Abstract
How animals achieve their specific body size is a fundamental, but still largely unresolved, biological question. Over the past decades, studies on the insect model system have provided some important insights into the process of body size determination and highlighted the importance of insulin/insulin-like growth factor signaling. Fat body, the Drosophila counterpart of liver and adipose tissue, senses nutrient availability and controls larval growth rate by modulating peripheral insulin signaling. Similarly, insulin-like growth factor I produced from liver and muscle promotes postnatal body growth in mammals. Organismal growth is tightly coupled with the process of sexual maturation wherein the sex steroid hormone attenuates body growth. This review summarizes some important findings from Drosophila and mammalian studies that shed light on the general mechanism of animal size determination.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the fundamental mysteries in biology is how animals know whether they have grown enough to reach appropriate body size. Recent studies combining cell growth, proliferation, organ formation, and endocrine regulation have provided new perspectives regarding the aforementioned query. A number of studies have employed Drosophila as a model system to investigate the process of animal size determination, thus enabling the integration of endocrinological studies with genetics. These studies have illuminated the intriguing parallels between the processes of body growth in flies and mammals and the involvement of surprisingly similar genes and signaling pathways. For instance, mass of the body exponentially increases during larval development, which corresponds to the juvenile period of the flies. As larvae prepare to maturate by initiating puparium formation, the activity of a maturation hormone increases concomitantly with the deceleration of body growth, essentially fixing the final body size at the end of the larval development. In parallel, human body growth rapidly occurs from birth until puberty, followed by high activities of sex hormones coinciding with deceleration of body growth until adulthood. Insulin signaling is highly conserved throughout the animal kingdom, mediating nutrient condition, energy metabolism, and cell proliferation. Insulin signaling plays a major role in causing rapid increase in larval body mass in Drosophila, and insulin-like growth factor I (IGF-I) is critical in pre- and postnatal body growth in mammals. In the present work, I will summarize some important findings that may provide a clue in understanding the regulation and determination of appropriate animal body size, which provide an important step towards understanding the general principle of animal size determination.
Insulin/insulin-like growth factor signaling
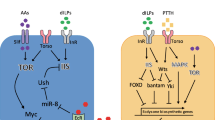
The insulin/IGF signaling (IIS) is a conserved signaling cascade in animals. It functions as a nutrient-sensing pathway, connecting dietary conditions to the control of cellular and organismal metabolism. Past decades have seen an expansion of our knowledge about this signaling pathway (Fig. 1). Insulin and insulin-like peptides (ILPs) such as IGFs are secreted and act in an endocrine manner by binding to insulin receptors (InR), triggering a phosphorylation cascade involving the insulin receptor substrate (IRS), phosphoinositide-3 kinase (PI3K), and Akt/PKB [1]. An active PI3K complex consists of a catalytic subunit (p110) and a regulatory subunit (p85a) [2]. PI3K promotes the conversion of phosphatidylinositol 4,5-bisphosphate (PIP2) to phosphatidylinositol 3,4,5-triphosphate (PIP3) in the cell membrane [3], which is catalytically reversed by phosphatase and tensin homolog (PTEN) [4]. Accumulation of PIP3 in cell membrane recruits phosphoinositide-dependent protein kinase 1 (PDK1) and Akt/PKB [1, 5]. Activation of Akt by phosphorylation (p-Akt) subsequently leads to phosphorylation of various downstream proteins. One of the downstream targets of Akt is a transcription factor FOXO. Phosphorylation of FOXO inhibits its nuclear localization, thus preventing its transcriptional activity [6].
The outline of the insulin/insulin-like growth factor signaling (IIS) pathway. The insulin/PI3K/Akt branch exerts its downstream effects through various ways. Akt phosphorylates FOXO transcription factor, prevents its nuclear localization, and thus inhibits the expression of a variety of FOXO target genes. Akt can also activate TOR by inhibition of either TSC2 or PRAS40, the two negative regulators of TOR, thereby allowing the connection between IIS and TOR branch. TOR inhibits 4EBP and activates S6K, together enhancing global translation and cellular growth. S6K can phosphorylate and inhibit IRS1, thus preventing over-activation of IIS. FOXO can also transcriptionally induce InR, contributing to feedback regulation of IIS. In this diagram, IIS factors are in blue, TOR-pathway components are in red. ILPs, insulin-like peptides
Target of rapamycin (TOR) protein kinase is another branch of signaling cascade mediating nutrient and cell growth, which is closely associated with insulin signaling. Activation of TOR promotes cell growth by enhancing global translation and ribosome biogenesis through phosphorylation of the translation initiation factor 4E-binding protein (4EBP) and ribosomal protein S6 kinase (S6K), respectively [7]. Each branch of IIS and TOR can function either independently [8] or together as linear insulin/Akt/TOR signaling network [9, 10]. TOR can be activated cell-autonomously in response to nutrient availability, especially at the level of cellular amino acids [11]. Moreover, insulin signaling can indirectly activate the TOR pathway. Activation of Akt by insulin signaling promotes the TOR pathway by suppressing the complex formed by tuberous sclerosis complex (TSC) 2, an inhibitor of TOR activity [12–15]. Recent studies also suggest that Akt phosphorylates PRAS40, an additional inhibitor of TOR, thereby allowing the IIS-induced activation of the TOR pathway [16, 17]. However, the linearity of the insulin/Akt/TOR pathway appears to be complicated, since separated regulation of IIS and TOR pathways by nutrient stimuli were sometimes observed under certain conditions [8, 18].
There is an elaborate feedback network among IIS, TOR, and their nutritional inputs. Activation of TOR, either by sensing of cellular amino acids or by input from insulin signaling, feeds back on IIS in several ways. First, activation of S6K by TOR phosphorylates and inhibits IRS, thus attenuating IIS [19]. This mechanism might prevent the over-activation of IIS by a plethora of nutrient stimuli. Second, activity of Akt induced by ILPs can be promoted by TOR [20], which presumably protects against harmful effects caused by a poor nutritional condition. Lastly, expression of InR is induced by FOXO [21], a transcription factor suppressed by Akt. Thus, nutrient deprivation can potentiate the effect of ILPs on IIS pathway by upregulating InR. For detailed information about the pathways of insulin signaling, the reader may refer to other reviews [22, 23].
Body size determination: lessons from Drosophila
Size determination during larval development
Drosophila has four morphologically distinct developmental states: embryo, larva, pupa, and adult. After embryogenesis, Drosophila goes through three larval stages called instars, following the larval stages, which last for about 4 days, it enters the pupal stage, during which there is formation of a rigid exoskeleton of the adult body. Subsequently, after about 10 days from hatching of embryo, a sexually mature adult fly is made. Progression of larval molting and pupal development is dictated by pulses of the steroid hormone 20-hydroxyecdysone (20E) [24]. The prothoracic gland (PG), an insect endocrine organ, produces ecdysone, which is released into larval hemolymph and modified in peripheral tissues into the active form, 20E [25, 26]. 20E acts through a heterodimer of the ecdysone receptor (EcR) and Ultraspiracle (Usp) nuclear receptor to trigger stage-specific transcriptional cascades, thus directing the progression of waves of stages in fly development [24].
Early in the third instar, the larva grows to reach a physiologically important developmental point, called the “critical weight” [27–29]. Upon the attainment of the critical weight, the larva has acquired sufficient nutrients to complete larval development without any requirement of further feeding. Whether the deprivation of food from developing larva prolongs the remaining time to puparium formation depends on the critical weight [29]. When larvae are starved before reaching critical weight, the animal pauses the developmental progression until normal nutritional condition is restored, thus delaying larval development. In contrast, when the larvae experience starvation after achieving critical weight, the animal still continues to develop into pupal stage without delaying development. To be precise, the nutritional condition past critical weight check point does not affect the speed of larval development.
There is an intriguing relationship between the time of starvation, duration of larval period, and final body size (Fig. 2). As briefly mentioned above, body size drastically increases during the larval period, contributing to most of the final body size. As such, duration of larval period is an important parameter in determining final body size. Interestingly, although starvation preceding critical weight check point extends the duration of larval development, this does not increase the final body size. On the other hand, starvation past the critical weight check point actually decreases the final body size without extending the duration of larval period. These observations suggest that starvation stimuli cause the decrease in actual growth rate (body mass increase per time) throughout the larval period, but in case of the starvation preceding the critical weight attainment, extension of larval period could compensate the lower growth rate to achieve normal body size. Perhaps there might be a specific period sensitive to nutritional condition during juvenile development that influences the final adult size in metazoans. Since nutritional condition is sensed and transduced through insulin signaling, it is tempting to speculate that temporal modulation of insulin signaling during larval development might similarly affect the final fly size as temporal starvation does. Indeed, transient inactivation of InR before critical weight check point lengthens the total larval period without affecting final body size, whereas InR inactivation after critical weight check point decreases final body size without affecting total developmental time [30]. Thus, insulin signaling in a specific time period during larval development is influential in determining the final fly size.
