Abstract
Androgens increase both the size and strength of skeletal muscle via diverse mechanisms. The aim of this review is to discuss the different cellular targets of androgens in skeletal muscle as well as the respective androgen actions in these cells leading to changes in proliferation, myogenic differentiation, and protein metabolism. Androgens bind and activate a specific nuclear receptor which will directly affect the transcription of target genes. These genes encode muscle-specific transcription factors, enzymes, structural proteins, as well as microRNAs. In addition, anabolic action of androgens is partly established through crosstalk with other signaling molecules such as Akt, myostatin, IGF-I, and Notch. Finally, androgens may also exert non-genomic effects in muscle by increasing Ca2+ uptake and modulating kinase activities. In conclusion, the anabolic effect of androgens on skeletal muscle is not only explained by activation of the myocyte androgen receptor but is also the combined result of many genomic and non-genomic actions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Androgens play crucial physiological roles in establishing and maintaining the male phenotype. Their actions are essential for the differentiation and growth of the male reproductive organs, initiation, and regulation of spermatogenesis, and the control of male sexual behavior. In addition, androgens also have anabolic actions on several extragenital structures including muscle and bone [1]. Indeed, testosterone, the main androgen in skeletal muscle [2], increases muscle size and strength both in young [3] and older men [4]. The testosterone-induced increase in muscle mass is partly due to muscle fiber hypertrophy, reflected by an increase in myonuclear number and cross-sectional area of both type I and type II muscle fibers [5]. The responsiveness of skeletal muscle to androgens could potentially be exploited clinically in the treatment of various chronic diseases that are accompanied by muscle wasting such as cancer cachexia, AIDS, chronic obstructive pulmonary disease, chronic renal disease, and burns [6]. Another important growing health issue associated with testosterone deficiency is the age-related increase in sarcopenia and frailty in elderly men and the accompanying risk for fractures due to increased falling [7]. Indeed, testosterone administration to frail elderly men may increase muscle strength [8]. These broad clinical potentials of androgens merit further review of the underlying cellular targets and mechanisms.
Although there is agreement that androgen administration increases muscle mass, data on the effects of testosterone supplementation on muscle performance and physical function are less clear. Meta-analyses indicate that testosterone therapy increases grip strength to a greater extent than placebo [9], but only few trials reported significant increases in maximal voluntary strength [10, 11]. While there is uncertainty about which measures of muscle performance are androgen-responsive [9], the tests of physical function used in most of the trials have serious limitations. Firstly, they have a low performance ceiling, so that at baseline the participants already perform above the test ceiling [12]. Secondly, sample size of most of the trials was relatively small. Therefore, it is likely that they did not have sufficient power to detect meaningful changes in physical function [9]. Finally, it has been suggested that the translation of muscle mass gain into improvements in physical function may require cognitive, behavioral, or functional training [12].
The protein hypothesis states that testosterone administration induces an increase in skeletal muscle protein synthesis [13, 14] and an improved recycling of intracellular amino acids [14, 15]. The proposed effects of androgens on muscle protein degradation, however, are less clear: short-term treatment does not appear to change the breakdown rate [14, 15], whereas treatment for several months decreases muscle protein breakdown [10, 16]. Testosterone-induced muscle hypertrophy may thus be explained by changes in muscle protein metabolism. However, androgens also mediate changes in body composition characterized by an increase in lean body mass accompanied by a concomitant decrease in fat mass [17], which are difficult to explain only by muscle protein synthesis and/or breakdown. The question therefore arises how androgens may induce differential anabolic actions such as changes in body composition as well as muscle hypertrophy.
Androgens exert their effects largely by binding to the nuclear androgen receptor (AR). The AR is a ligand-inducible transcription factor that binds to specific DNA sequences called androgen response elements (AREs) and recruits coactivators, which will help affect the transcription of target genes [18]. Androgens also interfere with other signaling pathways [19], and several non-genomic androgen effects are described [20]. It should be noted that some effects of testosterone can be explained by the activation of estrogen receptors after conversion into estrogens [21]. Here we will summarize the current views on how androgens might act on skeletal muscle. Better knowledge of these mechanisms could lead to more targeted therapeutics acting downstream of androgens in a muscle-specific way. To what extent anabolic androgen action is mediated directly through the AR of the different muscular cells or indirectly through other cells or tissues that affect muscle physiology, also remains an important research question.
Cellular targets of androgen action in skeletal muscle
Skeletal muscles differ markedly in their responsiveness to androgens. For example, the perineal skeletal muscles levator ani (LA) and bulbocavernosus (BC) are highly androgen responsive and depend on androgens for their normal maintenance and function, whereas the limb skeletal muscle extensor digitorum longus (EDL) is relatively unresponsive to androgens and does not depend on androgens to maintain fiber size [22]. Due to its high androgen responsiveness, the LA muscle is used widely as readout for androgen anabolic action in preclinical studies [6]. Immunohistochemical staining of muscle sections revealed that the BC/LA complex contains much more AR protein than do less responsive muscles like the EDL [23, 24]. Thus, differences in AR protein content of skeletal muscles seem to underlie differences in androgen responsiveness.
During growth and repair of the adult skeletal muscle, quiescent tissue-specific progenitor cells, also called satellite cells, are activated and start proliferating, at which stage they are often referred to as myoblasts [25]. Myoblasts further differentiate into myocytes that fuse to form multinucleated myotubes, which finally mature into contracting muscle fibers [26]. Satellite cells and myonuclei are reported to be the predominant sites of AR expression in muscle [27]. This observation supports the hypothesis that androgens might increase muscle mass mainly by stimulation of satellite cells [28]. However, other AR-expressing cell types may contribute to myogenic androgen action. Indeed, the AR is also expressed in CD34+ mesenchymal precursor cells within the human skeletal muscle that are capable of myogenic commitment [27], as well as in neurons that innervate skeletal muscle [29].
Satellite cells
Satellite cell biology
Satellite cells are located between the basal lamina and the plasma membrane of muscle fibers [30]. They can be identified as Pax7+ and CD34+ cells [31], but several other markers have been shown to be useful to isolate satellite cells such as SM/C-2.6, α7-integrin and caveolin-1 [32, 33]. During muscle development and regeneration, quiescent satellite cells become activated and start proliferating [25].
A progressive decline of skeletal muscle mass and strength is observed with ageing [34]. One potential underlying mechanism could be a decrease in the number of satellite cells [35, 36]. An alternative explanation may be a gradual age-related decline of the regenerative potential of skeletal muscle [37, 38], which may in large part be due to a decrease of Notch signaling [39]. Remarkably, the regenerative potential of satellite cells can be restored by exposure to a young systemic environment, suggesting that at least the intrinsic regenerative capacity of aged satellite cells remains intact [40]. Many factors, such as nitric oxide [41], interleukin-6 [42], and Notch signaling [43–45], may contribute to satellite cell activation but the exact underlying molecular mechanisms and interferences by androgens remain to be identified.
Androgen effects on satellite cell proliferation
Studies performed by Sinha-Hikim et al. [46] showed that testosterone-induced muscle hypertrophy is associated with an increase in satellite cell number, both in young and older men [47]. Moreover, AR upregulation has been observed following testosterone treatment of cultured satellite cells from men [27] as well as pigs [48]. Satellite cells are therefore considered to be a direct androgen target in skeletal muscle. Testosterone has also been shown to stimulate satellite cell proliferation in rat [49, 50] and pig models [51]. This cell proliferation was followed by a subsequent increase in the myonuclei number [49]. Also, in in vitro cultured rat primary myoblasts as well as in the mouse myoblast cell line C2C12, testosterone induced proliferation [52, 53]. However, other groups found no direct effects of testosterone on C2C12 proliferation, nor on cultured porcine satellite cells [54, 55]. All together, these data indicate that androgens act on satellite cells by increasing AR expression as well as satellite cell number. This raises the question how and at which stages androgens may impact satellite cell differentiation.
Androgen effects on satellite cell differentiation
In the male complete androgen receptor knockout (ARKO) mouse model, levels of Cdkn1c and Igf2, both drivers of terminal myogenic differentiation [56, 57], are upregulated in ARKO versus wild-type muscle, whereas expression of Itgb1bp3, a negative regulator of muscle differentiation [58], is decreased [59]. From these results, the authors concluded that androgens promote muscle growth by maintaining myoblasts in the proliferative state and delaying differentiation. However, further evidence of testosterone action on satellite cell differentiation is contradictory [48, 53, 54, 60].
In addition, several other studies suggest that it is not proliferation nor differentiation, but other satellite cell functions that are targeted by androgens. Treatment of cultured bovine satellite cells with the synthetic androgen trenbolone, e.g., resulted in a dose-dependent increase in protein synthesis rate and a decrease in protein degradation rate, effects that could be counteracted by AR antagonists [61]. In conclusion, further well-controlled studies are required to elucidate the exact effects of androgens on proliferation and differentiation of satellite cells, myoblasts, and myocytes.
Mesenchymal precursor cells
Alternative muscle progenitor cells
Satellite cells are considered to be the main source of myonuclei in postnatal muscle [62]. However, vascular and bone marrow cells [63, 64] as well as other muscle-resident stem cells with myogenic potential [65, 66] have been reported. The precise anatomical location of these non-satellite cell progenitors is difficult to define due to the lack of suitable cellular markers, with the exception of mesangioblasts, which can be identified as blood vessel-associated alkaline phosphatase (ALP) positive cells [67]. A recent study describes a population of muscle resident stem cells located in the interstitium and expressing PW1 but being negative for Pax7, which can contribute to muscle regeneration [68]. At this moment, the effects of androgens on these cell types under normal physiological conditions remain unexplored.
Pluripotent muscle-adipose progenitor cells
The increase in muscle mass observed upon testosterone administration is accompanied by a reciprocal decrease in fat mass [17]. Conversely, lowering of testosterone concentration below baseline leads to an increase in total body adipose tissue [69]. In patients suffering from androgen insensitivity syndrome (AIS) secondary to disrupted AR signaling, an increase in body fat is observed as well as a higher prevalence of obesity [70], suggesting that these androgen effects on body composition are mediated via the AR.
Several animal studies support this hypothesis. Indeed, the ARKO mouse model developed by the Kato group shows a decrease in lean mass accompanied by a marked increase in visceral and subcutaneous fat [71]. Similarly, Chang et al. [72] report an obese phenotype with enlarged gonadal and perirenal fat pads and larger adipocytes in their AR-null model. However, a third ARKO model shows a decreased muscle cross-sectional area accompanied by reduced potential of voluntary running but without increased adiposity or obesity [73, 74]. Surprisingly, myocyte-specific AR knockout (mARKO) mice not only have a lower muscle mass but also a lower intra-abdominal fat mass [75]. Thus, although AR-related androgen effects on body composition are well established, the underlying AR pathways remain controversial.
Since satellite cells are already committed to myogenesis and do not spontaneously adopt an adipogenic fate [76, 77], androgen action on these cells cannot account for the observed effects on body composition. Therefore, the Bhasin group hypothesized that, in addition to direct effects on satellite cells, testosterone may promote the commitment of pluripotent precursor cells into the myogenic lineage and inhibit their differentiation into the adipogenic lineage [78].
