signals that activate the PI3K-Akt-GLUT4 pathway [11]. One group also developed a type of GPR120 agonist, compound A (cpdA), that responded well to insulin sensitivity and improved glucose tolerance and decreased hyperinsulinemia [44]. Another small molecule agonist, TUG-891, and members of the new fatty acid ester of hydroxy fatty acid (9-PAHSA) lipid class have been designed to activate GPR120 and enhance glucose uptake [27, 62, 63]. Intriguingly, Liu et al. reported that in GPR120 knockdown 3T3-L1 cells, the expression of GLUT4 and insulin receptor substrate 1(IRS1) is decreased [64]. Ichimura et al. found that insulin signalling-related genes are remarkably decreased in adipose tissue of HFD-fed GPR120 −/− mice, especially IRS1 [10]. When the IRS1 level was lower than a specific amount, insulin signalling was abolished. Therefore, based on the abnormal insulin signal in adipose tissue and the liver, the GPR120-deficient mice fed with an HFD diet develop hyperglycaemia, glucose intolerance, and insulin resistance when compared with the control group. This suggests that dysfunction of GPR120 may impair normal insulin signalling and adversely impact glucose uptake in adipose tissue [16].
On the other hand, when mice are challenged by an HFD, the expression of GPR120 increased higher than reported in the previous work [12]. Some researchers, such as Chen et al., have reported an upstream mechanism to regulate GPR120 [65]. In context of the HFD, the mRNA and protein expression of GPR120 and PPARγ was increased and the binding of CCAAT/enhancer binding protein β (C/EBPβ) on the GPR120 core promoter was accelerated [65]. They speculate that the C/EBPβ-GPR120-PPARγ pathway might provide a way to regulate energy metabolism [65]. In addition, another group reported that EPA upregulated VEGF-A through GPR120-PPARγ or in a GPR120-independent manner in 3T3-L1 adipocytes [61]. Overexpression of VEGF-A in adipose tissue ameliorated glucose intolerance and showed an insulin-sensitizing effect under the challenge of HFD in mice [60]. Thus, this may demonstrate that in the HFD diet treatment, expression of GPR120 will be upregulated and activated GPR120 induced by an agonist will improve the metabolic status of adipose tissue. However, the interaction between GPR120 and PPARγ implicated in energy metabolism needs to be addressed in the white adipocyte.
Unlike the white adipocytes, the brown and beige adipocytes transfer stored chemical energy in the form of heat in response to cold or overfeeding [3]. Thus, we can confirm that the brown adipocytes thermogenic activity and the browning of white adipocytes are the key and promising mechanism to protect body from obesity and metabolic diseases. Notably, the BAT also contributes as an endocrine organ [66]. As recently reported, GPR120 may be the crucial mediator in the energy metabolism of brown adipocytes, since n-3 PUFAs- and synthetic agonists-mediated GPR120 activation induce the release of the hormonal factor fibroblast growth factor-21 (FGF21), resulting in the promotion of BAT activity and WAT browning [26].
These data indicate that GPR120 plays a role as a lipid sensor in vivo and senses the dietary fat to regulate glucose uptake and energy metabolism in adipose tissue, thus triggering a novel pathway of BAT activation and browning of WAT.
Taken together, the role of GPR120 in anti-inflammation, glucose uptake, energy metabolism, and other related biological events, such as angiogenesis, has so far seemed clear in some extent, although the precise mechanism of GPR120 needs to be further explored.
What are the inconsistencies during adipogenesis and adipocyte metabolism?