Timing of malnutrition differentially affects the progress of larval development and final body size in Drosophila. If a larva feeds on insufficient food and thus experiences a condition of low insulin signaling only before reaching critical weight, it exhibits slow development, but finally achieves normal body size. Conversely, if a larva experiences malnutrition and thus a condition of low insulin signaling only after attaining critical weight, it exhibits development at a normal rate but finally achieves decreased body size. Grey color indicates the timing of malnutrition during larval development. CW critical weight
Interestingly, the attainment of critical weight appears to coincide with the onset of three serial low-titer pulses of 20E in third-instar larval stage [30, 31] (Fig. 2). This implies that prothoracic gland (PG), the ecdysone producing organ, may play an important role in determining final body size by modulating the attainment of critical weight and total larval length. Indeed, inactivation of PI3K, Ras, or Raf activity specifically in PG attenuates ecdysone release, which results in increased body size by extending larval growth period without affecting the rate of body growth [27, 32]. Notably, these observations suggest that insulin signaling in various tissues can differentially affect final body size: Temporal inactivation of insulin signaling in whole larva before reaching critical weight has no effect on final body size, whereas inactivation of insulin signaling specifically in PG increases final body size.
Although most of the body size is established in the larval period, a small fraction of size increase appears to be additionally gained after the cessation of feeding of late third-instar larva. The marginal growth during postfeeding larval and pupal stages is shown to be mediated by Drosophila insulin-like peptide 6 (Dilp6), which is induced in fat body by pupariation signal and FOXO [33, 34]. Dilp6 may serve to mediate the tradeoff between body growth and storage of energy resources during fly maturation, thereby generating appropriately sized flies with resistance to nutritional stress.
Non-autonomous regulation of body growth by metabolic organs
The fat body is the characteristic organ in insects that functions as mammalian liver and adipose tissues. It is known to have the ability to directly sense the organismal condition of nutrition, thereby storing or mobilizing energy resources such as glycogen and lipids. Fat body also plays an important role in insect immune system by producing antimicrobial peptides in response to infectious bacteria [35]. Accumulated evidence indicates that fat body functions as an important endocrine organ producing some hormonal peptides into the hemolymph, thereby regulating the systemic homeostasis of metabolism. A seminal study by Leopold et al. [18] showed that inactivation of an amino acid transporter specifically in larval fat body attenuates the TOR signaling in this organ, which in turn decreases the organismal growth non-autonomously via secreted factor(s) emanating from fat body. They proposed that the secreted factor(s) diffuse into peripheral tissues and modulate their insulin signaling, thereby controlling body growth.
Several gene products may be raised as candidate humoral factors derived from fat body that regulate organismal growth during larval development. Acid-labile subunit (ALS) is a binding partner of IGF-1, which stabilizes and at the same time restrains the activity of IGF-1 in mammals [36]. Drosophila homolog of ALS was recently shown to form a complex with Dilps in a similar way as do mammalian counterparts [37]. It has been reported that ALS is expressed in larval fat body and its expression is severely suppressed by starvation [37]. Interestingly, depletion of ALS specifically in fat body affects final adult size, though in an opposite manner depending on rearing nutritional condition: ALS suppresses body growth in normal nutritional condition, whereas it promotes growth in starvation [37]. Imaginal morphogenesis protein-Late 2 (Imp-L2) is another binding partner of Dilps, consistent with its sequence homology to human IGF binding protein 7 (IGFBP-7) [38, 39]. Imp-L2 can form a ternary complex with Dilp2 and ALS, and appears to antagonize Dilps’ activity [38, 39]. Consistently, deletion of Imp-L2 increases final fly size [38]. Interestingly, Imp-L2 expression is induced in fat body in response to starvation, which is in stark contrast with the repression of ALS by starvation [38]. Neural Lazarillo (NLaz), the Drosophila Lipocalin family member homologous to the Retinol-Binding Protein 4, was found to be the secreted protein that suppresses insulin signaling [40]. The expression of NLaz is induced by oxidative stress, starvation, and JNK signaling [40]. NLaz mutant flies are bigger in size, and exhibit an increase in systemic insulin signaling [40]. Notably, overexpression of NLaz specifically in fat body decrease insulin signaling in oocytes nurse cells as well as final body size, and fat body specific depletion of NLaz prevents hyperglycemia induced by high sugar diet [40, 41]. These observations indicate the physiological role of fat body NLaz in organismal insulin signaling. The process by which secreted NLaz modulates peripheral insulin signaling remains to be unknown. In summary, ALS, Imp-L2, and NLaz are fat body-derived hormones that negatively regulate entire larval growth in normal nutritional condition, and their expressions are regulated by nutrient availability (Fig. 3).
Communication among metabolic organs controls body growth in Drosophila. The fat body produces various peptide hormones that modulate systemic insulin signaling and body growth, and their production depends on fat body’s nutritional signaling. Impl-L2 and ALS can forms a complex with Dilp (Drosophila insulin-like peptide), thereby inactivating Dilp. SDR produced by glial cells in the brain has a strong sequence homology with ectodomain of InR, whereby it can bind to and attenuate Dilp. Upd2 secreted from fat body activates JAK/STAT signaling in the neurons situated in the vicinity of IPC, which results in the activation of IPC to release Dilp. NLaz produced in response to stress condition suppresses systemic insulin signaling via unknown ways. Insulin signaling in muscle tissue can promote the growth of other unrelated organs and entire body. Some specific bacteria normally resident in the fly’s intestine promote body growth by activating systemic insulin signaling
Recent findings provide an additional way in which secreted hormone(s) from fat body modulate systemic insulin signaling and organismal growth. Leopold and colleagues [42] found that Dilp secretion from insulin-producing cells (IPCs) located on median neurosecretory cluster (mNSC) in the brain is promoted by humoral factor(s) derived from fat body. They showed by ex vivo organ culture that Dilps are released from IPCs by co-culturing with larval fat body from fed larva, and not by co-culturing with underfed larval fat body. Moreover, Dilp secretion from IPC is also induced by the addition of hemolymph derived from fed larva, and not from underfed larva, thus indicating that fat body in fed state emits its signal to the IPC via some hormone(s) secreted in hemolymph. A very recent study by Perrimon’s lab identified a hormone produced from fat body that promotes Dilp secretion from IPCs [43]. Unpaired 2 (Upd2), a protein with sequence similarity to type 1 cytokine, is secreted from the fat body of well-fed larva, which in turn activates JAK/STAT signaling in GABAergic neurons in the vicinity of IPCs. Activation of JAK/STAT signaling in these neurons then relieves their inhibitory effect on the IPC, thereby releasing Dilps into the hemolymph. Consistently, knockdown of fat body Upd2 decreases final adult size as well as prevents the release of Dilp2 from IPCs. One seemingly inconsistent observation is that Upd2 expression in fat body is not induced by amino acid, but rather induced by fat and sugar. Previous study by Leopold’s group [42] demonstrated that amino acid is the main nutrient that is sensed and relayed to IPCs by fat body. Thus, it is unclear whether Upd2 also mediates the relay of amino acid availability into IPCs or additional hormone may play that role (Fig. 3).
The fact that nutritional condition during growing larval period has a huge impact on final adult size and the fat body is the major endocrine organ that coordinates the systemic growth in response to rearing nutritional condition leads to the expectation that insulin/TOR signaling in fat body may play a central role in body size determination. Although it is less clear that insulin/PI3K/Akt signaling and TOR signaling act in parallel or in a linear pathway in larval fat body, suppression of either one in fat body can cause a decrease in the body size. Inactivation of an amino acid transporter, Slimfast (Slif), in fat body decreases final body size by suppressing fat body’s TOR activity without affecting PI3K [18]. However, suppression of InR or PI3K in fat body decreases the organismal growth [44, 45], and activation of Akt in fat body rescues the small body size induced by immune responses [46]. Whether insulin/PI3K/Akt signaling in fat body promotes body growth by activating the TOR pathway or independently of TOR remains to be determined.
Several genes downstream of insulin/TOR have been found that Myc act in fat body to regulate organismal growth. Myc is a well-known transcription factor that acts as a downstream effector of the TOR pathway, regulating ribosome biogenesis, and translational capacity [47–49]. Recent study has shown that overexpression or knockdown of Myc in fat body increases or decreases final pupal size, respectively, and that growth retardation as larva approaches the pupal stage coincides with repression of Myc in fat body [50]. The capacity of tRNA synthesis has been proposed to be an important factor for directing organismal growth. Maf1 is a conserved repressor of Pol III-dependent tRNA transcription, which is regulated by TOR signaling [51–53]. Knockdown of Maf1 increases larval growth and tRNA levels [54]. Interestingly, elevation of initiator of methionine tRNA \( ({\text{tRNA}}_{\text{i}}^{\text{met}} ) \) mimics the growth promoting effects of Maf1 knockdown [54]. Upon treatment of rapamycin, a TOR inhibitor, Maf1 forms a complex with Brf, a conserved component of the TFIIIB complex responsible for tRNA synthesis [55]. In this manner, Maf1 decreases tRNA levels by inhibiting Brf. Although Myc can also promote the synthesis of tRNA, Maf1/Brf appears to more dominantly regulate the tRNA synthesis in response to TOR activity than Myc does [55]. Recent study also identified the transcription factor DREF as an additional mediator that links TOR activity to ribosome biogenesis and growth [56]. FOXO is the crucial transcription factor that mediates downstream effects of insulin signaling, whereby Akt inhibits FOXO activity by its phosphorylation [6]. Consistent with positive regulation of body growth by fat body’s insulin signaling, knockdown of FOXO in fat body has been shown to increase final pupal size [50].