Pluripotent progenitor cell differentiation
In adult skeletal muscle, a population of uncommitted pluripotent progenitor cells of mesenchymal origin serves as a reservoir for the generation of new satellite cells during muscle regeneration or hypertrophy [79] and of adipocytes [80]. Immunofluorescence experiments showing AR expression in CD34+ mesenchymal cells within the human skeletal muscle [27] support the hypothesis that these pluripotent progenitors may be a target of androgen action. In addition, male mice with targeted AR overexpression in mesenchymal stem cells have reduced visceral and subcutaneous fat accumulation with a reciprocal increase in lean mass [81].
Thus, there is increasing evidence that the myogenic action of androgens is partly mediated through the regulation of mesenchymal precursor cell commitment, a model that may explain both the increase in muscle mass as well as the decrease in fat mass following testosterone treatment. However, other studies provide alternative hypotheses to explain the reciprocal changes in body composition. Indeed, a transgenic rat model with selective overexpression of AR in myocytes shows that increased androgen signaling in muscle cells is sufficient to increase lean mass and decrease adiposity by virtue of increased muscular and systemic oxidative metabolism [82]. In addition, co-culture experiments reveal that adipogenesis of mesenchymal progenitors is strongly inhibited by the presence of satellite cell-derived myofibers [77].
In vitro experiments using the C3H 10T1/2 pluripotent mesenchymal cell line provided further evidence for androgen action on the commitment of precursor cells. Indeed, testosterone treatment of C3H 10T1/2 cells upregulated and downregulated myogenic differentiation markers and markers of adipogenic differentiation, respectively [83]. β-catenin signaling may play an important role in the androgenic regulation of precursor cell differentiation [84], as will be discussed further.
Motoneurons
Skeletal muscle is innervated by neurons whose nuclei originate within the spinal cord. Sarcopenia in men is considered to be primarily driven by motoneuron death, subsequently leading to a decrease in muscle mass [85]. In addition, immunohistochemical staining reveals that these motoneurons also express AR [29], hereby further suggesting that androgen anabolic action may be mediated via muscle innervation. Moreover, testosterone causes a significant up-regulation of AR expression in these neurons [29] as well as an increase of the number and size of the motoneurons themselves [86, 87]. Androgen action on motoneurons may therefore contribute to their myogenic effects. However, whether androgen signaling in motoneurons is required for their anabolic action on muscle remains a matter of debate.
Muscle mass of mice selectively lacking AR expression in the nervous system does not differ significantly from that of their wild-type littermates [88]. In addition, complete denervation of the BC/LA complex in mice followed by testosterone administration did not prevent testosterone from sparing the muscle [89]. These data suggest that muscular maintenance is directly mediated by muscle and not by central androgen action. On the other hand, in another study, testosterone treatment failed to restore BC/LA weight following denervation [90], leaving open the possibility that androgens may also act upon motoneurons to affect muscle size. Thus, although AR expression has been demonstrated in motoneurons and both motoneuron number and size increase upon androgen administration, further studies are needed to fully elucidate the exact role of androgens in nerve cells and their relative contribution to the anabolic androgen action in skeletal muscle. A possible approach may consist of performing denervation studies also in limb muscles, as the validity of the perineal BC/LA complex as a general model of skeletal muscle is questionable.
In summary, the main cellular targets for androgens in skeletal muscle include satellite cells and myonuclei, but actions on pluripotent mesenchymal precursor cells and motoneurons may also contribute to the eventual outcome (Fig. 1).
Cellular targets of androgen action in skeletal muscle. Satellite cells and myonuclei are considered to be the main targets of androgen action in muscle. Other AR-expressing cell types such as pluripotent mesenchymal precursor cells and motoneurons may, however, contribute to myogenic androgen action as well. Apart from direct actions, including effects on genes regulating proliferation, myogenic differentiation, and muscle protein metabolism, indirect effects may explain at least part of the muscle hypertrophy observed following androgen administration. Non-genomic androgen pathways may be another mechanism by which androgens act on skeletal muscle. Full arrows indicate androgen action, dotted arrows depict cell differentiation. IGF-I insulin-like growth factor I, EGFR epidermal growth factor receptor, SHBGR sex hormone-binding globulin receptor
Genomic actions of androgens in skeletal muscle
Androgens act predominantly through binding of the classical nuclear AR, inducing receptor dimerization, nuclear translocation, and coactivator recruitment to promote transcription of target genes [18]. Although the role of coactivators in androgenic action has been clearly demonstrated in secondary sexual and reproductive tissues [91], their role in AR action in muscle has not been comprehensively demonstrated. A variety of AR coregulator-deficient mice has been generated in the past decades, but none of them showed an obvious muscular phenotype [92, 93]. In an attempt to identify muscle-specific or abundant coregulators of the AR, Chang et al. [94] screened the skeletal muscle cDNA library and proposed that several actin-associated proteins, such as gelsolin and supervillin, function as AR coregulators and might modulate AR transcriptional activity in skeletal muscle. Thus, although efforts are being made to unravel the role of AR coactivators in myogenic androgen effects, their exact contribution to the genomic action of androgens in skeletal muscle remains unclear. The next section details this genomic action, including tethering of the AR by other transcription factors and androgenic regulation of polyamines and microRNAs in muscle.
Actions involving direct and indirect DNA binding by the activated AR
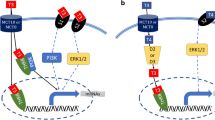
Wyce et al. [95] recently identified over 30,000 AR-binding sites in the chromatin of myoblasts upon stimulation with dihydrotestosterone. The majority of these binding sites contain sequences resembling the ARE consensus indicating direct AR-mediated gene regulation events by coactivator recruitment [95, 96]. However, binding sites for the myocyte enhancer factor 2 (Mef2) family of MADS-box transcription factors are also enriched in these sites [95], indicating that at least for part of the target genes, AR could be recruited indirectly by tethering via Mef2 factors. Similar AR tethering has been described via other transcription factors such as serum response factor (SRF) [97] and T cell factor (TCF) [98] (Fig. 2).
Genomic and non-genomic effects of androgens in skeletal muscle. Testosterone (T) enters the cell and binds to the AR. This can act as a canonical transcription factor via binding to AREs, or be tethered (e.g., by TCF, Mef2c, or SRF) to muscle-specific enhancers. In this way, transcription of androgen target genes is activated. Some of the myomiRs are androgen targets, explaining part of the effect of androgens on translation. SHBG-bound T, T alone, or the T-activated AR can also activate membrane receptors that will act through activating specific kinases and increasing Ca2+ uptake. DNA response elements are depicted in italics. XYZ represent genes implicated in muscle development and maintenance. SHBG(R) sex hormone-binding globulin (receptor), GPCR G-protein coupled receptor, Fst follistatin, Mst myostatin
AR-binding regions were found near genes encoding androgen-regulated microRNAs, as well as, e.g., the Mef2c gene, which controls muscle differentiation via regulating the expression of other muscle-specific genes [95]. AR binding was also observed near genes encoding factors involved in sarcomere integrity and muscle contraction, like myomesin, myotilin, and myozenin [95]. In conclusion, the definition of these binding sites results in a very valuable series of new putative androgen targets, but further in vivo validation experiments are needed.
Androgen-regulated polyamine biosynthesis
The polyamines putrescine, spermidine, and spermine play a role in cell proliferation and differentiation [99]. In skeletal muscle, too, several studies have demonstrated that hypertrophy is associated with increased polyamine levels [100, 101]. Conversely, decreased levels of putrescine, spermidine, and spermine have been observed in a rat model of muscle atrophy [102]. Androgens may directly regulate polyamine biosynthesis via an upregulation of the rate-limiting biosynthetic enzymes ornithine decarboxylase and S-adenosylmethionine decarboxylase, encoded by the genes Odc1 and Amd1. Indeed, orchidectomized male mice show a decreased expression of Odc1 and Amd1, which is restored by testosterone treatment [59]. Similarly, decreased expression of Odc1 and Amd1 is observed in male ARKO mice compared to their wild-type littermates [59]. In addition, expression of Odc1, which was recently shown to promote myoblast proliferation and delay myogenic differentiation, is also decreased in a muscle-specific AR knockout mouse model [103]. Finally, a putative ARE has been described near the promoter of the Odc1 gene [104], but this was not recovered as an AR-binding site by Wyce et al. [95].
Androgen-regulated microRNAs
MicroRNAs (miRNAs) are small (~22 nucleotides) non-coding RNA transcripts able to inhibit translation or promote messenger RNA (mRNA) degradation by annealing to complementary sequences in the 3′ untranslated regions of specific target mRNAs [105, 106]. MiRNAs are synthesized by RNA Pol II as primary miRNAs, which are converted to mature miRNAs by the RNAse enzymes Drosha and Dicer [107]. Deletion of Dicer in embryonic skeletal muscle results in perinatal lethality and a decreased skeletal muscle mass accompanied by abnormal myofiber morphology [108], hereby illustrating the essential role of miRNAs in muscle development and function. Since expression of a large number of miRNAs in rat LA muscle is reduced by orchidectomy [109], muscle-specific miRNAs, also called myomiRs, are hypothesized to be mediators of myogenic androgen action. Wyce et al. [95] identified AR-binding sites near four miRNA-encoding genes, namely miR-206, miR-133, miR-221, and miR-222. They were selected for further analysis, as they are known to be involved in myoblast differentiation [110, 111]. All four myomiRs exhibited increased expression upon dihydrotestosterone treatment [95], hereby further suggesting that their genes are direct targets of the AR in muscle. Androgen regulation of miRNAs does not seem to be restricted to the genomic level. Indeed, miRNA maturation could also be regulated by androgens, as suggested by the ligand-induced interaction between AR and Dicer in a co-immunoprecipitation assay [109]. Collectively, these data illustrate that specific myomiRs may be androgen targets in skeletal muscle.
In conclusion (Fig. 2), androgens induce AR binding to DNA, either directly to AREs, or indirectly by tethering via other transcription factors that bind to muscle-specific enhancers. In this way, protein encoding genes are upregulated and muscle-specific functions become expressed. Alternatively, the transcription of myomiR-encoding genes is upregulated, and these miRNAs may in turn serve as a feedback loop to attenuate the expression of target genes like SRF.
Crosstalk between androgens and other signaling pathways in skeletal muscle
This section will describe the crosstalk between androgens and other signaling pathways in skeletal muscle, including those of PI3K/Akt, myostatin, insulin-like growth factor I and Notch.
Phosphatidylinositol 3-kinase/Akt
PI3K/Akt and muscle
Activation of the phosphatidylinositol 3-kinase(PI3K)/Akt pathway induces an increase in skeletal muscle mass. Indeed, transgenic mice in which a mutant, constitutively active form of Akt is conditionally expressed in skeletal muscle show a dramatic increase in muscle size [112]. Stimulation of skeletal muscle development by Akt relies on two distinct mechanisms, i.e., activation of protein synthesis pathways and blocking of the transcriptional upregulation of key mediators of muscle atrophy. Indeed, activation of Akt leads to phosphorylation and activation of downstream molecules including mTOR and p70s6k, resulting in an increase in protein synthesis [113]. On the other hand, Akt activation also leads to phosphorylation and inhibition of forkhead box O (FoxO) transcription factors, which are required for the upregulation of the muscle-specific ubiquitin ligases MuRF-1 and MAFbx, resulting in a decrease in protein degradation [113]. These ubiquitin ligases induce the proteasome-mediated degradation of particular protein substrates, and have been shown to be induced in several models of skeletal muscle atrophy in both rodents and humans [113].