The normal expression of GPR120 may contribute to the normal functions
Several studies have reported that GPR120 is required for normal adipogenesis both in vivo and in vitro. As Gotoh et al. has shown, GPR120 was increased in four different adipose tissues from HFD-fed mice [12]. In addition, the expression of GPR120 was notably increased in subcutaneous and visceral adipose tissue from obese individuals [10]. In contrast, another group reported that morbidly obese individuals have lower mRNA and protein expression of GPR120 in visceral adipose tissue than lean humans, and the expression was reduced 3 h after a high-fat meal in obese people [56]. This is the first inconsistency between studies. As described above, GPR120 plays an important role in systemic homeostasis. In GPR120 −/− mice, it is easy to develop obesity, but decreased adipocyte differentiation with lower PPARγ level and lower content of triglyceride also occurs [10]. That means the adipogenesis process in GPR120-deficient mice is abnormal, which is consistent with the vitro experiment from the Gotoh group [10, 12]. Furthermore, hepatic lipogenesis is higher than in wild-type mice, which accounts for the final fat and other obesity-related metabolic problems of GPR120−/− mice. In addition, it has been indicated that adipogenesis fails to store all the excess nutrients, and the extra energy will be ectopically deposited into liver and muscle tissue [67]. Dysfunction of GPR120 may lead to lipid accumulation in the liver, resulting in worse insulin resistance [11]. Morbidly obese individuals may have an absence of functional GPR120 and the high-fat meal treatment may worsen the dysfunction of GPR120 and the insulin resistance. If so, the activation of normal GPR120 can be a way to facilitate positive adipogenesis and may improve insulin resistance. However, a direct method to measure the normal, functional GPR120 in obese people is still missing. Perhaps, the expression of GPR120 acts as an indicator to assess the state of metabolic homeostasis. Actually, mature adipocytes induce the production of C16:1n7 palmitoleate, which has been proposed to be a lipid hormone that modulates the interaction among adipose, muscle, and liver tissue [68]. It seems that dysfunction of GPR120 leads to insufficient production of palmitoleate [11, 68].
The contrariety between “the pro-adipogenic function” and “anti-inflammatory role”?
Another inconsistency is between the promoting function in adipogenesis and the anti-inflammatory role in adipocytes. The idea that obesity is involved in adipocyte hyperplasia has created a false notion in our mind: adipogenesis causes obesity [3]. However, it is worth noting that adipogenesis occurs in the obesity process but is not the primary driver. The root cause is the energy balance equation. Given extra nutrition, increased adipogenesis is driven by a requirement to store excess calories. Moreover, the formation of new adipocytes should be adapted to dispose of the surplus energy safely [3]. As mentioned before, normal GPR120 may be activated by FFAs to facilitate adipogenesis and lipogenesis in adipose tissue, thereby resulting in healthy metabolic homeostasis. Recently, a group of people called “metabolically healthy obese” (MHO) were identified, who tend to show reduced visceral adiposity and inflammation and improved glucose and lipid homeostasis [69]. Notably, they have smaller adipocytes than other “normal obese” people [70]. Another alternative hypothesis is that adipogenesis increases the number of new, smaller adipocytes that have functional glucose uptake and healthy adipokines to improve metabolic health [3]. Importantly, the agonist of PPARγ, thiazolidinedione (TZD), plays a pivotal role in regulating homeostasis and improving insulin resistance. Consequently, TZD increased adipocyte cell numbers and total adiposity [71], and unhealthy obese people had a diminished number of preadipocytes [72]. In our previous work, we found that the expression of GPR120, synchronously with PPARγ, is decreased after 6 days of MDI adipogenic induction [21]. In regard to the hypertrophy of mature adipocytes, the bigger the adipocyte grows, the worse their metabolic status. Thus, we speculate that the increase of GPR120 in adipogenesis may be used for forming new adipocytes, and the decrease of GPR120 in mature adipocytes may be a response to the dysfunction of adipocytes. However, it seems that making obese people more fat with new, smaller adipocytes is not a priority for medical research [3].
Despite some of the vital functions have been revealed, new roles are still being discovered. The contradictory nature of the different functions of GPR120 implies that there is much that is not known about this receptor. The precise underlying mechanisms of GPR120 signalling need to be explored. On the whole, it is certain that GPR120 is required for adipogenesis to format new adipocytes and functions in metabolic homeostasis via its important, but not detailed, role in maintaining normal adipose metabolism. Recently, the observation that GPR120 is prominently increased in murine brown adipose tissue in response to exposure to cold supports its role in energy expenditure [73]. Studies have shown that the GPR120 activated by agonists can trigger the BAT activation and browning of WAT [26, 29]. These findings indicate the importance of GPR120 in systemic metabolic and further stir the interest in the potential natural or synthetic agonists of GPR120 for fighting the metabolic diseases.