An important question that still remains to be unanswered is how these described downstream events of insulin/TOR signaling in fat body can non-autonomously regulate organismal growth. Might the protein biosynthetic capacity modulated by TOR signaling regulate the production of such hormones as ALS, Imp-L2, NLaz, or Upd2? If this is the case, the question arises as how? Or could these hormones be transcriptionally regulated by FOXO? Future studies should address these questions by investigating the connection between fat body-derived hormones and the downstream events of insulin/TOR signaling in fat body.
Glial cells in the central nervous system have been shown to play a role in organismal body growth in Drosophila. A very recent study by Okamoto et al. [57] has identified a new secreted protein in hemolymph that can suppress systemic insulin signaling and body growth. This protein was named as secreted decoy of InR (SDR), for its strong sequence similarity to the extracellular domain of InR. As expected from this sequence homology, SDR was shown to have a preferential binding affinity for a wide range of Dilps, thereby suppressing their activities. Interestingly, SDR in hemolymph mainly stems from glial cells in the central nervous system, and its expression appears to be constitutive regardless of nutritional condition. In addition to being larger body mass and structural size, SDR-null flies show increased mortality when raised in poor nutritional condition, probably due to abnormally high level of insulin signaling and lowered FOXO activity (Fig. 3).
It has been well established that commensal bacteria in the intestine have a huge impact on host physiology such as immunity and metabolism in metazoan [58–60]. Two recent papers demonstrate that certain bacterial strains among other commensal bacteria in the Drosophila gut play a critical role in larval growth and development. Storelli et al. [61] showed that the residence of a bacterium called Lactobacillus plantarum in fly’s gut promotes larval growth rate especially under poor nutritional conditions. They found that this bacterium in the gut promotes insulin signaling in larval peripheral tissues and this effect is dependent on TOR activity in the fat body. Meanwhile, Shin et al. [62] found that the colonization of a commensal bacterium, Acetobacter pomorum, in the gut is sufficient to rescue the growth retardation and reduced insulin signaling of germ-free fly. Furthermore, they found that acetic acid, a metabolic product of A. pomorum, in the gut is responsible for normal growth and proper insulin signaling in germ-free fly mono-associated with this bacterium [62]. Overall, these two studies indicate the potential role of fly’s intestine associated with some specific microorganisms in the regulation of systemic insulin signaling and organismal growth, although the exact mechanism requires further investigation (Fig. 3).
Muscle is another main metabolic organ that heavily responds to insulin. Demontis et al. [63] showed that insulin signaling in Drosophila larval muscle can non-autonomously regulate systemic growth. Increase in muscle size by modulating muscle specific InR can lead to an increase in size of unrelated organs such as salivary glands, gut, fat body, and epidermis. Modulating FOXO affects the muscle size and Myc activity in a consistent manner as InR modulation does. Interestingly, although overexpression of Myc increases the size of nucleolus and some Myc target genes’ transcripts known to promote growth, it cannot drive muscle growth and probably subsequent body growth. This appears to contrast with the fat body, in which Myc overexpression can promote systemic body growth [50]. The authors suggest that changes in muscle size affect larval feeding behavior, which in turn modifies the final body size [63]. However, possible endocrine function of muscle in body growth regulation should not be excluded (Fig. 3).
Connecting body growth to sexual maturation
Human body growth occurs rapidly from infant until puberty, after which growth gradually stops [64, 65]. This growth pattern parallels that of Drosophila, as mentioned above [66]. Although numerous studies have demonstrated that insulin/insulin-like growth factor signaling plays a major role in directing animal growth, it still remains mysterious as to why growth is largely restricted to the juvenile stage and how it is terminated upon sexual maturation. In general, the cessation of metazoan growth coincides with the rise in circulating steroid hormones that directs sexual maturation (e.g., ecdysone in flies and estrogen in human), implying the connection between steroid hormone and insulin/insulin-like growth factor signaling. In the mid-2000s, several studies on Drosophila began to identify the linkage between these two pathways. As mentioned above, modulating the PI3K, Ras, or Raf activity in prothoracic gland (PG) modifies the ecdysone production, which in turn changes the duration of larval growth period by altering timing of pupariation and thereby affecting the final body size [27, 32]. Interestingly, it was found that ecdysone could affect final body size not only by altering pupariation timing but also by changing larval growth rate via modifying insulin signaling. Leopold and colleagues [67] found that modulating PI3K in PG in some cases modifies final body size without changing the pupariation timing. Blocking ecdysone receptor (EcR) signaling increases the final body size, activates PI3K and Akt, and excludes FOXO from cellular nucleus. These observations suggest that maturating steroid hormone, ecdysone, not only directs the pupariation process but also suppresses insulin signaling during larval development [67]. A following study by Leopold’s group [50] further uncovered that circulating ecdysone mainly targets larval fat body, in which EcR signaling attenuates Myc activity. Subsequently, repression of fat body’s Myc activity non-autonomously suppresses peripheral insulin signaling and body growth.
Then, how does ecdysone signaling suppress insulin signaling and Myc activity in larval tissues? A microRNA was recently proposed as an important molecular linker between ecdysone and insulin signaling [68] (Fig. 4). MiR-8 is a highly conserved microRNA among nematodes, flies, and human, and was found to promote insulin signaling in both flies and human by suppressing a common target gene, u-shaped (ush) [44]. FOG2, a human ortholog of USH, was revealed to bind and interfere with active PI3K complex [44]. Interestingly, the promoter region of miR-8 has numerous binding sites for ecdysone’s early response genes, and promoter reporter assay showed that miR-8 is transcriptionally repressed by ecdysone signaling [68]. Modulating miR-8 level was shown to correlatively change the body size of fly. Importantly, overexpression of miR-8 antagonizes ecdysone-induced growth suppression while deletion of miR-8 abrogates EcR-mediated regulation of insulin signaling and growth. Perturbation of USH also consistently impedes ecdysone’s effect on body growth. Together, this study demonstrates that miR-8 is a critical molecular linker that mediates ecdysone regulation of insulin signaling and body growth [68].
Connection between steroid hormone signaling and body growth. In flies, a maturation signal, ecdysone, suppresses larval insulin signaling, thereby decelerating larval body growth. This process is mediated by a conserved microRNA-target axis regulating insulin signaling. miR-8 expression is transcriptionally repressed by ecdysone’s early response genes, thereby leading to the suppression of insulin signaling and body growth. Conversely, insulin signaling can also inhibit ecdysone signaling. DOR is a coactivator of ecdysone receptor (EcR) and also a target gene of FOXO. Increasing insulin signaling represses FOXO, decreases DOR expression, and can reduce ecdysone signaling. This relationship between the two signaling pathways may potentially create a bistable feedback loop: high insulin/low ecdysone state vs. low insulin/high ecdysone state. In mammals, estrogen signaling during puberty promotes the inactivation of growth plate, thereby terminating longitudinal bone growth. Sex steroid hormones such as androgen and estrogen during puberty have been known to be associated with insulin resistance, whose involvement in body growth requires further investigation. Glucocorticoid signaling in liver promotes IGF-1 expression and postnatal body growth. Interestingly, this signaling also affects the genes related to sexual maturation, raising the possible role of glucocorticoids in coupling mammalian body growth with sexual maturation
A recent study showed that insulin signaling can conversely suppress ecdysone signaling (Fig. 4). Drosophila DOR was identified as a novel coactivator of EcR for proper ecdysone signaling [69]. DOR-null flies display a number of ecdysone loss-of-function phenotypes like impaired salivary gland degradation and pupal lethality. Notably, DOR expression is suppressed by insulin signaling via FOXO, thereby enabling the insulin-induced suppression of ecdysone signaling. Although it was shown that DOR functions in the process of pupal development to promote adult flies’ adiposity as ecdysone signaling does, it is unclear whether this effect is through insulin signaling or through other factors [69]. Currently, the role of DOR in the regulation of body growth remains to be demonstrated.
The Drosophila imaginal discs are larval tissues that give rise to the adult appendage following metamorphosis. When growth of the imaginal disc is perturbed, the duration of larval growth period is extended by delaying timing of pupariation. This physiological change has been thought to allow the tissues to regenerate to their correct size, helping the organs to be in the same proportion [70–75]. Thus, the developing animal monitors organ growth and coordinates it with the timing of maturation. It has been postulated that damaged imaginal discs may emit some humoral signals that target ecdysone biosynthetic machinery, thereby communicating local growth perturbation to the center of developmental timing. Two recent studies have identified this humoral factor. It was demonstrated that this previously uncharacterized secreted peptide, named Dilp8 for its invariant 6-cysteine motif typical of Dilps, is produced in imaginal disc tissues that exhibit perturbed growth, which leads to delayed pupariation [76, 77]. Depletion of Dilp8 abrogates the developmental delay caused by local growth perturbation, while ectopic overexpression of Dilp8 decreases the expression of ecdysone biosynthetic genes and delays the pupariation timing. Therefore, local organ growth as well as the organismal growth is tightly coordinated with maturation process.