Crosstalk between PI3K/Akt and androgens
Several data sets indicate that androgens activate the PI3K/Akt pathway. Indeed, testosterone treatment of primary rat myotubes significantly increased Akt and mTOR phosphorylation [114], whereas decreased levels of phosphorylated Akt accompanied by an upregulation of MuRF-1 and MAFbx were observed following orchidectomy in both rats [115] and mice [116]. Both effects were reversed by testosterone replacement [115, 116]. Activation of Akt by androgens seems to be mediated by a direct interaction of the AR with the p85 regulatory subunit of PI3K, resulting in its activation and subsequent upregulation of Akt phosphorylation [117].
Thus, androgen-mediated increase in skeletal muscle mass is, at least partly, mediated through activation of PI3K/Akt signaling, resulting in both stimulation of protein synthesis and inhibition of protein degradation (Fig. 3).
Crosstalk between androgens and other signaling pathways in skeletal muscle. Testosterone activates PI3K/Akt signaling, either directly or through IGF-I stimulation. Activation of Akt leads to phosphorylation and activation of downstream molecules including mTOR and p70s6k, resulting in an increase in protein synthesis. Furthermore, Akt activation leads to phosphorylation and inhibition of FoxO transcription factors, which are required for upregulation of the ubiquitin ligases MuRF-1 and MAFbx, resulting in a decrease in protein degradation. Testosterone also inhibits expression and activity of Mst, which represses protein synthesis and stimulates muscle atrophy though inhibition of PI3K/Akt signaling and also negatively regulates myoblast proliferation and differentiation. Finally, testosterone increases Notch signaling, which is also a downstream effector of Akt and is essential for satellite cell proliferation and myogenic progression
Myostatin
Myostatin and muscle
Myostatin (Mst) is a member of the transforming growth factor-β (TGF-β) superfamily that is expressed specifically in skeletal muscle [118]. Mst is a strong negative regulator of muscle growth, since disruption of the Mst gene in mice, cattle and dogs induces a dramatic increase in muscle mass due to both muscle hypertrophy and hyperplasia [118–120]. Similarly, muscle-specific overexpression of Mst in mice is associated with lower muscle mass an decreased fiber size [121].
Mst seems to be involved in several processes that control muscle development and maintenance (Fig. 3). It inhibits both proliferation [122, 123] and differentiation of C2C12 myoblast [124, 125]. These inhibitory actions correlate with the upregulation of the cell cycle proteins p21 and p53 [126] and the downregulation of the myogenic factors MyoD and myogenin [125, 126]. In the same cell line, Mst was shown to dose-dependently inhibit DNA and protein synthesis [123]. Mst could also cause muscle cell atrophy by reversing the PI3K/Akt pathway, resulting in an increased FoxO transcriptional activity, which induces the expression of atrogenes [127].
The effect of Mst on satellite cells is still unresolved. As Mst knockout mice show increased satellite cell numbers [128], it has been proposed that Mst blocks the activation of satellite cells and also negatively regulates their self-renewal, thereby maintaining them in quiescence [129, 130]. However, another study showed that muscle hypertrophy in the absence of Mst involves no input from satellite cells [131].
Mst was also proposed to act on the commitment of pluripotent mesenchymal precursor cells, since the increased muscle mass in Mst knockout mice is associated with a significant reduction in adipogenesis and body fat [132, 133]. Moreover, Mst induces the expression of adipogenic markers in the pluripotent mesenchymal cell line C3H 10T1/2, whereas markers of myogenic differentiation are downregulated [134].
At the molecular level, Mst exerts its activity through the activin receptors type I and type II. Upon tetramerization of the receptor complex, the signal is relayed to the cytoplasm via SMAD proteins. Phosphorylated SMAD4 will translocate to the nucleus and regulate the expression of a specific set of target genes [135]. Moreover, the Mst-mediated effects are antagonized by follistatin (Fst) [136–138], a protein of which the expression is regulated through β-catenin signaling [84]. Fst antagonizes Mst by direct protein interaction, which prevents Mst from binding to its receptor [137].
Crosstalk between myostatin and androgens
The muscle hypertrophy observed in Mst knockout mice is more pronounced in males compared to females [118] and, conversely, muscle-specific Mst overexpression lowers muscle mass more in male than in female mice [121]. This gender specificity suggests a crosstalk between androgens and Mst, consistent with the finding of elevated Mst expression in LA muscle after orchidectomy [139]. Moreover, androgen regulation of Mst does not seem to be restricted to the repression of Mst expression at the gene level. Indeed, several studies support the hypothesis that androgens enhance β-catenin signaling, hereby increasing the expression of target genes including Fst, resulting in Mst inhibition [84]. A downregulation of axin, a negative regulator of β-catenin, is observed in orchidectomized rats treated with testosterone [140]. Moreover, co-immunoprecipitation assays showed direct interaction between AR and β-catenin, which might stabilize β-catenin and prevent it from degradation [84, 141], and might result in β-catenin-mediated tethering of the AR to specific target genes (Fig. 2). In addition, the activation of adenosine monophosphate-activated kinase (AMPK) by androgens might further contribute to the stabilization of β-catenin via phosphorylation at Ser552 [141]. A putative ARE has been identified by in silico analysis in the Mst gene promoter, but no further functional analysis has been presented [142].
Thus, there is some evidence that myogenic androgen action could, at least in part, be mediated through repression of both Mst expression and activity (Fig. 3).
Insulin-like growth factor I
IGF-I and muscle
Insulin-like growth factor I (IGF-I) is a well-characterized muscle growth-promoting factor produced mainly in the liver in response to growth hormone (GH) stimulation. It is also locally expressed in a variety of tissues including skeletal muscle, where it acts as an autocrine/paracrine growth factor under the control of multiple hormones [143]. IGF-I is regarded as an important regulator of muscle mass. Indeed, mice with targeted overexpression of IGF-I in skeletal muscle have a higher muscle mass compared to controls [144]. Stimulation of muscle mass development by IGF-I relies on multiple processes, including increases in protein synthesis and myogenesis and decreases in proteolysis and apoptosis [145, 146]. At the molecular level, IGF-I acts through binding of its specific receptor, the IGF-I receptor (IGF-IR), and subsequent activation of the PI3K/Akt pathway (Fig. 3), resulting in stimulation of protein synthesis and inhibition of FoxO nuclear translocation thereby suppressing the transcription of several atrogenes such as atrogin-1, MuRF-1 and cathepsin L [147]. Importantly, different isoforms of IGF-I can arise through alternative splicing.
Human skeletal muscle expresses two IGF-I variants, namely IGF-IEa, which is similar to the liver type or systemic form, and IGF-IEc, also called mechanogrowth factor (MGF), an autocrine/paracrine and mechanosensitive form [148, 149]. IGF-IEa and MGF are reported to have different myogenic actions. Indeed, MGF increases proliferation and inhibits terminal differentiation in C2C12 myoblast cell line, while the IGF-IEa isoform stimulates myoblast differentiation into myotubes with a smaller effect on proliferation [150]. A recent study suggests that IGF-I enhances β-catenin signaling, as treatment of C2C12 with IGF-IEa or MGF both increased nuclear β-catenin [140]. Thus, inhibition of Mst through enhanced β-catenin signaling could be an additional mechanism resulting in the stimulation of muscle mass development by IGF-I.
Crosstalk between IGF-I and androgens
Several clinical studies have demonstrated that testosterone therapy augments GH secretion [151, 152], which in turn correlates with an increase in serum IGF-I [153]. The androgen-induced stimulation of the GH/IGF-I axis has been studied extensively in animal models. It seems to be mediated centrally, since mice selectively lacking AR in the nervous system show a twofold reduction in serum IGF-I [88]. In addition, the crosstalk between IGF-I and androgens may in part be related to the aromatization of testosterone into the estrogen 17β-estradiol [21]. However, circulating GH and IGF-I may not be essential for the anabolic effects of androgens, as testosterone increases total body weight and LA muscle mass even in hypophysectomized rats that are deficient in GH and low in IGF-I serum levels [154]. Moreover, administration of high doses of dihydrotestosterone to orchidectomized rats did not change serum IGF-I concentrations although LA weight was restored to sham levels [155], and in ARKO male mice, serum IGF-I was not different from wild-type animals [59]. Collectively, these data suggest that circulating GH and IGF-I play only a minor role in mediating the anabolic effects of androgens.
There is increasing evidence that, in contrast to the circulating hormone, locally produced IGF-I is an important mediator of androgen action in muscle. Indeed, androgen treatment was found to increase IGF-I mRNA in bovine satellite cells [156] as well as in rat diaphragmatic muscle [19]. In addition, levels of IGF binding proteins (IGFBPs) were dramatically suppressed [155]. The presence of two AREs in the upstream promoter of the IGF-I gene [157] supports this hypothesis. IGF-IEa levels decreased upon orchidectomy both in LA and gastrocnemius muscle, while MGF levels remained constant [158], so IGF-IEa but not MGF expression is androgen-dependent in both perineal and limb muscles. In LA muscle of mice lacking myocytic AR a twofold reduction in IGF-IEa transcript levels was observed compared to control mice, whereas MGF levels were similar [158]. Surprisingly, in gastrocnemius muscle no difference in IGF-IEa expression was detected between mutant and control mice, suggesting that IGF-IEa expression depends on myocytic AR in perineal but not limb skeletal muscles.
A study investigating the effect of androgens on the phosphorylation of p70s6k provided further evidence that the muscular IGF-I system plays an important role in anabolic androgen action. The ribosomal protein kinase p70s6k is a downstream effector of IGF-I participating in the regulation of protein turnover in skeletal muscle [159]. Dihydrotestosterone was shown to induce phosphorylation of p70s6k in LA muscle of orchidectomized rats in a dose-dependent manner [155]. The phosphorylation status of p70s6k was decreased by the AR antagonist flutamide, suggesting that activation of intramuscular IGF-I signaling by androgens is AR-mediated.
Collectively, these data indicate that androgens interfere with the muscular IGF-I system at different levels. Moreover, the fact that IGF-I induces expression, phosphorylation, nuclear translocation and DNA binding activity of the AR in muscle [160, 161] indicates the existence of a feedback-loop between IGF-I and androgens.
Androgens and Notch signaling in muscle
Since the progressive decline of skeletal muscle mass with ageing is reported to be in large part due to a decline in Notch signaling, Notch regulation by androgens was proposed to be involved in the protective effect of androgens on age-associated muscle degradation [39]. Testosterone-induced muscle hypertrophy in mice is accompanied by an upregulation of the Notch ligand Delta1 and an activation of Notch signaling, as evidenced by the increase in activated forms of Notch1 and Notch2 [162]. Moreover, testosterone treatment inhibited c-Jun NH2-terminal kinase (JNK) and activated p38 mitogen-activated protein kinase (MAPK), two factors that are critical for the activation of Notch signaling [162]. Enhancement of Notch activation could also play a role in the androgen effects on satellite cells since Notch signaling is essential for satellite cell proliferation and myogenic progression [44, 45]. In addition, a study exploring androgen effects on aged muscle revealed that testosterone treatment can restore Notch signaling in old mice and reverse the age-associated increase in p21, a downstream member of the Notch cascade, which is known to interfere with satellite cell regenerative capacity [163]. As it has been demonstrated that Notch signaling is partly regulated by the PI3K/Akt cascade [164], Notch regulation by androgens could be either direct or through Akt activation (Fig. 3).