The application of GPR120 agonists in anti-obesity and anti-diabetic therapeutics
As mentioned before, GPR120 is a promising pharmaceutical target for anti-diabetic and anti-obesity treatments. Hirasawa and colleagues, for the first time, deorphanised this receptor and found that the ligand was LCFA [17]. After that, others have also identified several types of fatty acids and have determined that n-3 PUFA is not a unique ligand for GPR120 [11, 74]. Based on an ectopic GPR120-overexpression system in HEK293T cells, saturated and monounsaturated fatty acids have also been found to activate this receptor [11, 20, 75]. It needs careful consideration regarding the special kind of natural agonists. In some cell types that have high expression of GPR120, only LCPUFA responded well to GPR120. In mouse STC-1 cells, LCPUFA, but not saturated LCFA, could evoke a clear response to the secretion of glucagon-like peptide 1(GLP-1) [17]. Surprisingly, both n-3 and n-6 PUFAs could strongly activate GPR120 with different signalling events in human CaCo-2 cells, which are used as an intestinal epithelial model [76]. Thus, the conclusions should be treated with caution. In lipidomic analysis experiments, Yore et al. identified a new class of endogenous agonist, called 9-PAHSA, and found that it can activate GPR120 in a concentration-dependent manner [62]. Although 9-PAHSA shares similar properties with DHA and another agonist, GW9508, the pharmacological characteristics of this endogenous agonist have not been well illustrated.
Based on the uncertainty of the natural ligand, it is necessary to explore synthetic ligands with high potency and selectivity for GPR120. Several recent reviews have well summarized the development progress of GPR120 agonists. For instance, Briscoe et al. described GW9508, a small molecule for both GPR120 and GPR40 [74]. In cells expressing GPR120, but not GPR40, GW9508 can be a specific agonist for GPR120 [11, 77]. NCG21, another agonist product, was not widely used in subsequent research, and notably, it is identified among the derivatives of PPARγ agonists [31]. This may also show the relationship between GPR120 and PPARγ. Noticeably, TUG-891 was recently identified as a GPR120 agonist, which has better selective potency on human and mice GPR120 than GW9508 and NCG21 [27, 63]. In our previous work, using TUG-891 to activate porcine GPR120, we found that this agonist shares the strong response to GPR120 similar to DHA and ALA [21]. Furthermore, TUG-891 can stimulate intracellular calcium release in a concentration-dependent manner in 2-day differentiated 3T3-L1 adipocytes [24]. Both in the ectopically expressing HEK293T cells and the endogenously expressing adipocytes, ERK1/2 signal can be well phosphorylated by TUG-891 treatment [21, 24]. However, the selectivity of TUG-891 is limited for mice and is still unknown for rat GPR120. In addition, the orally available and in vivo tested GPR120 agonist cpdA has been developed [44]. cpdA mimics the anti-inflammatory effects with DHA, as Oh et al. reported, and showed a GPR120-mediated anti-inflammation role in vivo [44].
It is certain that GPR120 improves glucose uptake and provides anti-inflammatory/insulin-sensitizing effects. Uncovering the detailed mechanism of GPR120-n-3 PUFA will lead to a more direct and potent way to guide the proper supplementation of fish oil. Especially, in regard to the anti-inflammatory effect, n-3 PUFA could activate GPR120 in the hypothalamus in a diet-induced obesity mice model, and further studies are still needed to shed light on the role of GPR120 to alleviate inflammation in the central nervous system (CNS) [78, 79]. As a “druggable” GPCR, the development of a highly efficient and functional GPR120 agonist both in vitro and in vivo could lead to a new therapeutic approach for the treatment of systemic metabolic diseases (Fig. 2) [80].