Body growth regulation in mammals
Growth hormone/insulin-like growth factor I axis
The growth hormone (GH) is a peptide hormone produced mainly from the anterior region of the pituitary. The secretion of GH is governed by the neurosecretory signal from hypothalamus. GH is known to enhance muscle and longitudinal bone growth mainly by promoting IGF-1 production in liver, muscle, and many other tissues expressing GH receptor (GHR) [78–80] (Fig. 5). In these tissue cells, GH binding to GHR triggers intracellular signaling involving Janus kinase 2 (JAK2) and the signal transducers and activators of transcription 5 (STAT5) [81–84], which results in the expression of IGF-1 [85–90]. Numerous studies have pointed to the critical role of the GH/IGF-1 axis in directing mammalian growth. Deletion of GHR in mice reduces adult body mass by about 50 %, whereas deletion of IGF-1 reduces adult body mass by about 70 % [91, 92]. Conversely, overexpression of GH or IGF-1 in mice increases the mass of the adult mice [93, 94]. Although IGF-1 is the main mediator of growth promoting effects of GH, separate effects of GH and IGF-1 on body growth and other animal physiology have been observed. Knockout of IGF-1 results in severe intrauterine growth retardation in mice [92, 95], which is in sharp contrast to the minimal effect on birth size by GH mutation [96]. IGF-1-null mice showed about 60 % of normal birth size, followed by further decrease of postnatal growth that resulted in 30 % of normal adult size [92, 95]. Thus, IGF-1 has an additional role in promoting prenatal growth besides the role in mediating the effects of GH on postnatal body growth. Moreover, mice lacking both GHR and IGF-1 exhibited small birth weight to a similar extent with that of mice lacking IGF-1 alone, but showed more severe retardation of postnatal body growth than IGF-1-null mice [91]. This observation indicates that GH has additional ways independent of IGF-1 in promoting postnatal body growth. In line with this, there have been suggestions that GH can exert direct effects on some target tissues that are not mediated by IGF-1. For example, Green et al. [97] proposed that GH increases tissue formation by promoting the differentiation of precursor cells into the cells that can respond to IGF-1, meaning that GH can also act upstream of IGF-1 in promoting tissue growth. Moreover, GH and IGF-1 are known to have different metabolic effects in that GH administration caused hyperglycemia while IGF-I administration caused hypoglycemia [98].
Schematic diagram of growth hormone (GH)/insulin-like growth factor I (IGF-I) axis. GH is synthesized in the anterior pituitary in response to the signal from hypothalamus. GH acts on liver, muscle, and other organs to induce the synthesis of IGF-1. Circulating IGF-1 mainly stems from liver, contributing to postnatal body growth. IGF-1 produced from muscle and other tissues promotes the growth of local organ tissues in an autocrine/paracrine manner. GH can also promote tissue growth in an IGF-I-independent way
Several studies on other vertebrate species corroborate the critical role of IGF-1 in body growth and adult size determination. Small breeds of domestic dogs have been frequently observed to harbor a common IGF-1 single-nucleotide polymorphism not observed in larger breeds [99], and positive correlation between plasma IGF-1 concentration and adult body size was observed in many free-ranging animals such as turtles, deer, and snakes [100–102]. Moreover, domesticated sheep that have been artificially selected for high level of plasma IGF-1 exhibit increased postnatal growth rate [103, 104], and conversely, domesticated chickens that have been artificially selected for high postnatal growth rate exhibit increased level of plasma IGF-1 [105].
IGF-1 promotes body growth in both in an endocrine and para/autocrine manner. Circulating IGF-1 in serum is supplied mainly from the liver, which is responsible for approximately 75 % of serum IGF-1 [106]. There have been some debates on the extent of contribution of circulating IGF-1 to the postnatal body growth. Initial studies surprisingly showed that liver-specific deletion of IGF-1 did not result in severe retardation of growth despite about a 75 % decrease in circulating IGF-1 [106, 107]. However, additional mutation of ALS, which binds to and stabilizes IGF-1, in IGF-1 knockout mouse decreased the IGF-1 even further (~90 %) and significantly retarded postnatal growth [108]. More recently, it was shown that liver-specific knock-in of IGF-1 in IGF-1-null mice substantially rescues the growth retardation postnatally, demonstrating the significant contribution of circulating IGF-1 emanating from the liver in postnatal body growth [109]. IGF-1 produced from skeletal muscle was also shown to significantly contribute to postnatal body growth. Skeletal muscle-specific deletion of STAT5 reduced IGF-1 mRNA levels by 60 % in muscle, and caused about 20 % reduction in mouse body size. Interestingly, this mouse also showed reduced sizes of skeletons despite only slight reduction in circulating IGF-1 levels [110]. In summary, both circulating and locally produced IGF-1 appear to significantly contribute to mammalian body growth, the extents of contributions by which are roughly the same [109].
IGF-2 has a structural similarity with IGF-1 and insulin, exerting its mitogenic effects by binding to IGF-1 receptor [111]. IGF-2-null mice exhibited severe retardation of embryonic growth and decreased birth weight, to similar extents to those observed in IGF-1-null mice [92]. Interestingly, in contrast to IGF-1, IGF-2-null mice did not show a further reduction of body growth after birth, following normal growth rate throughout life [96]. In accordance with this, IGF-2 expression is largely restricted to the period of embryogenesis in rodents and is absent throughout the rest of life [96]. Thus, IGF-2 appears to play a major role in prenatal growth in mice, not constituting the GH/IGF axis in postnatal period. However, in humans, it was found that IGF-2 expression continues postnatally [112]. Notably, IGF-2 is an imprinted gene, expressed only in paternal allele, and loss of IGF-2 imprinting has been found in a number of cancers [111]. Currently, the role of IGF-2 in postnatal body growth in human remains enigmatic.
The bioactivity of IGFs can be modulated by their binding partner proteins. There are at least six specific IGF-binding proteins (IGFBPs) in mammals (IGFBP-1 to IGFBP-6). Some are soluble and while others are tethered in the cell membrane [113]. Most of the IGFs (~90 %) are in complex with IGFBPs in human body, among which IGFBP-3 is the most abundant. These proteins can promote or inhibit the action of IGFs in different ways. By binding IGFs, IGFBPs promote IGFs’ activity both by increasing the half-life of IGFs and by aiding in delivery of the IGFs to the receptor, while IGFBPs also inhibit IGFs’ action by preventing their binding to the receptor. In addition, specific proteases were found to regulate the degradation of the IGFBPs, providing an additional layer of IGF regulation [113].
Modulation of GH/IGF-1 axis
Glucocorticoids are the steroid hormones secreted from the adrenal cortex and comprise the final effectors for the hypothalamic–pituitary–adrenal axis. Recent studies have demonstrated that glucocorticoids act to potentiate STAT5 activity in the liver cell, thereby promoting postnatal body growth [114, 115]. Liver-specific deletion of glucocorticoids receptor (GR) showed severe growth retardation to a similar degree as liver-specific STAT5 deletion in mice. GR was shown to physically interact with STAT5 and act as its coactivator and mediate IGF-1 and ALS expression in the liver. Thus, these studies corroborate the critical role of the growth-promoting hormones emanating from the liver in the postnatal mammalian growth. Interestingly, GR-STAT5 interaction in hepatocyte not only affects genes involved in growth but also those involved in sexual maturation, thus raising the possibility of glucocorticoids’ role in coupling mammalian body growth with sexual maturation [115] (Fig. 4).
Cell-to-cell contact-mediated signaling also appears to be involved in postnatal body growth. Very recent studies by Jing et al. [116] have demonstrated that mutation of Epha4, a receptor for ephrins anchored in cell membrane, causes significant retardation of organismal growth in mice. They found that Epha4 can activate STAT5B in both JAK2-dependent and JAK2-independent manners, thereby enhancing the synthesis of IGF-1. This study suggests that local cell–cell contact signal mediated by EphA4 is also important in IGF-1 production and body growth.
Mammalian adipose tissues have been well known to secrete several humoral peptides, commonly called as adipokines, influencing the homeostasis of energy metabolism in an organism. Cybulski et al. [117] recently provided the evidence that TOR signaling in adipose tissue can non-autonomously control whole body growth. They showed that adipose-specific knockout of Rictor, a component of mammalian TOR complex 2 (mTORC2), unexpectedly increases whole body growth in mice. This genetically engineered mouse showed an increase in size of non-adipose organs, like heart and bone as well as the elevation of IGF-1 and IGF-1 binding protein 3 (IGFBP3). Although the exact molecular mechanism via which the disruption of mTORC2 in fat tissues increases circulating IGF-1 and IGFBP3 remains elusive, this study suggests the existence of mTORC2-dependent adipose/liver axis that controls whole-body growth [117].
Puberty and growth
As mentioned above, the rise in sex steroid hormone levels during puberty is accompanied shortly by a gradual decrease in body growth. Estrogen does play a role in growth deceleration, especially by acting on the growth plate. Upon puberty, in both boys and girls, a rise in estrogen levels causes a temporal burst of linear growth partly due to increased GH [118, 119]. Soon afterward, the growth rate rapidly declines, approaching zero as puberty progresses. At this time, growth plates are inactivated and converted into bone [120] (Fig. 4). Interestingly, when the estrogen effect is absent, this growth deceleration occurs more slowly. For example, a man harboring mutations in estrogen receptor showed persistent activity of growth plate later in adulthood [121]. Rodents whose growth plate appeared not to respond to estrogen similarly showed slower decline in linear growth rate [122]. Thus, estrogen diminishes body growth following pubertal period by inactivation of growth plate. The underlying mechanism for the regulation of the growth plate activity by estrogen receptor (ER)-mediated signaling requires further investigations.