In conclusion, several pathways seem to contribute to the myogenic action of androgens. The crosstalk between androgens and other signaling molecules in skeletal muscle is summarized in Fig. 3.
Non-genomic androgen action
Androgens act predominantly through binding of the classical nuclear AR, inducing receptor dimerization, nuclear translocation and coactivator recruitment to promote transcription of target genes [18]. Increasing evidence suggests that, in addition to this genomic mode of action, androgens may also exert fast non-genomic effects within seconds to minutes after hormone administration [165, 166]. Such non-genomic effects may occur (i) through interactions between the AR and the tyrosine kinase c-Src, inducing the MAPK signaling cascade [167, 168], (ii) through interaction of the AR with the sex hormone-binding globulin (SHBG) receptor (SHBGR), increasing protein kinase A (PKA) activity [169] or (iii) by activation of a distinct non-classical receptor associated with the plasma membrane, triggering an increase in intracellular Ca2+ levels [170, 171].
AR–c-Src interaction and MAPK signaling
Stimulation of the MAPK pathway through interaction of the AR with c-Src may contribute to myogenic androgen action in several ways. Firstly, it is possible that this non-genomic action of the AR ultimately influences AR transcriptional activity in skeletal muscle. Indeed, AR phosphorylation by extracellular signal-regulated kinase (ERK), a downstream member of the MAPK signaling cascade, is associated with enhanced AR transcriptional activity and an increased ability to recruit the coactivator ARA70 [172]. In addition, phosphorylation of the steroid receptor coactivators (SRCs) by MAPK results in an increased ability of these coactivators to recruit additional coactivator complexes to the DNA-bound receptor [173]. Secondly, the c-Src-mediated activation of MAPK is involved in multiple cellular processes, including myoblast proliferation and differentiation [174, 175]. A recent study suggests an AR-independent mechanism of MAPK activation by androgens [176]. Dihydrotestosterone treatment of isolated intact mammalian skeletal muscle fiber bundles increased both twitch and tetanic contractions in fast twitch fibers, and these changes were accompanied by an increase in MAPK/ERK phosphorylation. Interestingly, these effects were insensitive to inhibitors of c-Src and AR, but abolished by an inhibitor of the epidermal growth factor receptor (EGFR), suggesting that the non-genomic effects of androgens on skeletal muscle involve the EGFR.
AR–SHBGR interaction and PKA activity
The majority of testosterone and dihydrotestosterone in human serum is complexed to SHBG [177]. Its action as a steroid transporter is well known, but it could also affect target cells via a specific cell surface receptor for SHBG which has been reported in a number of tissues including skeletal muscle [178]. The intracellular interaction of the AR complex with this SHBGR was proposed to increase PKA activity [169] and this could influence AR-mediated transcription by altering the phosphorylation of the AR and its coactivators [179–181]. However, whether SHBG has similar effects on the skeletal muscle has not been demonstrated yet [181, 182].
Activation of a non-classical plasma membrane receptor
Testosterone induces a rapid increase in intracellular Ca2+ level in several cell types [171]. Possibly, this involves a membrane binding site which is saturable and selective for androgens but immunologically and functionally different from the classical intracellular AR [170]. This transient Ca2+ increase is sensitive to the G-protein coupled receptor (GPCR) inhibitor pertussis toxin, suggesting that this membrane androgen-binding protein is either a GPCR or that its function is closely linked to one [183].
A rapid increase in intracellular Ca2+ in response to androgens has also been observed in primary cultures of rat myotubes treated with testosterone and this Ca2+ increase was preceded by an increase in inositol 1,4,5-triphosphate (IP3) [20]. In addition, exposure of these myotubes to androgens produced an IP3-Ca2+ dependent and pertussis toxin-sensitive increase in ERK phosphorylation [184]. Indeed, the increase in intracellular Ca2+ is followed by the activation of several signal transduction cascades, including PKA and MAPK [185]. As already mentioned, PKA and MAPK/ERK activity influence AR-mediated transcription by altering the phosphorylation of the AR and its coactivators.
Androgens have also been reported to exert AR-independent effects on skeletal muscle. In the AR-negative rat L6 myoblast cell line, testosterone promotes both proliferation and differentiation. Bovine serum albumin (BSA)-linked testosterone, which does not cross the plasma membrane, has similar effects as free testosterone. The inhibition of these effects by pertussis toxin further suggests the involvement of a GPCR. Using specific inhibitors, it is shown that the stimulation of L6 proliferation by testosterone involves the MAPK/ERK pathway, whereas PKA signaling plays a role in androgen-mediated stimulation of L6 differentiation [186].
Thus, myogenic androgen effects are partly mediated through non-genomic pathways (Fig. 2), including activation of a non-classical G-protein linked binding site on the plasma membrane of myoblasts resulting in Ca2+-dependent activation of several kinases. However, the relative contribution as well as the exact mechanisms through which these non-genomic effects impact skeletal muscle growth and maintenance have to be further elucidated.
Selective androgen receptor modulators
As discussed in the previous sections, it is now well established that androgen administration increases muscular and lean body mass. Thus, testosterone could potentially be exploited in the treatment of muscle wasting caused by various underlying diseases. However, these therapies may have severe side-effects, including the stimulation of prostate hyperplasia in men, as well as virilization in women [187]. Therefore, therapeutic agents that could achieve anabolic effects on skeletal muscle without androgenic activities such as prostatic effects and virilization are of great clinical interest. A first approach to achieve tissue selectivity is to elucidate the mechanisms of androgen action in skeletal muscle and prostate, and to identify signaling molecules that are downstream of the AR and which activate pathways involved in skeletal muscle hypertrophy but not in prostatic growth. From this point of view, Mst is an ideal target molecule, since β-catenin and TGF-β/SMAD signaling play essential roles in mediating testosterone effects on myogenic differentiation (see previous sections). This strategy is currently being explored [188, 189].
A second approach to dissociate anabolic and androgenic activities of androgens is the development of tissue-selective AR-ligands, also called selective androgen receptor modulators (SARMs). A well-established body of evidence supports their in vivo tissue selectivity in animal models [190]. However, the mechanisms by which SARMs achieve the observed tissue selectivity are not fully elucidated. Several hypotheses have been proposed, although these hypotheses are not mutually exclusive. Firstly, most SARMs are nonsteroidal and are therefore not substrates for reduction by 5α-reductase, an enzyme highly expressed in androgenic tissues including the prostate and responsible for amplification of testosterone action in these organs by its conversion to the more potent dihydrotestosterone [191]. Secondly, several studies have shown that SARMs induce a conformational change of the AR that is distinct from testosterone, thus recruiting different coregulator complexes [192]. Finally, the earlier mentioned non-genomic pathways may play a role in the mode of action of SARMs [193].
Although the mechanisms by which SARMs work at the molecular level are still debated, the immense interest regarding the therapeutic potential of SARMs in humans has culminated in the development of several compounds being evaluated in phase I clinical trials [190]. Recently, Dalton et al. [194] have reported a phase II clinical trial where GTx-024, an orally available nonsteroidal SARM, has been tested in healthy elderly men and postmenopausal women. GTx-024 treatment significantly increased total lean body mass and improved physical function, whereas no increased adverse effects were observed compared to placebo. The effects of SARMs on body composition and muscle strength are not only promising for future muscle-wasting treatment strategies, but also illuminate the mechanisms of anabolic androgen action. Indeed, unlike testosterone, nonsteroidal SARMs are not substrates for 5α-reductase or for aromatase, indicating that, although reduction to dihydrotestosterone and aromatization to estradiol may contribute to myogenic androgen action to some extent, these conversions are not essential for mediating androgen response in skeletal muscle.
Outlook
The effects of androgens on skeletal muscle are very diverse and are mediated via different cellular targets as well as biochemical pathways, as summarized in Figs. 1 and 2. Clinical studies complemented with animal models and in vitro cell cultures continue to enhance our understanding of these processes. However, despite growing clinical interest in anabolic action of androgens, many research questions remain largely unresolved. What is the relative importance of the many pathways that may cross talk with androgen action in skeletal muscle? How large is the non-genomic contribution of androgen action? What are the main cellular targets under normal physiological as well as clinical conditions? What are the direct AR targets in the different cell types? Which pathways can safely be used in therapeutic strategies for the treatment of disease or age-related muscle wasting? It is expected that the development of cell- and stage-specific knockout or knockin approaches combined with recently developed techniques like ChIP-seq, transcriptomics, proteomics and metabolomics in model organisms as well as human subjects will provide new insights which will serve as inspiration for the development of clinical applications.