Conclusion and perspective
Over the last decade, recent studies have illustrated that GPR120 acts as a positive regulator of adipogenesis and improves glucose uptake in adipocytes, as well as having an anti-inflammatory effect in ATMs. Its different roles during the development and metabolism of adipocytes may suggest that the expression and activity of GPR120 is capable of promoting normal lipid and glucose metabolism (Fig. 2). Therefore, useful approaches to modulating GPR120 activity may be developed as promising anti-obesity and anti-diabetic agents. Currently, highly efficient and potent drugs targeting GPR120 are being developed, though one kind of agonist, cpdA, shows functional effects both in vivo and in vitro in the regulation of glucose uptake, anti-inflammatory activity, and insulin resistance. Although some important issues have been solved to uncover the role of GPR120, other questions remain to be addressed in the future.
First, some studies demonstrate that the signals transduced from membrane-associated GPR120 into the cells result in the regulation of the nuclear receptor PPARγ. What are the specific events in this putative GPR120-PPARγ pathway? Calcium and ERK1/2 may be two second messengers that help transduce the signal into the cell, but the subsequent mechanism that activates PPARγ needs to be dissected. GPR120 and PPARγ exhibit a similar pocket structure, and n-3 PUFA can activate both of them, which means that co-activation might be a model to investigate in further research, such as in studies of anti-inflammatory properties. Furthermore, the precise downstream signalling mediated by GPR120 in brown adipocytes adipogenesis is worth being uncovered.
Second, as an important endocrine organ, adipose tissue is distributed or intertwined in different organs, such as muscle, heart, kidney, liver, or even cancer cells. The interactions between adipose tissue and other organs are being explored and show promise [3]. GPR120 maintains the normal adipose-liver-muscle interactions, and the dysfunction of GPR120 may lead to insufficient production of palmitoleate (C16:1n7), leading to disorders in lipid metabolism in the liver and muscle [10]. The systemic role of GPR120 in adipose tissue interactions with other organs needs to be further verified.
Finally, GPR120 signals via both β-arrestin 2 and G proteins in the reported studies. As is well known, diverse proteins are coupled in different biological events. β-Arrestin2 mediates the anti-inflammatory effect through internalization with the receptor, while in lipid and glucose metabolism, two downstream signals, intracellular calcium mobilization, and phosphorylation of ERK1/2 are the consequence of G protein coupling. However, direct evidence for the receptor and G protein interaction is lacking. Furthermore, there are few reports about the roles of other possible protein interactions in these events, such as G protein-coupled receptor kinase 6 (GRK6) [81, 82]. Thus, it is of great interest to clarify the precise downstream signals in different processes. This may help us better understand the actual function of GPR120 and better design agents for the treatment of metabolic diseases.
References
Hajer GR, van Haeften TW, Visseren FLJ (2008) Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J 29(24):2959–2971
Cannon B, Nedergaard J (2004) Brown adipose tissue: Function and physiological significance. Physiol Rev 84(1):277–359
Rosen ED, Spiegelman BM (2014) What we talk about when we talk about fat. Cell 156(1–2):20–44
Kershaw EE, Flier JS (2004) Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 89(6):2548–2556
Harwood HJ (2012) The adipocyte as an endocrine organ in the regulation of metabolic homeostasis. Neuropharmacology 63(1):57–75
Duplus E, Forest C (2002) Is there a single mechanism for fatty acid regulation of gene transcription? Biochem Pharmacol 64(5–6):893–901
Lee JH et al (2009) Omega-3 fatty acids: cardiovascular benefits, sources and sustainability. Nat Rev Cardiol 6(12):753–758
Scorletti E, Byrne CD (2013) Omega-3 fatty acids, hepatic lipid metabolism, and nonalcoholic fatty liver disease. Annual Rev Nutr 33: 231–248.