It has long been documented that a state of insulin resistance occurs during puberty. Insulin resistance peaks at mid-puberty and declines to nearly prepubertal levels by adulthood [123, 124]. The elevated serum levels of sex steroids in puberty have been thought to mediate the insulin resistance. Indeed, many studies indicated that high levels of sex steroids induce insulin resistance. Administration of testosterones or estrogens caused a reduction in peripheral glucose uptake and hyperinsulinemia, indicative of insulin resistance [125–128]. Similarly, abuse of anabolic steroids was observed to cause reduced insulin sensitivity [129]. Notably, the polycystic ovarian syndrome, a disorder with excessive androgen production, manifests insulin resistance, which is ameliorated by treatment of an anti-androgen drug [130]. The molecular basis of this sex hormone-induced insulin resistance is poorly understood, although some studies indicate that IRS protein in insulin signaling components fails to function normally in conditions of cellular insulin resistance induced by steroid treatment [131, 132]. Despite the well-known phenomenon of insulin resistance in puberty, it remains to be determined whether the molecular mechanism underlying sex hormone-induced insulin resistance contributes to the growth regulation in puberty (Fig. 4).
Recent genome-wide association studies have discovered a candidate gene that might play a critical role in integrating puberty and body growth. The sequence variants around the LIN28B locus have been demonstrated to be closely associated with the timing of puberty and height in human [133–137]. LIN28B and its paralogs LIN28A are the RNA-binding proteins that regulate the translation of mRNA and the metabolism of various RNAs, and have been recently highlighted for its importance in stem cell pluripotency, cancer progression, and biogenesis of microRNA [138]. Zhu et al. [139] generated the transgenic mouse expressing LIN28A and found that this mouse indeed showed the phenotypes of body size and puberty. The moderate expression of LIN28A from the tetON promoter without Doxycline led to an increase in lean body mass, sizes of many organs, and bone mineral density in mice. Moreover, these mice showed delayed onset of puberty as measured by timing of virginal opening or first estrus. The mRNA expression of LIN28A was especially increased in such organs as hypothalamus, ovary, and muscle, which might underlie the growth and maturation phenotypes. Interestingly, these transgenic mice showed an increase in liver Igf2 mRNA, glucose uptake, and insulin sensitivity, which appear partly due to the decrease in let-7 microRNA [139, 140].
Conclusions and perspectives
Numerous studies in both insects and mammals have uncovered the important factors and their action mechanisms in regulation of organismal growth and body size determination. Insulin/insulin-like growth factor signaling is the main effector pathway in driving body growth in metazoans ranging from insects to mammals. Nutritional conditions and developmental signals modulate the insulin/insulin-like growth factor signaling, thus affecting growth and final body size. In Drosophila, determination of body size is prone to the nutritional condition in specific time period during larval development. A maturation signal, ecdysone, continuously attenuates the peripheral insulin signaling and body growth rate as larva reaches pupal stage, which may underlie the formation of the appropriately sized adult. Similarly, in mammals, estrogen decelerates body growth following puberty via gradual inactivation of growth plates. Moreover, the elevated serum levels of sex steroids in puberty are known to be associated with the insulin resistance, which might underlie the growth deceleration following puberty. Meanwhile, genome-wide association studies and transgenic mice reveal LIN28 as a potential candidate gene that coordinates timing of puberty and adult height. Several metabolic organs, such as liver, muscle, and the adipose tissue have been suggested to play critical roles in controlling body growth via diverse means involving several hormones in both flies and humans.
One of the directions that future studies should focus on is the convergence of insect and mammalian studies, thereby synergizing the discovery that would break new ground in this field. The applicability of the many of the findings from Drosophila regarding body growth and adult size determination in mammalian system remains unknown. For example, would miR-200 and FOG2, the mammalian counterpart of fly miR-8 and USH microRNA/target axis, play a similar role in mediating pubertal growth in mammals in response to increased activities of sex hormones like miR-8 and USH in flies [68]? The literature indicates that miR-200 expression is repressed by estrogen in human cells, raising the possibility that miR-200 links estrogen and insulin signaling in human [141–143]. Whether mammals also have a similar post-embryonic developmental checkpoint like critical weight is an interesting question that merits the aims of future studies. This issue is related with the question about the existence of specific time during adult size determination that might be susceptible to dietary conditions in mammals. In addition, the significance of communication among metabolic organs via various hormones in postnatal mammalian growth should be an important issue, which could be investigated based on the findings in Drosophila studies.
Modern sequencing technology can provide the most valuable information in revealing the genes and their variants associated with the abnormalities in body growth and sexual maturation. Identifying this genetic information should undoubtedly unveil the new mechanisms via which body growth and final body size are regulated. LIN28 identified using this technology might open a new avenue in studying the mechanism connecting sexual maturation and adult body size.
It should be noted that most of the genes and hormones involved in body growth are known to be associated with cancer and aging. Enhanced activity of insulin/insulin-like growth factor signaling is highly correlated with proliferative capacity of tumor growth and also negatively correlated with lifespans in many animals [144]. Therefore, elucidation of the fundamental mechanism in body growth involving insulin/insulin-like growth factor signaling may help in treating the pathophysiology of many growth disorders, unrestrained cancer growth, and many aging-related diseases.
References
Oldham S, Hafen E (2003) Insulin/IGF and target of rapamycin signaling: a TOR de force in growth control. Trends Cell Biol 13(2):79–85
Carpenter CL, Duckworth BC, Auger KR, Cohen B, Schaffhausen BS, Cantley LC (1990) Purification and characterization of phosphoinositide 3-kinase from rat liver. J Biol Chem 265(32):19704–19711
Auger KR, Serunian LA, Soltoff SP, Libby P, Cantley LC (1989) PDGF-dependent tyrosine phosphorylation stimulates production of novel polyphosphoinositides in intact cells. Cell 57(1):167–175
Lee JO, Yang H, Georgescu MM, Di Cristofano A, Maehama T, Shi Y, Dixon JE, Pandolfi P, Pavletich NP (1999) Crystal structure of the PTEN tumor suppressor: implications for its phosphoinositide phosphatase activity and membrane association. Cell 99(3):323–334
Mora A, Komander D, van Aalten DM, Alessi DR (2004) PDK1, the master regulator of AGC kinase signal transduction. Semin Cell Dev Biol 15(2):161–170
Arden KC (2008) FOXO animal models reveal a variety of diverse roles for FOXO transcription factors. Oncogene 27(16):2345–2350
Hay N, Sonenberg N (2004) Upstream and downstream of mTOR. Genes Dev 18(16):1926–1945
Radimerski T, Montagne J, Hemmings-Mieszczak M, Thomas G (2002) Lethality of Drosophila lacking TSC tumor suppressor function rescued by reducing dS6K signaling. Genes Dev 16(20):2627–2632
Dann SG, Thomas G (2006) The amino acid sensitive TOR pathway from yeast to mammals. FEBS Lett 580(12):2821–2829
Sengupta S, Peterson TR, Sabatini DM (2010) Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell 40(2):310–322
Wullschleger S, Loewith R, Hall MN (2006) TOR signaling in growth and metabolism. Cell 124(3):471–484
Potter CJ, Pedraza LG, Xu T (2002) Akt regulates growth by directly phosphorylating Tsc2. Nat Cell Biol 4(9):658–665
Manning BD, Tee AR, Logsdon MN, Blenis J, Cantley LC (2002) Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol Cell 10(1):151–162