Abbreviations
- AIS:
-
Androgen insensitivity syndrome
- ALP:
-
Alkaline phosphatase
- AMPK:
-
Adenosine monophosphate-activated kinase
- AR:
-
Androgen receptor
- ARE:
-
Androgen response element
- ARKO:
-
Androgen receptor knockout
- BC:
-
Bulbocavernosus
- BSA:
-
Bovine serum albumin
- c-Src:
-
Cellular sarcoma
- EDL:
-
Extensor digitorum logus
- EGFR:
-
Epidermal growth factor receptor
- ERK:
-
Extracellular signal-regulated kinase
- FoxO:
-
Forkhead box O
- Fst:
-
Follistatin
- GH:
-
Growth hormone
- GPCR:
-
G-protein coupled receptor
- IGFBP:
-
IGF binding protein
- IGF-I:
-
Insulin-like growth factor I
- IGF-IR:
-
IGF-I receptor
- IP3:
-
Inositol 1,4,5-triphosphate
- JNK:
-
c-Jun NH2-terminal kinase
- LA:
-
Levator ani
- MADS:
-
Mcm1 agamous deficiens serum response factor
- MAFbx:
-
Muscle Atrophy F-box
- MAPK:
-
Mitogen-activated protein kinase
- mARKO:
-
Myocyte-specific AR knockout
- Mef:
-
Myocyte enhancer factor
- MGF:
-
Mechanogrowth factor
- miR:
-
microRNA
- mRNA:
-
Messenger RNA
- Mst:
-
Myostatin
- mTOR:
-
Mammalian target of rapamycin
- MuRF-1:
-
Muscle Ring Finger 1
- PI3K:
-
Phosphatidylinositol 3-kinase
- PKA:
-
Protein kinase A
- SARM:
-
Selective AR modulator
- SHBG:
-
Sex hormone-binding globulin
- SHBGR:
-
SHBG receptor
- SRC:
-
Steroid receptor coactivator
- SRE:
-
Serum response element
- SRF:
-
Serum response factor
- TCF:
-
T cell factor
- TGF-β:
-
Transforming growth factor-β
- WRE:
-
Wnt response element
- SRE:
-
Serum response element
- SARM:
-
Selective AR modulator
References
Yin D, Gao W, Kearbey JD, Xu H, Chung K, He Y, Marhefka CA, Veverka KA, Miller DD, Dalton JT (2003) Pharmacodynamics of selective androgen receptor modulators. J Pharmacol Exp Ther 304:1334–1340
Bhasin S, Woodhouse L, Storer TW (2003) Androgen effects on body composition. Growth Horm IGF Res 13 (Suppl A):S63–S71
Bhasin S, Storer TW, Berman N, Callegari C, Clevenger B, Phillips J, Bunnell TJ, Tricker R, Shirazi A, Casaburi R (1996) The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. N Engl J Med 335:1–7
Bhasin S, Woodhouse L, Casaburi R, Singh AB, Mac RP, Lee M, Yarasheski KE, Sinha-Hikim I, Dzekov C, Dzekov J, Magliano L, Storer TW (2005) Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle. J Clin Endocrinol Metab 90:678–688
Sinha-Hikim I, Artaza J, Woodhouse L, Gonzalez-Cadavid N, Singh AB, Lee MI, Storer TW, Casaburi R, Shen R, Bhasin S (2002) Testosterone-induced increase in muscle size in healthy young men is associated with muscle fiber hypertrophy. Am J Physiol Endocrinol Metab 283:E154–E164
MacLean HE, Handelsman DJ (2009) Unraveling androgen action in muscle: genetic tools probing cellular mechanisms. Endocrinology 150:3437–3439
Cawthon PM, Ensrud KE, Laughlin GA, Cauley JA, Dam TT, Barrett-Connor E, Fink HA, Hoffman AR, Lau E, Lane NE, Stefanick ML, Cummings SR, Orwoll ES (2009) Sex hormones and frailty in older men: the osteoporotic fractures in men (MrOS) study. J Clin Endocrinol Metab 94:3806–3815
Srinivas-Shankar U, Roberts SA, Connolly MJ, O’Connell MD, Adams JE, Oldham JA, Wu FC (2010) Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab 95:639–650
Bhasin S, Calof OM, Storer TW, Lee ML, Mazer NA, Jasuja R, Montori VM, Gao W, Dalton JT (2006) Drug insight: testosterone and selective androgen receptor modulators as anabolic therapies for chronic illness and aging. Nat Clin Pract Endocrinol Metab 2:146–159
Ferrando AA, Sheffield-Moore M, Yeckel CW, Gilkison C, Jiang J, Achacosa A, Lieberman SA, Tipton K, Wolfe RR, Urban RJ (2002) Testosterone administration to older men improves muscle function: molecular and physiological mechanisms. Am J Physiol Endocrinol Metab 282:E601–E607
Bhasin S, Storer TW, Javanbakht M, Berman N, Yarasheski KE, Phillips J, Dike M, Sinha-Hikim I, Shen R, Hays RD, Beall G (2000) Testosterone replacement and resistance exercise in HIV-infected men with weight loss and low testosterone levels. JAMA 283:763–770
Storer TW, Woodhouse L, Magliano L, Singh AB, Dzekov C, Dzekov J, Bhasin S (2008) Changes in muscle mass, muscle strength, and power but not physical function are related to testosterone dose in healthy older men. J Am Geriatr Soc 56:1991–1999
Urban RJ, Bodenburg YH, Gilkison C, Foxworth J, Coggan AR, Wolfe RR, Ferrando A (1995) Testosterone administration to elderly men increases skeletal muscle strength and protein synthesis. Am J Physiol 269:E820–E826
Ferrando AA, Tipton KD, Doyle D, Phillips SM, Cortiella J, Wolfe RR (1998) Testosterone injection stimulates net protein synthesis but not tissue amino acid transport. Am J Physiol 275:E864–E871
Sheffield-Moore M, Urban RJ, Wolf SE, Jiang J, Catlin DH, Herndon DN, Wolfe RR, Ferrando AA (1999) Short-term oxandrolone administration stimulates net muscle protein synthesis in young men. J Clin Endocrinol Metab 84:2705–2711
Ferrando AA, Sheffield-Moore M, Paddon-Jones D, Wolfe RR, Urban RJ (2003) Differential anabolic effects of testosterone and amino acid feeding in older men. J Clin Endocrinol Metab 88:358–362
Wittert GA, Chapman IM, Haren MT, Mackintosh S, Coates P, Morley JE (2003) Oral testosterone supplementation increases muscle and decreases fat mass in healthy elderly males with low-normal gonadal status. J Gerontol A Biol Sci Med Sci 58:618–625
Claessens F, Denayer S, Van Tilborgh N, Kerkhofs S, Helsen C, Haelens A (2008) Diverse roles of androgen receptor (AR) domains in AR-mediated signaling. Nucl Recept Signal 6:e008
Lewis MI, Horvitz GD, Clemmons DR, Fournier M (2002) Role of IGF-I and IGF-binding proteins within diaphragm muscle in modulating the effects of nandrolone. Am J Physiol Endocrinol Metab 282:E483–E490
Estrada M, Liberona JL, Miranda M, Jaimovich E (2000) Aldosterone- and testosterone-mediated intracellular calcium response in skeletal muscle cell cultures. Am J Physiol Endocrinol Metab 279:E132–E139
Callewaert F, Sinnesael M, Gielen E, Boonen S, Vanderschueren D (2010) Skeletal sexual dimorphism: relative contribution of sex steroids, GH-IGF1, and mechanical loading. J Endocrinol 207:127–134
Lubischer JL, Bebinger DM (1999) Regulation of terminal Schwann cell number at the adult neuromuscular junction. J Neurosci 19: RC46
Monks DA, O’Bryant EL, Jordan CL (2004) Androgen receptor immunoreactivity in skeletal muscle: enrichment at the neuromuscular junction. J Comp Neurol 473:59–72
Johansen JA, Breedlove SM, Jordan CL (2007) Androgen receptor expression in the levator ani muscle of male mice. J Neuroendocrinol 19:823–826
Charge SB, Rudnicki MA (2004) Cellular and molecular regulation of muscle regeneration. Physiol Rev 84:209–238
Chen Y, Zajac JD, MacLean HE (2005) Androgen regulation of satellite cell function. J Endocrinol 186:21–31
Sinha-Hikim I, Taylor WE, Gonzalez-Cadavid NF, Zheng W, Bhasin S (2004) Androgen receptor in human skeletal muscle and cultured muscle satellite cells: up-regulation by androgen treatment. J Clin Endocrinol Metab 89:5245–5255
Niel L, Willemsen KR, Volante SN, Monks DA (2008) Sexual dimorphism and androgen regulation of satellite cell population in differentiating rat levator ani muscle. Dev Neurobiol 68:115–122
Matsumoto A, Arai Y, Prins GS (1996) Androgenic regulation of androgen receptor immunoreactivity in motoneurons of the spinal nucleus of the bulbocavernosus of male rats. J Neuroendocrinol 8:553–559
Mauro A (1961) Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol 9:493–495
Montarras D, Morgan J, Collins C, Relaix F, Zaffran S, Cumano A, Partridge T, Buckingham M (2005) Direct isolation of satellite cells for skeletal muscle regeneration. Science 309:2064–2067
Fukada S, Higuchi S, Segawa M, Koda K, Yamamoto Y, Tsujikawa K, Kohama Y, Uezumi A, Imamura M, Miyagoe-Suzuki Y, Takeda S, Yamamoto H (2004) Purification and cell-surface marker characterization of quiescent satellite cells from murine skeletal muscle by a novel monoclonal antibody. Exp Cell Res 296:245–255
Gnocchi VF, White RB, Ono Y, Ellis JA, Zammit PS (2009) Further characterisation of the molecular signature of quiescent and activated mouse muscle satellite cells. PLoS One 4:e5205
Hughes VA, Frontera WR, Roubenoff R, Evans WJ, Singh MA (2002) Longitudinal changes in body composition in older men and women: role of body weight change and physical activity. Am J Clin Nutr 76:473–481
Brooks NE, Schuenke MD, Hikida RS (2009) No change in skeletal muscle satellite cells in young and aging rat soleus muscle. J Physiol Sci 59:465–471
Day K, Shefer G, Shearer A, Yablonka-Reuveni Z (2010) The depletion of skeletal muscle satellite cells with age is concomitant with reduced capacity of single progenitors to produce reserve progeny. Dev Biol 340:330–343
Grounds MD (1998) Age-associated changes in the response of skeletal muscle cells to exercise and regeneration. Ann N Y Acad Sci 854:78–91
Welle S (2002) Cellular and molecular basis of age-related sarcopenia. Can J Appl Physiol 27:19–41
Conboy IM, Conboy MJ, Smythe GM, Rando TA (2003) Notch-mediated restoration of regenerative potential to aged muscle. Science 302:1575–1577
Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA (2005) Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 433:760–764
Filippin LI, Moreira AJ, Marroni NP, Xavier RM (2009) Nitric oxide and repair of skeletal muscle injury. Nitric Oxide 21:157–163
Pedersen BK, Edward F (2009) Adolph distinguished lecture: muscle as an endocrine organ: IL-6 and other myokines. J Appl Physiol 107:1006–1014
Conboy IM, Rando TA (2002) The regulation of Notch signaling controls satellite cell activation and cell fate determination in postnatal myogenesis. Dev Cell 3:397–409
Buas MF, Kadesch T (2010) Regulation of skeletal myogenesis by Notch. Exp Cell Res 316:3028–3033
Tsivitse S (2010) Notch and Wnt signaling, physiological stimuli and postnatal myogenesis. Int J Biol Sci 6:268–281
Sinha-Hikim I, Roth SM, Lee MI, Bhasin S (2003) Testosterone-induced muscle hypertrophy is associated with an increase in satellite cell number in healthy, young men. Am J Physiol Endocrinol Metab 285:E197–E205