Hirasawa A, Tsujimoto G (2005) Ligand identification and functional analysis for orphan GPCR GPR120. Yakugaku Zasshi J Pharm Soc Jpn 125: 122–123
Ichimura A et al (2012) Dysfunction of lipid sensor GPR120 leads to obesity in both mouse and human. Nature 483(7389): 350–354
Oh DY et al (2010) GPR120 Is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 142(5):687–698
Gotoh C et al (2007) The regulation of adipogenesis through GPR120. Biochem Biophys Res Commun 354(2):591–597
Im DS (2016) Functions of omega-3 fatty acids and FFA4 (GPR120) in macrophages. Eur J Pharmacol 785:36–43
Moniri NH (2016) Free-fatty acid receptor-4 (GPR120): Cellular and molecular function and its role in metabolic disorders. Biochem Pharmacol 110:1–15
Ulven T, Christiansen E (2015) Dietary fatty acids and their potential for controlling metabolic diseases through activation of FFA4/GPR120. Annual Rev Nutr 35: 239–263
Zhang D, Leung PS (2014) Potential roles of GPR120 and its agonists in the management of diabetes. Drug Design Dev Therapy 8: 1013–1027
Hirasawa A et al (2005) Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med 11(1):90–94
Tanaka T et al (2008) Cloning and characterization of the rat free fatty acid receptor GPR120: in vivo effect of the natural ligand on GLP-1 secretion and proliferation of pancreatic beta cells. Naunyn Schmiedebergs Arch Pharmacol 377(4–6):515–522
Moore K et al (2009) Cloning, expression, and pharmacological characterization of the GPR120 free fatty acid receptor from cynomolgus monkey: comparison with human GPR120 splice variants. Comp Biochem Physiol B-Biochem Mol Biol 154(4):419–426
Watson SJ, A.J.H. Brown, Holliday ND (2012) Differential signaling by splice variants of the human free fatty acid receptor GPR120. Mol Pharmacol 81(5):631–642
Song TX et al (2015) Cloning and characterization of spliced variants of the porcine G protein coupled receptor 120. Biomed Res Int 2015:1–10
Tanaka T et al (2008) Free fatty acids induce cholecystokinin secretion through GPR120. Naunyn Schmiedebergs Arch Pharmacol 377(4–6):523–527
Otto TC, Lane MD (2005) Adipose development: From stem cell to adipocyte. Crit Rev Biochem Mol Biol 40(4):229–242
Song TX, et al. (2016) GPR120 promotes adipogenesis through intracellular calcium and extracellular signal-regulated kinase 1/2 signal pathway. Mol Cell Endocrinol 434(C): 1–13.
Ichimura A, Hara T, Hirasawa A (2014) Regulation of energy homeostasis via GPR120. Front Endocrinol 5:111
Quesada-Lopez T et al (2016) The lipid sensor GPR120 promotes brown fat activation and FGF21 release from adipocytes. Nat Commun 7:13479
Hudson BD et al (2013) The pharmacology of TUG-891, a potent and selective agonist of the free fatty acid receptor 4 (FFA4/GPR120), demonstrates both potential opportunity and possible challenges to therapeutic agonism. Mol Pharmacol 84(5):710–725
Gao B et al (2015) GPR120: A bi-potential mediator to modulate the osteogenic and adipogenic differentiation of BMMSCs. Sci Rep 5:14080
Kim J et al (2016) Eicosapentaenoic acid potentiates brown thermogenesis through FFAR4-dependent up-regulation of miR-30b and miR-378. J Biol Chem 291(39):20551–20562
Gim HJ et al (2013) Design and synthesis of alkoxyindolyl-3-acetic acid analogs as peroxisome proliferator-activated receptor-gamma/delta agonists. Bioorganic Med Chem Lett 23(2):513–517
Suzuki T et al (2008) Identification of G protein-coupled receptor 120-selective agonists derived from PPAR gamma agonists. J Med Chem 51(23):7640–7644
Hudson BD et al (2014) The molecular basis of ligand interaction at free fatty acid receptor 4 (FFA4/GPR120). J Biol Chem 289(29):20345–20358
Bost F et al (2005) The role of MAPKs in adipocyte differentiation and obesity. Biochimie 87(1):51–56
Prusty D et al (2002) Activation of MEK/ERK signaling promotes adipogenesis by enhancing peroxisome proliferator-activated receptor gamma (PPAR gamma) and C/EBP alpha gene expression during the differentiation of 3T3-L1 preadipocytes. J Biol Chem 277(48):46226–46232