Inoki K, Li Y, Zhu T, Wu J, Guan KL (2002) TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol 4(9):648–657
Cai SL, Tee AR, Short JD, Bergeron JM, Kim J, Shen J, Guo R, Johnson CL, Kiguchi K, Walker CL (2006) Activity of TSC2 is inhibited by AKT-mediated phosphorylation and membrane partitioning. J Cell Biol 173(2):279–289
Vander Haar E, Lee SI, Bandhakavi S, Griffin TJ, Kim DH (2007) Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat Cell Biol 9(3):316–323
Sancak Y, Thoreen CC, Peterson TR, Lindquist RA, Kang SA, Spooner E, Carr SA, Sabatini DM (2007) PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol Cell 25(6):903–915
Colombani J, Raisin S, Pantalacci S, Radimerski T, Montagne J, Leopold P (2003) A nutrient sensor mechanism controls Drosophila growth. Cell 114(6):739–749
Bhaskar PT, Hay N (2007) The two TORCs and Akt. Dev Cell 12(4):487–502
Hietakangas V, Cohen SM (2007) Re-evaluating AKT regulation: role of TOR complex 2 in tissue growth. Genes Dev 21(6):632–637
Puig O, Tjian R (2005) Transcriptional feedback control of insulin receptor by dFOXO/FOXO1. Genes Dev 19(20):2435–2446
Teleman AA (2010) Molecular mechanisms of metabolic regulation by insulin in Drosophila. Biochem J 425(1):13–26
Hietakangas V, Cohen SM (2009) Regulation of tissue growth through nutrient sensing. Annu Rev Genet 43:389–410
Thummel CS (2001) Molecular mechanisms of developmental timing in C. elegans and Drosophila. Dev Cell 1(4):453–465
Gilbert LI, Warren JT (2005) A molecular genetic approach to the biosynthesis of the insect steroid molting hormone. Vitam Horm 73:31–57
Huang X, Warren JT, Gilbert LI (2008) New players in the regulation of ecdysone biosynthesis. J Genet Genomics 35(1):1–10
Mirth C, Truman JW, Riddiford LM (2005) The role of the prothoracic gland in determining critical weight for metamorphosis in Drosophila melanogaster. Curr Biol 15(20):1796–1807
Nijhout HF (2003) The control of body size in insects. Dev Biol 261(1):1–9
Mirth CK, Riddiford LM (2007) Size assessment and growth control: how adult size is determined in insects. BioEssays 29(4):344–355
Shingleton AW, Das J, Vinicius L, Stern DL (2005) The temporal requirements for insulin signaling during development in Drosophila. PLoS Biol 3(9):e289
Tennessen JM, Thummel CS (2011) Coordinating growth and maturation—insights from Drosophila. Curr Biol 21(18):R750–R757
Caldwell PE, Walkiewicz M, Stern M (2005) Ras activity in the Drosophila prothoracic gland regulates body size and developmental rate via ecdysone release. Curr Biol 15(20):1785–1795
Slaidina M, Delanoue R, Gronke S, Partridge L, Leopold P (2009) A Drosophila insulin-like peptide promotes growth during nonfeeding states. Dev Cell 17(6):874–884
Okamoto N, Yamanaka N, Yagi Y, Nishida Y, Kataoka H, O’Connor MB, Mizoguchi A (2009) A fat body-derived IGF-like peptide regulates postfeeding growth in Drosophila. Dev Cell 17(6):885–891
Ferrandon D, Imler JL, Hetru C, Hoffmann JA (2007) The Drosophila systemic immune response: sensing and signalling during bacterial and fungal infections. Nat Rev Immunol 7(11):862–874
Boisclair YR, Rhoads RP, Ueki I, Wang J, Ooi GT (2001) The acid-labile subunit (ALS) of the 150 kDa IGF-binding protein complex: an important but forgotten component of the circulating IGF system. J Endocrinol 170(1):63–70
Arquier N, Geminard C, Bourouis M, Jarretou G, Honegger B, Paix A, Leopold P (2008) Drosophila ALS regulates growth and metabolism through functional interaction with insulin-like peptides. Cell Metab 7(4):333–338
Honegger B, Galic M, Kohler K, Wittwer F, Brogiolo W, Hafen E, Stocker H (2008) Imp-L2, a putative homolog of vertebrate IGF-binding protein 7, counteracts insulin signaling in Drosophila and is essential for starvation resistance. J Biol 7(3):10
Alic N, Hoddinott MP, Vinti G, Partridge L (2011) Lifespan extension by increased expression of the Drosophila homologue of the IGFBP7 tumour suppressor. Aging Cell 10(1):137–147
Hull-Thompson J, Muffat J, Sanchez D, Walker DW, Benzer S, Ganfornina MD, Jasper H (2009) Control of metabolic homeostasis by stress signaling is mediated by the lipocalin NLaz. PLoS Genet 5(4):e1000460
Pasco MY, Leopold P (2012) High sugar-induced insulin resistance in Drosophila relies on the lipocalin Neural Lazarillo. PLoS ONE 7(5):e36583
Geminard C, Rulifson EJ, Leopold P (2009) Remote control of insulin secretion by fat cells in Drosophila. Cell Metab 10(3):199–207
Rajan A, Perrimon N (2012) Drosophila cytokine unpaired 2 regulates physiological homeostasis by remotely controlling insulin secretion. Cell 151(1):123–137
Hyun S, Lee JH, Jin H, Nam J, Namkoong B, Lee G, Chung J, Kim VN (2009) Conserved microRNA miR-8/miR-200 and its target USH/FOG2 control growth by regulating PI3K. Cell 139(6):1096–1108
Britton JS, Lockwood WK, Li L, Cohen SM, Edgar BA (2002) Drosophila’s insulin/PI3-kinase pathway coordinates cellular metabolism with nutritional conditions. Dev Cell 2(2):239–249
DiAngelo JR, Bland ML, Bambina S, Cherry S, Birnbaum MJ (2009) The immune response attenuates growth and nutrient storage in Drosophila by reducing insulin signaling. Proc Natl Acad Sci USA 106(49):20853–20858
Teleman AA, Hietakangas V, Sayadian AC, Cohen SM (2008) Nutritional control of protein biosynthetic capacity by insulin via Myc in Drosophila. Cell Metab 7(1):21–32
Li L, Edgar BA, Grewal SS (2010) Nutritional control of gene expression in Drosophila larvae via TOR, Myc and a novel cis-regulatory element. BMC Cell Biol 11:7
Parisi F, Riccardo S, Daniel M, Saqcena M, Kundu N, Pession A, Grifoni D, Stocker H, Tabak E, Bellosta P (2011) Drosophila insulin and target of rapamycin (TOR) pathways regulate GSK3 beta activity to control Myc stability and determine Myc expression in vivo. BMC Biol 9:65
Delanoue R, Slaidina M, Leopold P (2010) The steroid hormone ecdysone controls systemic growth by repressing dMyc function in Drosophila fat cells. Dev Cell 18(6):1012–1021
Upadhya R, Lee J, Willis IM (2002) Maf1 is an essential mediator of diverse signals that repress RNA polymerase III transcription. Mol Cell 10(6):1489–1494
Michels AA, Robitaille AM, Buczynski-Ruchonnet D, Hodroj W, Reina JH, Hall MN, Hernandez N (2010) mTORC1 directly phosphorylates and regulates human MAF1. Mol Cell Biol 30(15):3749–3757
Kantidakis T, Ramsbottom BA, Birch JL, Dowding SN, White RJ (2010) mTOR associates with TFIIIC, is found at tRNA and 5S rRNA genes, and targets their repressor Maf1. Proc Natl Acad Sci USA 107(26):11823–11828
Rideout EJ, Marshall L, Grewal SS (2012) Drosophila RNA polymerase III repressor Maf1 controls body size and developmental timing by modulating tRNAiMet synthesis and systemic insulin signaling. Proc Natl Acad Sci USA 109(4):1139–1144
Marshall L, Rideout EJ, Grewal SS (2012) Nutrient/TOR-dependent regulation of RNA polymerase III controls tissue and organismal growth in Drosophila. EMBO J 31(8):1916–1930
Killip LE, Grewal SS (2012) DREF is required for cell and organismal growth in Drosophila and functions downstream of the nutrition/TOR pathway. Dev Biol 371(2):191–202
Okamoto N, Nakamori R, Murai T, Yamauchi Y, Masuda A, Nishimura T (2013) A secreted decoy of InR antagonizes insulin/IGF signaling to restrict body growth in Drosophila. Genes Dev 27(1):87–97
Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI (2005) Host-bacterial mutualism in the human intestine. Science 307(5717):1915–1920
Koropatnick TA, Engle JT, Apicella MA, Stabb EV, Goldman WE, McFall-Ngai MJ (2004) Microbial factor-mediated development in a host-bacterial mutualism. Science 306(5699):1186–1188
Round JL, Lee SM, Li J, Tran G, Jabri B, Chatila TA, Mazmanian SK (2011) The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science 332(6032):974–977
Storelli G, Defaye A, Erkosar B, Hols P, Royet J, Leulier F (2011) Lactobacillus plantarum promotes Drosophila systemic growth by modulating hormonal signals through TOR-dependent nutrient sensing. Cell Metab 14(3):403–414
Shin SC, Kim SH, You H, Kim B, Kim AC, Lee KA, Yoon JH, Ryu JH, Lee WJ (2011) Drosophila microbiome modulates host developmental and metabolic homeostasis via insulin signaling. Science 334(6056):670–674
Demontis F, Perrimon N (2009) Integration of insulin receptor/foxo signaling and dMyc activity during muscle growth regulates body size in Drosophila. Development 136(6):983–993
Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, Mei Z, Curtin LR, Roche AF, Johnson CL (2000) CDC growth charts: United States. Adv Data 314:1–27