Sinha-Hikim I, Cornford M, Gaytan H, Lee ML, Bhasin S (2006) Effects of testosterone supplementation on skeletal muscle fiber hypertrophy and satellite cells in community-dwelling older men. J Clin Endocrinol Metab 91:3024–3033
Doumit ME, Cook DR, Merkel RA (1996) Testosterone up-regulates androgen receptors and decreases differentiation of porcine myogenic satellite cells in vitro. Endocrinology 137:1385–1394
Joubert Y, Tobin C (1989) Satellite cell proliferation and increase in the number of myonuclei induced by testosterone in the levator ani muscle of the adult female rat. Dev Biol 131:550–557
Joubert Y, Tobin C, Lebart MC (1994) Testosterone-induced masculinization of the rat levator ani muscle during puberty. Dev Biol 162:104–110
Mulvaney DR, Marple DN, Merkel RA (1988) Proliferation of skeletal muscle satellite cells after castration and administration of testosterone propionate. Proc Soc Exp Biol Med 188:40–45
Powers ML, Florini JR (1975) A direct effect of testosterone on muscle cells in tissue culture. Endocrinology 97:1043–1047
Diel P, Baadners D, Schlupmann K, Velders M, Schwarz JP (2008) C2C12 myoblastoma cell differentiation and proliferation is stimulated by androgens and associated with a modulation of myostatin and Pax7 expression. J Mol Endocrinol 40:231–241
Chen Y, Lee NK, Zajac JD, MacLean HE (2008) Generation and analysis of an androgen-responsive myoblast cell line indicates that androgens regulate myotube protein accretion. J Endocrinol Invest 31:910–918
Desler MM, Jones SJ, Smith CW, Woods TL (1996) Effects of dexamethasone and anabolic agents on proliferation and protein synthesis and degradation in C2C12 myogenic cells. J Anim Sci 74:1265–1273
Zhang P, Wong C, Liu D, Finegold M, Harper JW, Elledge SJ (1999) p21CIP1 and p57KIP2 control muscle differentiation at the myogenin step. Genes Dev 13:213–224
Schmid C, Steiner T, Froesch ER (1983) Preferential enhancement of myoblast differentiation by insulin-like growth factors (IGF I and IGF II) in primary cultures of chicken embryonic cells. FEBS Lett 161:117–121
Li J, Mayne R, Wu C (1999) A novel muscle-specific beta 1 integrin binding protein (MIBP) that modulates myogenic differentiation. J Cell Biol 147:1391–1398
MacLean HE, Chiu WS, Notini AJ, Axell AM, Davey RA, McManus JF, Ma C, Plant DR, Lynch GS, Zajac JD (2008) Impaired skeletal muscle development and function in male, but not female, genomic androgen receptor knockout mice. FASEB J 22:2676–2689
Wannenes F, Caprio M, Gatta L, Fabbri A, Bonini S, Moretti C (2008) Androgen receptor expression during C2C12 skeletal muscle cell line differentiation. Mol Cell Endocrinol 292:11–19
Kamanga-Sollo E, White ME, Hathaway MR, Weber WJ, Dayton WR (2011) Effect of trenbolone acetate on protein synthesis and degradation rates in fused bovine satellite cell cultures. Domest Anim Endocrinol 40:60–66
Zammit PS, Partridge TA, Yablonka-Reuveni Z (2006) The skeletal muscle satellite cell: the stem cell that came in from the cold. J Histochem Cytochem 54:1177–1191
De Angelis L, Berghella L, Coletta M, Lattanzi L, Zanchi M, Cusella-De Angelis MG, Ponzetto C, Cossu G (1999) Skeletal myogenic progenitors originating from embryonic dorsal aorta coexpress endothelial and myogenic markers and contribute to postnatal muscle growth and regeneration. J Cell Biol 147:869–878
LaBarge MA, Blau HM (2002) Biological progression from adult bone marrow to mononucleate muscle stem cell to multinucleate muscle fiber in response to injury. Cell 111:589–601
Asakura A, Seale P, Girgis-Gabardo A, Rudnicki MA (2002) Myogenic specification of side population cells in skeletal muscle. J Cell Biol 159:123–134
Tamaki T, Okada Y, Uchiyama Y, Tono K, Masuda M, Nitta M, Hoshi A, Akatsuka A (2008) Skeletal muscle-derived CD34 +/45- and CD34-/45- stem cells are situated hierarchically upstream of Pax7 + cells. Stem Cells Dev 17:653–667
Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, Perani L, Innocenzi A, Galvez BG, Messina G, Morosetti R, Li S, Belicchi M, Peretti G, Chamberlain JS, Wright WE, Torrente Y, Ferrari S, Bianco P, Cossu G (2007) Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol 9:255–267
Mitchell KJ, Pannerec A, Cadot B, Parlakian A, Besson V, Gomes ER, Marazzi G, Sassoon DA (2010) Identification and characterization of a non-satellite cell muscle resident progenitor during postnatal development. Nat Cell Biol 12:257–266
Woodhouse LJ, Gupta N, Bhasin M, Singh AB, Ross R, Phillips J, Bhasin S (2004) Dose-dependent effects of testosterone on regional adipose tissue distribution in healthy young men. J Clin Endocrinol Metab 89:718–726
Dati E, Baroncelli GI, Mora S, Russo G, Baldinotti F, Parrini D, Erba P, Simi P, Bertelloni S (2009) Body composition and metabolic profile in women with complete androgen insensitivity syndrome. Sex Dev 3:188–193
Sato T, Matsumoto T, Yamada T, Watanabe T, Kawano H, Kato S (2003) Late onset of obesity in male androgen receptor-deficient (AR KO) mice. Biochem Biophys Res Commun 300:167–171
Lin HY, Xu Q, Yeh S, Wang RS, Sparks JD, Chang C (2005) Insulin and leptin resistance with hyperleptinemia in mice lacking androgen receptor. Diabetes 54:1717–1725
Callewaert F, Venken K, Ophoff J, De Gendt K, Torcasio A, van Lenthe GH, Van Oosterwyck H, Boonen S, Bouillon R, Verhoeven G, Vanderschueren D (2009) Differential regulation of bone and body composition in male mice with combined inactivation of androgen and estrogen receptor-alpha. FASEB J 23:232–240
Ophoff J, Callewaert F, Venken K, De Gendt K, Ohlsson C, Gayan-Ramirez G, Decramer M, Boonen S, Bouillon R, Verhoeven G, Vanderschueren D (2009) Physical activity in the androgen receptor knockout mouse: evidence for reversal of androgen deficiency on cancellous bone. Biochem Biophys Res Commun 378:139–144
Ophoff J, Van Proeyen K, Callewaert F, De Gendt K, De Bock K, Vanden Bosch A, Verhoeven G, Hespel P, Vanderschueren D (2009) Androgen signaling in myocytes contributes to the maintenance of muscle mass and fiber type regulation but not to muscle strength or fatigue. Endocrinology 150:3558–3566
Starkey JD, Yamamoto M, Yamamoto S, Goldhamer DJ (2011) Skeletal muscle satellite cells are committed to myogenesis and do not spontaneously adopt nonmyogenic fates. J Histochem Cytochem 59:33–46
Uezumi A, Fukada S, Yamamoto N, Takeda S, Tsuchida K (2010) Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat Cell Biol 12:143–152
Herbst KL, Bhasin S (2004) Testosterone action on skeletal muscle. Curr Opin Clin Nutr Metab Care 7:271–277
Grounds MD, White JD, Rosenthal N, Bogoyevitch MA (2002) The role of stem cells in skeletal and cardiac muscle repair. J Histochem Cytochem 50:589–610
Jankowski RJ, Deasy BM, Huard J (2002) Muscle-derived stem cells. Gene Ther 9:642–647
Semirale AA, Zhang X, Wiren KM (2011) Body composition changes and inhibition of fat development in vivo implicates androgen in regulation of stem cell lineage allocation. J Cell Biochem 112:1773–1786
Fernando SM, Rao P, Niel L, Chatterjee D, Stagljar M, Monks DA (2010) Myocyte androgen receptors increase metabolic rate and improve body composition by reducing fat mass. Endocrinology 151:3125–3132
Singh R, Artaza JN, Taylor WE, Gonzalez-Cadavid NF, Bhasin S (2003) Androgens stimulate myogenic differentiation and inhibit adipogenesis in C3H 10T1/2 pluripotent cells through an androgen receptor-mediated pathway. Endocrinology 144:5081–5088
Singh R, Bhasin S, Braga M, Artaza JN, Pervin S, Taylor WE, Krishnan V, Sinha SK, Rajavashisth TB, Jasuja R (2009) Regulation of myogenic differentiation by androgens: cross talk between androgen receptor/beta-catenin and follistatin/transforming growth factor-beta signaling pathways. Endocrinology 150:1259–1268
Narici MV, Maffulli N, Maganaris CN (2008) Ageing of human muscles and tendons. Disabil Rehabil 30:1548–1554
Hadi Mansouri S, Siegford JM, Ulibarri C (2003) Early postnatal response of the spinal nucleus of the bulbocavernosus and target muscles to testosterone in male gerbils. Brain Res Dev Brain Res 142:129–139
Fraley GS, Ulibarri CM (2002) Long-term castration effects motoneuron size but not number in the spinal nucleus of the bulbocavernosus in the adult male Mongolian gerbil. Brain Res 953:265–271
Raskin K, de Gendt K, Duittoz A, Liere P, Verhoeven G, Tronche F, Mhaouty-Kodja S (2009) Conditional inactivation of androgen receptor gene in the nervous system: effects on male behavioral and neuroendocrine responses. J Neurosci 29:4461–4470
Fishman RB, Breedlove SM (1988) Neonatal androgen maintains sexually dimorphic muscles in the absence of innervation. Muscle Nerve 11:553–560
Rand MN, Breedlove SM (1992) Androgen locally regulates rat bulbocavernosus and levator ani size. J Neurobiol 23:17–30
Heemers HV, Tindall DJ (2007) Androgen receptor (AR) coregulators: a diversity of functions converging on and regulating the AR transcriptional complex. Endocr Rev 28:778–808
Xu J, Qiu Y, DeMayo FJ, Tsai SY, Tsai MJ, O’Malley BW (1998) Partial hormone resistance in mice with disruption of the steroid receptor coactivator-1 (SRC-1) gene. Science 279:1922–1925
Gehin M, Mark M, Dennefeld C, Dierich A, Gronemeyer H, Chambon P (2002) The function of TIF2/GRIP1 in mouse reproduction is distinct from those of SRC-1 and p/CIP. Mol Cell Biol 22:5923–5937
Ting HJ, Chang C (2008) Actin associated proteins function as androgen receptor coregulators: an implication of androgen receptor’s roles in skeletal muscle. J Steroid Biochem Mol Biol 111:157–163
Wyce A, Bai Y, Nagpal S, Thompson CC (2010) Research resource: the androgen receptor modulates expression of genes with critical roles in muscle development and function. Mol Endocrinol 24:1665–1674
Helsen C, Kerkhofs S, Clinckemalie L, Spans L, Laurent M, Boonen S, Vanderschueren D, Claessens F (2011) Structural basis for nuclear hormone receptor DNA binding. Mol Cell Endocrinol (Epub ahead of print)
Vlahopoulos S, Zimmer WE, Jenster G, Belaguli NS, Balk SP, Brinkmann AO, Lanz RB, Zoumpourlis VC, Schwartz RJ (2005) Recruitment of the androgen receptor via serum response factor facilitates expression of a myogenic gene. J Biol Chem 280:7786–7792