Neal JW, Clipstone NA (2002) Calcineurin mediates the calcium-dependent inhibition of adipocyte differentiation in 3T3-L1 cells. J Biol Chem 277(51):49776–49781
Shi H et al (2000) Role of intracellular calcium in human adipocyte differentiation. Physiol Genomics 3(2):75–82
Strissel KJ et al (2007) Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes 56(12):2910–2918
Ouchi N et al (2011) Adipokines in inflammation and metabolic disease. Nat Rev Immunol 11(2):85–97
Lumeng CN, Saltiel AR (2011) Inflammatory links between obesity and metabolic disease. J Clin Invest 121(6):2111–2117
Weisberg SP et al (2003) Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112(12):1796–1808
Weisberg S et al (2003) Obesity leads to macrophage accumulation in adipose tissue. Obes Res 11:A6–A7
Xu HY et al (2003) Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112(12):1821–1830
Harkins JM et al (2004) Expression of interleukin-6 is greater in preadipocytes than in adipocytes of 3T3-L1 cells and C57BL/6 J and ob/ob mice. J Nutr 134(10):2673–2677
Oh DY et al (2014) A Gpr120-selective agonist improves insulin resistance and chronic inflammation in obese mice. Nat Med 20(8):942–947
Liu YQ et al (2014) The fish oil ingredient, docosahexaenoic acid, activates cytosolic phospholipase A(2) via GPR120 receptor to produce prostaglandin E-2 and plays an anti-inflammatory role in macrophages. Immunology 143(1):81–95
Li XZ, Yu Y, Funk CD (2013) Cyclooxygenase-2 induction in macrophages is modulated by docosahexaenoic acid via interactions with free fatty acid receptor 4 (FFA4). Faseb J 27(12):4987–4997
Buysschaert M et al (2000) Hyperhomocysteinemia in type 2 diabetes: relationship to macroangiopathy, nephropathy, and insulin resistance. Diabetes Care 23(12):1816–1822
Li Y et al (2008) Homocysteine upregulates resistin production from adipocytes in vivo and in vitro. Diabetes 57(4):817–827
Li Y et al (2013) Hyperhomocysteinemia promotes insulin resistance by inducing endoplasmic reticulum stress in adipose tissue. J Biol Chem 288(14):9583–9592
Ozcan U et al (2006) Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313(5790):1137–1140
Karpe F, Dickmann JR, Frayn KN (2011) Fatty acids, obesity, and insulin resistance: time for a reevaluation. Diabetes 60(10):2441–2449
Vieira WA, Sadie-Van Gijsen H, Ferris WF (2016) Free fatty acid G-protein coupled receptor signaling in M1 skewed white adipose tissue macrophages. CMLS Cell Mol Life Sci 73(19):3665–3676
Iyer A et al (2010) Inflammatory lipid mediators in adipocyte function and obesity. Nat Rev Endocrinol 6(2):71–82
Boden G, Shulman GI (2002) Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and beta-cell dysfunction. Eur J Clin Invest 32:14–23
Rodriguez-Pacheco F et al (2016) The pro-/anti-inflammatory effects of different fatty acids on visceral adipocytes are partially mediated by GPR120. Eur J Nutr 1–10. doi:10.1007/s00394-016-1222-0
Rodriguez-Pacheco F et al (2014) Effects of obesity/fatty acids on the expression of GPR120. Mol Nutr Food Res 58(9):1852–1860
Cornall LM et al (2011) Diet-induced Obesity up-regulates the abundance of GPR43 and GPR120 in a tissue specific manner. Cell Physiol Biochem 28(5):949–958
Trayhurn P (2013) Hypoxia and adipose tissue function and dysfunction in obesity. Physiol Rev 93(1):1–21
Krishnan J et al (2012) Dietary obesity-associated Hif1 alpha activation in adipocytes restricts fatty acid oxidation and energy expenditure via suppression of the Sirt2-NAD(+) system. Genes Dev 26(3):259–270
Sun K et al (2012) Dichotomous effects of VEGF-A on adipose tissue dysfunction. Proc Natl Acad Sci USA 109(15):5874–5879
Hasan AU et al. (2015) Eicosapentaenoic acid upregulates VEGF-A through both GPR120 and PPAR gamma mediated pathways in 3T3-L1 adipocytes. Mol Cell Endocrinol 406(C):10–18.