Tanner JM, Davies PS (1985) Clinical longitudinal standards for height and height velocity for North American children. J Pediatr 107(3):317–329
King-Jones K, Thummel CS (2005) Developmental biology. Less steroids make bigger flies. Science 310(5748):630–631
Colombani J, Bianchini L, Layalle S, Pondeville E, Dauphin-Villemant C, Antoniewski C, Carre C, Noselli S, Leopold P (2005) Antagonistic actions of ecdysone and insulins determine final size in Drosophila. Science 310(5748):667–670
Jin H, Kim VN, Hyun S (2012) Conserved microRNA miR-8 controls body size in response to steroid signaling in Drosophila. Genes Dev 26(13):1427–1432
Francis VA, Zorzano A, Teleman AA (2010) dDOR is an EcR coactivator that forms a feed-forward loop connecting insulin and ecdysone signaling. Curr Biol 20(20):1799–1808
Russell MA (1974) Pattern formation in the imaginal discs of a temperature-sensitive cell-lethal mutant of Drosophila melanogaster. Dev Biol 40(1):24–39
Kunkel JG (1977) Cockroach molting. II. The nature of regeneration-induced delay of molting hormone secretion. Biol Bull 153(1):145–162
Simpson P, Berreur P, Berreur-Bonnenfant J (1980) The initiation of pupariation in Drosophila: dependence on growth of the imaginal discs. J Embryol Exp Morphol 57:155–165
Halme A, Cheng M, Hariharan IK (2010) Retinoids regulate a developmental checkpoint for tissue regeneration in Drosophila. Curr Biol 20(5):458–463
Stieper BC, Kupershtok M, Driscoll MV, Shingleton AW (2008) Imaginal discs regulate developmental timing in Drosophila melanogaster. Dev Biol 321(1):18–26
Parker NF, Shingleton AW (2011) The coordination of growth among Drosophila organs in response to localized growth-perturbation. Dev Biol 357(2):318–325
Garelli A, Gontijo AM, Miguela V, Caparros E, Dominguez M (2012) Imaginal discs secrete insulin-like peptide 8 to mediate plasticity of growth and maturation. Science 336(6081):579–582
Colombani J, Andersen DS, Leopold P (2012) Secreted peptide Dilp8 coordinates Drosophila tissue growth with developmental timing. Science 336(6081):582–585
D’Ercole AJ, Stiles AD, Underwood LE (1984) Tissue concentrations of somatomedin C: further evidence for multiple sites of synthesis and paracrine or autocrine mechanisms of action. Proc Natl Acad Sci USA 81(3):935–939
Gosteli-Peter MA, Winterhalter KH, Schmid C, Froesch ER, Zapf J (1994) Expression and regulation of insulin-like growth factor-I (IGF-I) and IGF-binding protein messenger ribonucleic acid levels in tissues of hypophysectomized rats infused with IGF-I and growth hormone. Endocrinology 135(6):2558–2567
Mathews LS, Norstedt G, Palmiter RD (1986) Regulation of insulin-like growth factor I gene expression by growth hormone. Proc Natl Acad Sci USA 83(24):9343–9347
Argetsinger LS, Campbell GS, Yang X, Witthuhn BA, Silvennoinen O, Ihle JN, Carter-Su C (1993) Identification of JAK2 as a growth hormone receptor-associated tyrosine kinase. Cell 74(2):237–244
Waxman DJ, Ram PA, Park SH, Choi HK (1995) Intermittent plasma growth hormone triggers tyrosine phosphorylation and nuclear translocation of a liver-expressed, Stat 5-related DNA binding protein. Proposed role as an intracellular regulator of male-specific liver gene transcription. J Biol Chem 270(22):13262–13270
Ram PA, Park SH, Choi HK, Waxman DJ (1996) Growth hormone activation of Stat 1, Stat 3, and Stat 5 in rat liver. Differential kinetics of hormone desensitization and growth hormone stimulation of both tyrosine phosphorylation and serine/threonine phosphorylation. J Biol Chem 271(10):5929–5940
Silva CM, Lu H, Day RN (1996) Characterization and cloning of STAT5 from IM-9 cells and its activation by growth hormone. Mol Endocrinol 10(5):508–518
Woelfle J, Chia DJ, Rotwein P (2003) Mechanisms of growth hormone (GH) action. Identification of conserved Stat5 binding sites that mediate GH-induced insulin-like growth factor-I gene activation. J Biol Chem 278(51):51261–51266
Davey HW, Xie T, McLachlan MJ, Wilkins RJ, Waxman DJ, Grattan DR (2001) STAT5b is required for GH-induced liver IGF-I gene expression. Endocrinology 142(9):3836–3841
Woelfle J, Billiard J, Rotwein P (2003) Acute control of insulin-like growth factor-I gene transcription by growth hormone through Stat5b. J Biol Chem 278(25):22696–22702
Woelfle J, Rotwein P (2004) In vivo regulation of growth hormone-stimulated gene transcription by STAT5b. Am J Physiol Endocrinol Metab 286(3):E393–E401
Chia DJ, Ono M, Woelfle J, Schlesinger-Massart M, Jiang H, Rotwein P (2006) Characterization of distinct Stat5b binding sites that mediate growth hormone-stimulated IGF-I gene transcription. J Biol Chem 281(6):3190–3197
Frost RA, Nystrom GJ, Lang CH (2002) Regulation of IGF-I mRNA and signal transducers and activators of transcription-3 and -5 (Stat-3 and -5) by GH in C2C12 myoblasts. Endocrinology 143(2):492–503
Lupu F, Terwilliger JD, Lee K, Segre GV, Efstratiadis A (2001) Roles of growth hormone and insulin-like growth factor 1 in mouse postnatal growth. Dev Biol 229(1):141–162
Baker J, Liu JP, Robertson EJ, Efstratiadis A (1993) Role of insulin-like growth factors in embryonic and postnatal growth. Cell 75(1):73–82
Brem G, Wanke R, Wolf E, Buchmuller T, Muller M, Brenig B, Hermanns W (1989) Multiple consequences of human growth hormone expression in transgenic mice. Mol Biol Med 6(6):531–547
Mathews LS, Hammer RE, Behringer RR, D’Ercole AJ, Bell GI, Brinster RL, Palmiter RD (1988) Growth enhancement of transgenic mice expressing human insulin-like growth factor I. Endocrinology 123(6):2827–2833
Woods KA, Camacho-Hubner C, Savage MO, Clark AJ (1996) Intrauterine growth retardation and postnatal growth failure associated with deletion of the insulin-like growth factor I gene. N Engl J Med 335(18):1363–1367
Efstratiadis A (1998) Genetics of mouse growth. Int J Dev Biol 42(7):955–976
Green H, Morikawa M, Nixon T (1985) A dual effector theory of growth-hormone action. Differentiation 29(3):195–198
Clemmons DR, Smith-Banks A, Underwood LE (1992) Reversal of diet-induced catabolism by infusion of recombinant insulin-like growth factor-I in humans. J Clin Endocrinol Metab 75(1):234–238
Sutter NB, Bustamante CD, Chase K, Gray MM, Zhao K, Zhu L, Padhukasahasram B, Karlins E, Davis S, Jones PG, Quignon P, Johnson GS, Parker HG, Fretwell N, Mosher DS, Lawler DF, Satyaraj E, Nordborg M, Lark KG, Wayne RK, Ostrander EA (2007) A single IGF1 allele is a major determinant of small size in dogs. Science 316(5821):112–115
Crain DA, Bolten AB, Bjorndal KA, Guillette LJ Jr, Gross TS (1995) Size-dependent, sex-dependent, and seasonal changes in insulin-like growth factor I in the loggerhead sea turtle (Caretta caretta). Gen Comp Endocrinol 98(2):219–226
Ditchkoff SS, Spicer LJ, Masters RE, Lochmiller RL (2001) Concentrations of insulin-like growth factor-I in adult male white-tailed deer (Odocoileus virginianus): associations with serum testosterone, morphometrics and age during and after the breeding season. Comp Biochem Physiol A Mol Integr Physiol 129(4):887–895
Sparkman AM, Vleck CM, Bronikowski AM (2009) Evolutionary ecology of endocrine-mediated life-history variation in the garter snake Thamnophis elegans. Ecology 90(3):720–728
Kenyon PR, Blair HT, Breier BH, Gluckman PD (2007) The influence of maternal IGF-1 genotype on birthweight and growth rate of lambs. N Z J Agricult Res 50(3):291–297
Kenyon PR, Jenkinson CMC, Blair HT, Morel PCH, Breier BH, Gluckman PD (2009) Reproductive performance of progesterone synchronised IGF-1 selection line ewes. N Z J Agricult Res 52(3):307–314
Beccavin C, Chevalier B, Cogburn LA, Simon J, Duclos MJ (2001) Insulin-like growth factors and body growth in chickens divergently selected for high or low growth rate. J Endocrinol 168(2):297–306
Sjogren K, Liu JL, Blad K, Skrtic S, Vidal O, Wallenius V, LeRoith D, Tornell J, Isaksson OG, Jansson JO, Ohlsson C (1999) Liver-derived insulin-like growth factor I (IGF-I) is the principal source of IGF-I in blood but is not required for postnatal body growth in mice. Proc Natl Acad Sci USA 96(12):7088–7092
Yakar S, Liu JL, Stannard B, Butler A, Accili D, Sauer B, LeRoith D (1999) Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc Natl Acad Sci USA 96(13):7324–7329
Yakar S, Rosen CJ, Beamer WG, Ackert-Bicknell CL, Wu Y, Liu JL, Ooi GT, Setser J, Frystyk J, Boisclair YR, LeRoith D (2002) Circulating levels of IGF-1 directly regulate bone growth and density. J Clin Invest 110(6):771–781
Stratikopoulos E, Szabolcs M, Dragatsis I, Klinakis A, Efstratiadis A (2008) The hormonal action of IGF1 in postnatal mouse growth. Proc Natl Acad Sci USA 105(49):19378–19383