Amir AL, Barua M, McKnight NC, Cheng S, Yuan X, Balk SP (2003) A direct beta-catenin-independent interaction between androgen receptor and T cell factor 4. J Biol Chem 278:30828–30834
Igarashi K, Kashiwagi K (2000) Polyamines: mysterious modulators of cellular functions. Biochem Biophys Res Commun 271:559–564
Turchanowa L, Rogozkin VA, Milovic V, Feldkoren BI, Caspary WF, Stein J (2000) Influence of physical exercise on polyamine synthesis in the rat skeletal muscle. Eur J Clin Invest 30:72–78
Cepero M, Cubria JC, Reguera R, Balana-Fouce R, Ordonez C, Ordonez D (1998) Plasma and muscle polyamine levels in aerobically exercised rats treated with salbutamol. J Pharm Pharmacol 50:1059–1064
Bardocz S, Brown DS, Grant G, Pusztai A, Stewart JC, Palmer RM (1992) Effect of the beta-adrenoceptor agonist clenbuterol and phytohaemagglutinin on growth, protein synthesis and polyamine metabolism of tissues of the rat. Br J Pharmacol 106:476–482
Lee NK, Skinner JP, Zajac JD, Maclean HE (2011) Ornithine decarboxylase is up-regulated by the androgen receptor in skeletal muscle and regulates myoblast proliferation. Am J Physiol Endocrinol Metab 301:E172–E179
Crozat A, Palvimo JJ, Julkunen M, Janne OA (1992) Comparison of androgen regulation of ornithine decarboxylase and S-adenosylmethionine decarboxylase gene expression in rodent kidney and accessory sex organs. Endocrinology 130:1131–1144
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297
Stark A, Brennecke J, Bushati N, Russell RB, Cohen SM (2005) Animal MicroRNAs confer robustness to gene expression and have a significant impact on 3′UTR evolution. Cell 123:1133–1146
Jinek M, Doudna JA (2009) A three-dimensional view of the molecular machinery of RNA interference. Nature 457:405–412
O’Rourke JR, Georges SA, Seay HR, Tapscott SJ, McManus MT, Goldhamer DJ, Swanson MS, Harfe BD (2007) Essential role for Dicer during skeletal muscle development. Dev Biol 311:359–368
Narayanan R, Jiang J, Gusev Y, Jones A, Kearbey JD, Miller DD, Schmittgen TD, Dalton JT (2010) MicroRNAs are mediators of androgen action in prostate and muscle. PLoS One 5:e13637
van Rooij E, Liu N, Olson EN (2008) MicroRNAs flex their muscles. Trends Genet 24:159–166
Williams AH, Liu N, van Rooij E, Olson EN (2009) MicroRNA control of muscle development and disease. Curr Opin Cell Biol 21:461–469
Lai KM, Gonzalez M, Poueymirou WT, Kline WO, Na E, Zlotchenko E, Stitt TN, Economides AN, Yancopoulos GD, Glass DJ (2004) Conditional activation of akt in adult skeletal muscle induces rapid hypertrophy. Mol Cell Biol 24:9295–9304
Glass DJ (2005) Skeletal muscle hypertrophy and atrophy signaling pathways. Int J Biochem Cell Biol 37:1974–1984
Allemand MC, Irving BA, Asmann YW, Klaus KA, Tatpati L, Coddington CC, Nair KS (2009) Effect of testosterone on insulin stimulated IRS1 Ser phosphorylation in primary rat myotubes—a potential model for PCOS-related insulin resistance. PLoS One 4:e4274
Jones A, Hwang DJ, Narayanan R, Miller DD, Dalton JT (2010) Effects of a novel selective androgen receptor modulator on dexamethasone-induced and hypogonadism-induced muscle atrophy. Endocrinology 151:3706–3719
Ibebunjo C, Eash JK, Li C, Ma Q, Glass DJ (2011) Voluntary running, skeletal muscle gene expression, and signaling inversely regulated by orchidectomy and testosterone replacement. Am J Physiol Endocrinol Metab 300:E327–E340
Baron S, Manin M, Beaudoin C, Leotoing L, Communal Y, Veyssiere G, Morel L (2004) Androgen receptor mediates non-genomic activation of phosphatidylinositol 3-OH kinase in androgen-sensitive epithelial cells. J Biol Chem 279:14579–14586
McPherron AC, Lawler AM, Lee SJ (1997) Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 387:83–90
McPherron AC, Lee SJ (1997) Double muscling in cattle due to mutations in the myostatin gene. Proc Natl Acad Sci USA 94:12457–12461
Mosher DS, Quignon P, Bustamante CD, Sutter NB, Mellersh CS, Parker HG, Ostrander EA (2007) A mutation in the myostatin gene increases muscle mass and enhances racing performance in heterozygote dogs. PLoS Genet 3:e79
Reisz-Porszasz S, Bhasin S, Artaza JN, Shen R, Sinha-Hikim I, Hogue A, Fielder TJ, Gonzalez-Cadavid NF (2003) Lower skeletal muscle mass in male transgenic mice with muscle-specific overexpression of myostatin. Am J Physiol Endocrinol Metab 285:E876–E888
Thomas M, Langley B, Berry C, Sharma M, Kirk S, Bass J, Kambadur R (2000) Myostatin, a negative regulator of muscle growth, functions by inhibiting myoblast proliferation. J Biol Chem 275:40235–40243
Taylor WE, Bhasin S, Artaza J, Byhower F, Azam M, Willard DH Jr, Kull FC Jr, Gonzalez-Cadavid N (2001) Myostatin inhibits cell proliferation and protein synthesis in C2C12 muscle cells. Am J Physiol Endocrinol Metab 280:E221–E228
Rios R, Carneiro I, Arce VM, Devesa J (2002) Myostatin is an inhibitor of myogenic differentiation. Am J Physiol Cell Physiol 282:C993–C999
Langley B, Thomas M, Bishop A, Sharma M, Gilmour S, Kambadur R (2002) Myostatin inhibits myoblast differentiation by down-regulating MyoD expression. J Biol Chem 277:49831–49840
Joulia D, Bernardi H, Garandel V, Rabenoelina F, Vernus B, Cabello G (2003) Mechanisms involved in the inhibition of myoblast proliferation and differentiation by myostatin. Exp Cell Res 286:263–275
McFarlane C, Plummer E, Thomas M, Hennebry A, Ashby M, Ling N, Smith H, Sharma M, Kambadur R (2006) Myostatin induces cachexia by activating the ubiquitin proteolytic system through an NF-kappaB-independent, FoxO1-dependent mechanism. J Cell Physiol 209:501–514
Wagner KR, Liu X, Chang X, Allen RE (2005) Muscle regeneration in the prolonged absence of myostatin. Proc Natl Acad Sci USA 102:2519–2524
McCroskery S, Thomas M, Maxwell L, Sharma M, Kambadur R (2003) Myostatin negatively regulates satellite cell activation and self-renewal. J Cell Biol 162:1135–1147
McFarlane C, Hennebry A, Thomas M, Plummer E, Ling N, Sharma M, Kambadur R (2008) Myostatin signals through Pax7 to regulate satellite cell self-renewal. Exp Cell Res 314:317–329
Amthor H, Otto A, Vulin A, Rochat A, Dumonceaux J, Garcia L, Mouisel E, Hourde C, Macharia R, Friedrichs M, Relaix F, Zammit PS, Matsakas A, Patel K, Partridge T (2009) Muscle hypertrophy driven by myostatin blockade does not require stem/precursor-cell activity. Proc Natl Acad Sci USA 106:7479–7484
McPherron AC, Lee SJ (2002) Suppression of body fat accumulation in myostatin-deficient mice. J Clin Invest 109:595–601
Lin J, Arnold HB, Della-Fera MA, Azain MJ, Hartzell DL, Baile CA (2002) Myostatin knockout in mice increases myogenesis and decreases adipogenesis. Biochem Biophys Res Commun 291:701–706
Artaza JN, Bhasin S, Magee TR, Reisz-Porszasz S, Shen R, Groome NP, Meerasahib MF, Gonzalez-Cadavid NF (2005) Myostatin inhibits myogenesis and promotes adipogenesis in C3H 10T(1/2) mesenchymal multipotent cells. Endocrinology 146:3547–3557
Seuntjens E, Umans L, Zwijsen A, Sampaolesi M, Verfaillie CM, Huylebroeck D (2009) Transforming Growth Factor type beta and Smad family signaling in stem cell function. Cytokine Growth Factor Rev 20:449–458
Gilson H, Schakman O, Kalista S, Lause P, Tsuchida K, Thissen JP (2009) Follistatin induces muscle hypertrophy through satellite cell proliferation and inhibition of both myostatin and activin. Am J Physiol Endocrinol Metab 297:E157–E164
Amthor H, Nicholas G, McKinnell I, Kemp CF, Sharma M, Kambadur R, Patel K (2004) Follistatin complexes Myostatin and antagonises Myostatin-mediated inhibition of myogenesis. Dev Biol 270:19–30
Hill JJ, Davies MV, Pearson AA, Wang JH, Hewick RM, Wolfman NM, Qiu Y (2002) The myostatin propeptide and the follistatin-related gene are inhibitory binding proteins of myostatin in normal serum. J Biol Chem 277:40735–40741
Mendler L, Baka Z, Kovacs-Simon A, Dux L (2007) Androgens negatively regulate myostatin expression in an androgen-dependent skeletal muscle. Biochem Biophys Res Commun 361:237–242
Gentile MA, Nantermet PV, Vogel RL, Phillips R, Holder D, Hodor P, Cheng C, Dai H, Freedman LP, Ray WJ (2010) Androgen-mediated improvement of body composition and muscle function involves a novel early transcriptional program including IGF1, mechano growth factor, and induction of {beta}-catenin. J Mol Endocrinol 44:55–73
Zhao JX, Hu J, Zhu MJ, Du M (2011) Trenbolone enhances myogenic differentiation by enhancing beta-catenin signaling in muscle-derived stem cells of cattle. Domest Anim Endocrinol 40:222–229
Ma K, Mallidis C, Artaza J, Taylor W, Gonzalez-Cadavid N, Bhasin S (2001) Characterization of 5′-regulatory region of human myostatin gene: regulation by dexamethasone in vitro. Am J Physiol Endocrinol Metab 281:E1128–E1136
Laviola L, Natalicchio A, Giorgino F (2007) The IGF-I signaling pathway. Curr Pharm Des 13:663–669
Shavlakadze T, Winn N, Rosenthal N, Grounds MD (2005) Reconciling data from transgenic mice that overexpress IGF-I specifically in skeletal muscle. Growth Horm IGF Res 15:4–18
Florini JR, Ewton DZ, Coolican SA (1996) Growth hormone and the insulin-like growth factor system in myogenesis. Endocr Rev 17:481–517
Frost RA, Lang CH (2003) Regulation of insulin-like growth factor-I in skeletal muscle and muscle cells. Minerva Endocrinol 28:53–73
Sacheck JM, Ohtsuka A, McLary SC, Goldberg AL (2004) IGF-I stimulates muscle growth by suppressing protein breakdown and expression of atrophy-related ubiquitin ligases, atrogin-1 and MuRF1. Am J Physiol Endocrinol Metab 287:E591–E601
Hameed M, Lange KH, Andersen JL, Schjerling P, Kjaer M, Harridge SD, Goldspink G (2004) The effect of recombinant human growth hormone and resistance training on IGF-I mRNA expression in the muscles of elderly men. J Physiol 555:231–240
Goldspink G, Harridge SD (2004) Growth factors and muscle ageing. Exp Gerontol 39:1433–1438
Yang SY, Goldspink G (2002) Different roles of the IGF-I Ec peptide (MGF) and mature IGF-I in myoblast proliferation and differentiation. FEBS Lett 522:156–160
Keenan BS, Richards GE, Ponder SW, Dallas JS, Nagamani M, Smith ER (1993) Androgen-stimulated pubertal growth: the effects of testosterone and dihydrotestosterone on growth hormone and insulin-like growth factor-I in the treatment of short stature and delayed puberty. J Clin Endocrinol Metab 76:996–1001