Yore MM et al (2014) Discovery of a class of endogenous mammalian lipids with anti-diabetic and anti-inflammatory effects. Cell 159(2):318–332
Shimpukade B et al (2012) Discovery of a potent and selective GPR120 agonist. J Med Chem 55(9):4511–4515
Liu D et al (2012) G-protein coupled receptor 120 Is involved in glucose metabolism in fat cells. Cell Mol Biol 58:1757–1762
Chen K et al (2016) Transcription factor C/EBP beta promotes the transcription of the porcine GPR120 gene. J Mol Endocrinol 56(2):91–100
Kajimura S (2017) Adipose tissue in 2016: advances in the understanding of adipose tissue biology. Nat Rev Endocrinol 13(2):69–70
Danforth E Jr (2000) Failure of adipocyte differentiation causes type II diabetes mellitus? Nat Genet 26(1):13
Cao HM et al (2008) Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell 134(6):933–944
Denis GV, Obin MS (2013) ‘Metabolically healthy obesity’: origins and implications. Mol Aspects Med 34(1):59–70
Kloting N et al (2010) Insulin-sensitive obesity. Am J Physiol Endocrinol Metab 299(3):E506–E515
Tang W et al (2011) Thiazolidinediones Regulate Adipose Lineage Dynamics. Cell Metab 14(1):116–122
Gustafson B et al (2013) Restricted adipogenesis in hypertrophic obesity the role of WISP2, WNT, and BMP4. Diabetes 62(9):2997–3004
Rosell M et al (2014) Brown and white adipose tissues: intrinsic differences in gene expression and response to cold exposure in mice. Am J Physiol Endocrinol Metab 306(8):E945–E964
Briscoe CP et al (2006) Pharmacological regulation of insulin secretion in MIN6 cells through the fatty acid receptor GPR40: identification of agonist and antagonist small molecules. Br J Pharmacol 148(5):619–628
Galindo MM et al (2012) G protein-coupled receptors in human fat taste perception. Chem Senses 37(2):123–139
Mobraten K et al (2013) Omega-3 and omega-6 PUFAs induce the same GPR120-mediated signalling events, but with different kinetics and intensity in Caco-2 cells. Lipids Health Disease 12(1):101
Wu Q et al (2013) Identification of G-protein-coupled receptor 120 as a tumor-promoting receptor that induces angiogenesis and migration in human colorectal carcinoma. Oncogene 32(49):5541–5550
Cintra DE et al (2012) Unsaturated fatty acids revert diet-induced hypothalamic inflammation in obesity. Plos One 7(1):e30571
Wellhauser L, Belsham DD (2014) Activation of the omega-3 fatty acid receptor GPR120 mediates anti-inflammatory actions in immortalized hypothalamic neurons. J Neuroinflammation 11(1):60
Oh DY, Walenta E (2014) Omega-3 fatty acids and FFAR4. Front Endocrinol 5(4):115
Shenoy SK, Lefkowitz RJ (2011) beta-arrestin-mediated receptor trafficking and signal transduction. Trends Pharmacol Sci 32(9):521–533
Strachan RT et al (2014) Divergent transducer-specific molecular efficacies generate biased agonism at a G Protein-coupled receptor (GPCR). J Biol Chem 289(20):14211–14224
Acknowledgements
We apologize to those authors whose excellent work we could not reference directly in this review due to limited text space. This study was jointly supported by the National Natural Science Foundation of China (Nos. 31472075 and 31402085); Hubei Provincial Creative Team Project of Agricultural Science and Technology (No. 2007-620); the Key Technology Research and Development Program of Hubei Province (Nos. 2014ABB014 and 2014ABC012).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Song, T., Yang, Y., Zhou, Y. et al. GPR120: a critical role in adipogenesis, inflammation, and energy metabolism in adipose tissue. Cell. Mol. Life Sci. 74, 2723–2733 (2017). https://doi.org/10.1007/s00018-017-2492-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-017-2492-2