Klover P, Hennighausen L (2007) Postnatal body growth is dependent on the transcription factors signal transducers and activators of transcription 5a/b in muscle: a role for autocrine/paracrine insulin-like growth factor I. Endocrinology 148(4):1489–1497
O’Dell SD, Day IN (1998) Insulin-like growth factor II (IGF-II). Int J Biochem Cell Biol 30(7):767–771
Gray A, Tam AW, Dull TJ, Hayflick J, Pintar J, Cavenee WK, Koufos A, Ullrich A (1987) Tissue-specific and developmentally regulated transcription of the insulin-like growth factor 2 gene. DNA 6(4):283–295
Clemmons DR (1997) Insulin-like growth factor binding proteins and their role in controlling IGF actions. Cytokine Growth Factor Rev 8(1):45–62
Tronche F, Opherk C, Moriggl R, Kellendonk C, Reimann A, Schwake L, Reichardt HM, Stangl K, Gau D, Hoeflich A, Beug H, Schmid W, Schutz G (2004) Glucocorticoid receptor function in hepatocytes is essential to promote postnatal body growth. Genes Dev 18(5):492–497
Engblom D, Kornfeld JW, Schwake L, Tronche F, Reimann A, Beug H, Hennighausen L, Moriggl R, Schutz G (2007) Direct glucocorticoid receptor-Stat5 interaction in hepatocytes controls body size and maturation-related gene expression. Genes Dev 21(10):1157–1162
Jing X, Miyajima M, Sawada T, Chen Q, Iida K, Furushima K, Arai D, Chihara K, Sakaguchi K (2012) Crosstalk of humoral and cell–cell contact-mediated signals in postnatal body growth. Cell Rep 2(3):652–665
Cybulski N, Polak P, Auwerx J, Ruegg MA, Hall MN (2009) mTOR complex 2 in adipose tissue negatively controls whole-body growth. Proc Natl Acad Sci USA 106(24):9902–9907
Marin G, Domene HM, Barnes KM, Blackwell BJ, Cassorla FG, Cutler GB Jr (1994) The effects of estrogen priming and puberty on the growth hormone response to standardized treadmill exercise and arginine-insulin in normal girls and boys. J Clin Endocrinol Metab 79(2):537–541
Leung KC, Johannsson G, Leong GM, Ho KK (2004) Estrogen regulation of growth hormone action. Endocr Rev 25(5):693–721
Parfitt AM (2002) Misconceptions (1): epiphyseal fusion causes cessation of growth. Bone 30(2):337–339
Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B, Williams TC, Lubahn DB, Korach KS (1994) Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med 331(16):1056–1061
Nilsson O, Abad V, Chrysis D, Ritzen EM, Savendahl L, Baron J (2002) Estrogen receptor-alpha and -beta are expressed throughout postnatal development in the rat and rabbit growth plate. J Endocrinol 173(3):407–414
Amiel SA, Sherwin RS, Simonson DC, Lauritano AA, Tamborlane WV (1986) Impaired insulin action in puberty. A contributing factor to poor glycemic control in adolescents with diabetes. N Engl J Med 315(4):215–219
Caprio S, Bronson M, Sherwin RS, Rife F, Tamborlane WV (1996) Co-existence of severe insulin resistance and hyperinsulinaemia in pre-adolescent obese children. Diabetologia 39(12):1489–1497
Diamond MP, Grainger D, Diamond MC, Sherwin RS, Defronzo RA (1998) Effects of methyltestosterone on insulin secretion and sensitivity in women. J Clin Endocrinol Metab 83(12):4420–4425
Polderman KH, Gooren LJ, Asscheman H, Bakker A, Heine RJ (1994) Induction of insulin resistance by androgens and estrogens. J Clin Endocrinol Metab 79(1):265–271
Landon J, Wynn V, Samols E (1963) The effect of anabolic steroids on blood sugar and plasma insulin levels in man. Metabolism 12:924–935
Woodard TL, Burghen GA, Kitabchi AE, Wilimas JA (1981) Glucose intolerance and insulin resistance in aplastic anemia treated with oxymetholone. J Clin Endocrinol Metab 53(5):905–908
Cohen JC, Hickman R (1987) Insulin resistance and diminished glucose tolerance in powerlifters ingesting anabolic steroids. J Clin Endocrinol Metab 64(5):960–963
Buffington CK, Kitabchi AE (1994) Evidence for a defect in insulin metabolism in hyperandrogenic women with polycystic ovarian syndrome. Metabolism 43(11):1367–1372
Collison M, Campbell IW, Salt IP, Dominiczak AF, Connell JM, Lyall H, Gould GW (2000) Sex hormones induce insulin resistance in 3T3-L1 adipocytes by reducing cellular content of IRS proteins. Diabetologia 43(11):1374–1380
Clark SF, Molero JC, James DE (2000) Release of insulin receptor substrate proteins from an intracellular complex coincides with the development of insulin resistance. J Biol Chem 275(6):3819–3826
Sulem P, Gudbjartsson DF, Rafnar T, Holm H, Olafsdottir EJ, Olafsdottir GH, Jonsson T, Alexandersen P, Feenstra B, Boyd HA, Aben KK, Verbeek AL, Roeleveld N, Jonasdottir A, Styrkarsdottir U, Steinthorsdottir V, Karason A, Stacey SN, Gudmundsson J, Jakobsdottir M, Thorleifsson G, Hardarson G, Gulcher J, Kong A, Kiemeney LA, Melbye M, Christiansen C, Tryggvadottir L, Thorsteinsdottir U, Stefansson K (2009) Genome-wide association study identifies sequence variants on 6q21 associated with age at menarche. Nat Genet 41(6):734–738
Lettre G, Jackson AU, Gieger C, Schumacher FR, Berndt SI, Sanna S, Eyheramendy S, Voight BF, Butler JL, Guiducci C, Illig T, Hackett R, Heid IM, Jacobs KB, Lyssenko V, Uda M, Boehnke M, Chanock SJ, Groop LC, Hu FB, Isomaa B, Kraft P, Peltonen L, Salomaa V, Schlessinger D, Hunter DJ, Hayes RB, Abecasis GR, Wichmann HE, Mohlke KL, Hirschhorn JN (2008) Identification of ten loci associated with height highlights new biological pathways in human growth. Nat Genet 40(5):584–591
Ong KK, Elks CE, Li S, Zhao JH, Luan J, Andersen LB, Bingham SA, Brage S, Smith GD, Ekelund U, Gillson CJ, Glaser B, Golding J, Hardy R, Khaw KT, Kuh D, Luben R, Marcus M, McGeehin MA, Ness AR, Northstone K, Ring SM, Rubin C, Sims MA, Song K, Strachan DP, Vollenweider P, Waeber G, Waterworth DM, Wong A, Deloukas P, Barroso I, Mooser V, Loos RJ, Wareham NJ (2009) Genetic variation in LIN28B is associated with the timing of puberty. Nat Genet 41(6):729–733
He C, Kraft P, Chen C, Buring JE, Pare G, Hankinson SE, Chanock SJ, Ridker PM, Hunter DJ, Chasman DI (2009) Genome-wide association studies identify loci associated with age at menarche and age at natural menopause. Nat Genet 41(6):724–728
Perry JR, Stolk L, Franceschini N, Lunetta KL, Zhai G, McArdle PF, Smith AV, Aspelund T, Bandinelli S, Boerwinkle E, Cherkas L, Eiriksdottir G, Estrada K, Ferrucci L, Folsom AR, Garcia M, Gudnason V, Hofman A, Karasik D, Kiel DP, Launer LJ, van Meurs J, Nalls MA, Rivadeneira F, Shuldiner AR, Singleton A, Soranzo N, Tanaka T, Visser JA, Weedon MN, Wilson SG, Zhuang V, Streeten EA, Harris TB, Murray A, Spector TD, Demerath EW, Uitterlinden AG, Murabito JM (2009) Meta-analysis of genome-wide association data identifies two loci influencing age at menarche. Nat Genet 41(6):648–650
Huang Y (2012) A mirror of two faces: Lin28 as a master regulator of both miRNA and mRNA. Wiley Interdiscip Rev RNA 3(4):483–494
Zhu H, Shah S, Shyh-Chang N, Shinoda G, Einhorn WS, Viswanathan SR, Takeuchi A, Grasemann C, Rinn JL, Lopez MF, Hirschhorn JN, Palmert MR, Daley GQ (2010) Lin28a transgenic mice manifest size and puberty phenotypes identified in human genetic association studies. Nat Genet 42(7):626–630
Zhu H, Shyh-Chang N, Segre AV, Shinoda G, Shah SP, Einhorn WS, Takeuchi A, Engreitz JM, Hagan JP, Kharas MG, Urbach A, Thornton JE, Triboulet R, Gregory RI, Altshuler D, Daley GQ (2011) The Lin28/let-7 axis regulates glucose metabolism. Cell 147(1):81–94
Maillot G, Lacroix-Triki M, Pierredon S, Gratadou L, Schmidt S, Benes V, Roche H, Dalenc F, Auboeuf D, Millevoi S, Vagner S (2009) Widespread estrogen-dependent repression of microRNAs involved in breast tumor cell growth. Cancer Res 69(21):8332–8340
Yamagata K, Fujiyama S, Ito S, Ueda T, Murata T, Naitou M, Takeyama K, Minami Y, O’Malley BW, Kato S (2009) Maturation of microRNA is hormonally regulated by a nuclear receptor. Mol Cell 36(2):340–347
Nothnick WB, Healy C (2010) Estrogen induces distinct patterns of microRNA expression within the mouse uterus. Reprod Sci 17(11):987–994
Kenyon CJ (2010) The genetics of ageing. Nature 464(7288):504–512
Acknowledgments
I apologize to the authors whose publications have not been cited due to space limitations. I thank Dr. Yoosik Kim, Dr. Sunhoe Bang, Wonho Kim, Sekyu Choi, and Gang Jun Lee for critical reading of the manuscript. This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2012R1A1A1009732 to S.H.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hyun, S. Body size regulation and insulin-like growth factor signaling. Cell. Mol. Life Sci. 70, 2351–2365 (2013). https://doi.org/10.1007/s00018-013-1313-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-013-1313-5