Ulloa-Aguirre A, Blizzard RM, Garcia-Rubi E, Rogol AD, Link K, Christie CM, Johnson ML, Veldhuis JD (1990) Testosterone and oxandrolone, a nonaromatizable androgen, specifically amplify the mass and rate of growth hormone (GH) secreted per burst without altering GH secretory burst duration or frequency or the GH half-life. J Clin Endocrinol Metab 71:846–854
Bhasin S, Woodhouse L, Casaburi R, Singh AB, Bhasin D, Berman N, Chen X, Yarasheski KE, Magliano L, Dzekov C, Dzekov J, Bross R, Phillips J, Sinha-Hikim I, Shen R, Storer TW (2001) Testosterone dose-response relationships in healthy young men. Am J Physiol Endocrinol Metab 281:E1172–E1181
Serra C, Bhasin S, Tangherlini F, Barton ER, Ganno M, Zhang A, Shansky J, Vandenburgh HH, Travison TG, Jasuja R, Morris C (2011) The role of GH and IGF-I in mediating anabolic effects of testosterone on androgen-responsive muscle. Endocrinology 152:193–206
Xu T, Shen Y, Pink H, Triantafillou J, Stimpson SA, Turnbull P, Han B (2004) Phosphorylation of p70s6 kinase is implicated in androgen-induced levator ani muscle anabolism in castrated rats. J Steroid Biochem Mol Biol 92:447–454
Kamanga-Sollo E, Pampusch MS, Xi G, White ME, Hathaway MR, Dayton WR (2004) IGF-I mRNA levels in bovine satellite cell cultures: effects of fusion and anabolic steroid treatment. J Cell Physiol 201:181–189
Wu Y, Zhao W, Zhao J, Pan J, Wu Q, Zhang Y, Bauman WA, Cardozo CP (2007) Identification of androgen response elements in the insulin-like growth factor I upstream promoter. Endocrinology 148:2984–2993
Chambon C, Duteil D, Vignaud A, Ferry A, Messaddeq N, Malivindi R, Kato S, Chambon P, Metzger D (2010) Myocytic androgen receptor controls the strength but not the mass of limb muscles. Proc Natl Acad Sci USA 107:14327–14332
Dardevet D, Sornet C, Vary T, Grizard J (1996) Phosphatidylinositol 3-kinase and p70 s6 kinase participate in the regulation of protein turnover in skeletal muscle by insulin and insulin-like growth factor I. Endocrinology 137:4087–4094
Kim HJ, Lee WJ (2009) Insulin-like growth factor-I induces androgen receptor activation in differentiating C2C12 skeletal muscle cells. Mol Cells 28:189–194
Lee WJ (2009) Insulin-like growth factor-I-induced androgen receptor activation is mediated by the PI3 K/Akt pathway in C2C12 skeletal muscle cells. Mol Cells 28:495–499
Brown D, Hikim AP, Kovacheva EL, Sinha-Hikim I (2009) Mouse model of testosterone-induced muscle fiber hypertrophy: involvement of p38 mitogen-activated protein kinase-mediated Notch signaling. J Endocrinol 201:129–139
Kovacheva EL, Hikim AP, Shen R, Sinha I, Sinha-Hikim I (2010) Testosterone supplementation reverses sarcopenia in aging through regulation of myostatin, c-Jun NH2-terminal kinase, Notch, and Akt signaling pathways. Endocrinology 151:628–638
Bedogni B, Warneke JA, Nickoloff BJ, Giaccia AJ, Powell MB (2008) Notch1 is an effector of Akt and hypoxia in melanoma development. J Clin Invest 118:3660–3670
Wehling M (1997) Specific, nongenomic actions of steroid hormones. Annu Rev Physiol 59:365–393
Cato AC, Nestl A, Mink S (2002) Rapid actions of steroid receptors in cellular signaling pathways. Sci STKE 2002(138): re9
Migliaccio A, Castoria G, Di Domenico M, de Falco A, Bilancio A, Lombardi M, Barone MV, Ametrano D, Zannini MS, Abbondanza C, Auricchio F (2000) Steroid-induced androgen receptor-oestradiol receptor beta-Src complex triggers prostate cancer cell proliferation. EMBO J 19:5406–5417
Kousteni S, Bellido T, Plotkin LI, O’Brien CA, Bodenner DL, Han L, Han K, DiGregorio GB, Katzenellenbogen JA, Katzenellenbogen BS, Roberson PK, Weinstein RS, Jilka RL, Manolagas SC (2001) Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell 104:719–730
Nakhla AM, Rosner W (1996) Stimulation of prostate cancer growth by androgens and estrogens through the intermediacy of sex hormone-binding globulin. Endocrinology 137:4126–4129
Kampa M, Papakonstanti EA, Hatzoglou A, Stathopoulos EN, Stournaras C, Castanas E (2002) The human prostate cancer cell line LNCaP bears functional membrane testosterone receptors that increase PSA secretion and modify actin cytoskeleton. FASEB J 16:1429–1431
Benten WP, Lieberherr M, Giese G, Wrehlke C, Stamm O, Sekeris CE, Mossmann H, Wunderlich F (1999) Functional testosterone receptors in plasma membranes of T cells. FASEB J 13:123–133
Yeh S, Lin HK, Kang HY, Thin TH, Lin MF, Chang C (1999) From HER2/Neu signal cascade to androgen receptor and its coactivators: a novel pathway by induction of androgen target genes through MAP kinase in prostate cancer cells. Proc Natl Acad Sci USA 96:5458–5463
Lopez GN, Turck CW, Schaufele F, Stallcup MR, Kushner PJ (2001) Growth factors signal to steroid receptors through mitogen-activated protein kinase regulation of p160 coactivator activity. J Biol Chem 276:22177–22182
Schneider MD, Olson EN (1988) Control of myogenic differentiation by cellular oncogenes. Mol Neurobiol 2:1–39
Falcone G, Alema S, Tato F (1991) Transcription of muscle-specific genes is repressed by reactivation of pp60v-src in postmitotic quail myotubes. Mol Cell Biol 11:3331–3338
Hamdi MM, Mutungi G (2010) Dihydrotestosterone activates the MAPK pathway and modulates maximum isometric force through the EGF receptor in isolated intact mouse skeletal muscle fibres. J Physiol 588:511–525
Siiteri PK, Murai JT, Hammond GL, Nisker JA, Raymoure WJ, Kuhn RW (1982) The serum transport of steroid hormones. Recent Prog Horm Res 38:457–510
Krupenko SA, Krupenko NI, Danzo BJ (1994) Interaction of sex hormone-binding globulin with plasma membranes from the rat epididymis and other tissues. J Steroid Biochem Mol Biol 51:115–124
Nazareth LV, Weigel NL (1996) Activation of the human androgen receptor through a protein kinase A signaling pathway. J Biol Chem 271:19900–19907
Sadar MD (1999) Androgen-independent induction of prostate-specific antigen gene expression via cross-talk between the androgen receptor and protein kinase A signal transduction pathways. J Biol Chem 274:7777–7783
Fortunati N (1999) Sex hormone-binding globulin: not only a transport protein. What news is around the corner? J Endocrinol Invest 22:223–234
Rosner W, Hryb DJ, Kahn SM, Nakhla AM, Romas NA (2010) Interactions of sex hormone-binding globulin with target cells. Mol Cell Endocrinol 316:79–85
Benten WP, Lieberherr M, Stamm O, Wrehlke C, Guo Z, Wunderlich F (1999) Testosterone signaling through internalizable surface receptors in androgen receptor-free macrophages. Mol Biol Cell 10:3113–3123
Estrada M, Espinosa A, Muller M, Jaimovich E (2003) Testosterone stimulates intracellular calcium release and mitogen-activated protein kinases via a G protein-coupled receptor in skeletal muscle cells. Endocrinology 144:3586–3597
Mellstrom B, Naranjo JR (2001) Mechanisms of Ca(2 +)-dependent transcription. Curr Opin Neurobiol 11:312–319
Fu R, Liu J, Fan J, Li R, Li D, Yin J, Cui S (2011) Novel evidence that testosterone promotes cell proliferation and differentiation via G protein-coupled receptors in the rat L6 skeletal muscle myoblast cell line. J Cell Physiol 227:98–107
Thum T, Springer J (2011) Breakthrough in cachexia treatment through a novel selective androgen receptor modulator?! J Cachex Sarcopenia Muscle 2:121–123
Whittemore LA, Song K, Li X, Aghajanian J, Davies M, Girgenrath S, Hill JJ, Jalenak M, Kelley P, Knight A, Maylor R, O’Hara D, Pearson A, Quazi A, Ryerson S, Tan XY, Tomkinson KN, Veldman GM, Widom A, Wright JF, Wudyka S, Zhao L, Wolfman NM (2003) Inhibition of myostatin in adult mice increases skeletal muscle mass and strength. Biochem Biophys Res Commun 300:965–971
Rose FF Jr, Mattis VB, Rindt H, Lorson CL (2009) Delivery of recombinant follistatin lessens disease severity in a mouse model of spinal muscular atrophy. Hum Mol Genet 18:997–1005
Mohler ML, Bohl CE, Jones A, Coss CC, Narayanan R, He Y, Hwang DJ, Dalton JT, Miller DD (2009) Nonsteroidal selective androgen receptor modulators (SARMs): dissociating the anabolic and androgenic activities of the androgen receptor for therapeutic benefit. J Med Chem 52:3597–3617
Wright AS, Douglas RC, Thomas LN, Lazier CB, Rittmaster RS (1999) Androgen-induced regrowth in the castrated rat ventral prostate: role of 5alpha-reductase. Endocrinology 140:4509–4515
Kazmin D, Prytkova T, Cook CE, Wolfinger R, Chu TM, Beratan D, Norris JD, Chang CY, McDonnell DP (2006) Linking ligand-induced alterations in androgen receptor structure to differential gene expression: a first step in the rational design of selective androgen receptor modulators. Mol Endocrinol 20:1201–1217
Narayanan R, Coss CC, Yepuru M, Kearbey JD, Miller DD, Dalton JT (2008) Steroidal androgens and nonsteroidal, tissue-selective androgen receptor modulator, S-22, regulate androgen receptor function through distinct genomic and nongenomic signaling pathways. Mol Endocrinol 22:2448–2465
Dalton JT, Barnette KG, Bohl CE, Hancock ML, Rodriguez D, Dodson ST, Morton RA, Steiner MS (2011) The selective androgen receptor modulator GTx-024 (enobosarm) improves lean body mass and physical function in healthy elderly men and postmenopausal women: results of a double-blind, placebo-controlled phase II trial. J Cachex Sarcopenia Muscle 2:153–161
Acknowledgments
We thank our colleagues of the Molecular Endocrinology Laboratory for many helpful discussions. V. Dubois is holder of a doctoral fellowship of the Fund for Scientific Research Flanders (FWO). S. Boonen and D. Vanderschueren are, respectively, senior clinical investigators of the Fund for Scientific Research Flanders (FWO) and the University hospital of Leuven. S. Boonen is holder of the Leuven University Chair in Gerontology and Geriatrics.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dubois, V., Laurent, M., Boonen, S. et al. Androgens and skeletal muscle: cellular and molecular action mechanisms underlying the anabolic actions. Cell. Mol. Life Sci. 69, 1651–1667 (2012). https://doi.org/10.1007/s00018-011-0883-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-011-0883-3