Abstract
Background
Dysfunctional immune regulation plays a crucial role in the pathogenesis of airway allergies. Macrophages are one of the components of the immune regulation cells. The aim of this study is to elucidate the role of lysine demethylase 5 A (KDM5A) in maintaining macrophages’ immune regulatory ability.
Methods
DNA was extracted from Lactobacillus rhamnosus GG to be designated as LgDNA. LgDNA was administered to the mice through nasal instillations. M2 macrophages (M2 cells) were isolated from the airway tissues using flow cytometry.
Results
We found that airway M2 cells of mice with airway Th2 polarization had reduced amounts of IL-10 and KDM5A. Mice with Kdm5a deficiency in M2 cells showed the airway Th2 polarization. The expression of Kdm5a in airway M2 cells was enhanced by nasal instillations containing LgDNA. KDM5A mediated the effects of LgDNA on inducing the Il10 expression in airway M2 cells. Administration of LgDNA mitigated experimental airway allergy.
Conclusions
M2 macrophages in the airway tissues of mice with airway allergy show low levels of KDM5A. By upregulating KDM5A expression, LgDNA can increase Il10 expression and reconcile airway Th2 polarization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Airway Th2 polarization is the canonical pathological feature of airway allergy (AA), which is an adverse reaction to the harmless airborne antigens by the immune system in the airway tissues [1]. The mechanism is unclear [2]. AA mainly includes allergic rhinitis and allergic asthma [3]. AA attacks have a paroxysmal nature. In the early stages, the attack of airway allergy is self-limiting. In advanced stages, medical help is needed to stop airway allergy attacks [1]. If AA is not properly managed, complications may arise. [1]. Symptom control is the main focus of current treatment for airway allergies. Allergen specific immunotherapy is an etiology targeting therapy for AA. However, its effectiveness needs to be improved [4]. In fact, airway allergies have had a significant negative impact on human health. The mechanism of AA needs to be further investigated and new remedies developed for its treatment.
The basic pathological feature of AA is the Th2 polarization in the airway tissues [5, 6]. Th2 polarization indicates that a large number of Th2 cells aggregate in the local tissue to produce more than needed amounts of Th2 cells [7]. Th2 cytokines facilitate the development of plasma cells and the production of IgE. IgE sensitizes mast cells. Re-exposure to specific antigen induces the sensitized mast cells to release allergic mediators to evoke allergy attacks [8]. The immune regulatory system is responsible for tightly regulating immune reactions in the body. The Th2 polarization is a sign that the immune regulatory system in the local tissue is not functioning properly [5, 6].
The immune regulatory system consists of several cell fractions. Regulatory T cells, regulatory B cells, and M2 macrophages are recognized having immune regulatory functions [9,10,11]. The dysfunctional M2 cells in AA subjects have been reported [11]. Insufficient production of IL-10, a cytokine that regulates immune response, was observed in M2 cells of asthma patients [11]. There is still a need to investigate the underlying mechanism further.
Probiotics have been used as supplements to improve health conditions or as joint treatment alongside other remedies [12]. Probiotics are beneficial microbes that reside in the body and contribute to overall health. The mediators in probiotics that enhance immune functions are not fully comprehended yet. The current method of administration for live probiotics is through ingestion [13]. Some of these bacteria, if not all, may die in the digestive tract, especially during passage through the harsh environment of the stomach. DNA is then released from dead probiotics. It is well known that DNA can activate Toll like receptor 9 (TLR9) to modulate targeted cell functions [14]. Whether probiotic DNA regulates immune functions to affect the ongoing inflammatory response has not yet been fully understood.
In this study, we found that airway M2 cells from AA mice showed lower levels of Lysine Demethylase 5 A (KDM5A). KDM5A plays a crucial role in gene transcription in many cases [15]. We hypothesize that upregulating KDM5A may improve the immune regulatory environment in the airways, and consequently reconcile airway Th2 polarization. To test the hypothesis, we treated mice with LgDNA, the DAN from probiotics, Lactobacillus rhamnosus GG. This remedy increased the expression of KDM5A and IL-10 in M2 cells, and thus, mitigated experimental airway Th2 polarization and airway allergy.
Materials and methods
Reagents
Antibodies (Abs) of F4/80 (Cat#sc-52664, fluorochrome: AF488), arginase (sc-271430, AF546), CD206 (sc-58986, AF594), IL-10 (sc-57245, AF648), KDM5A (sc-365993, AF680), H3K9me3 (sc-130356) and Pol II (sc-47701) were bought from Santa Cruz Biotech (Santa Cruz, CA). A neutralizing anti-IL-10 Ab was purchased from abcam (AF519). ELISA kits of IL-4, IL-5, IL-9, IL-13, KDM5A, IL-10, sIgE, EPX and Mcpt1 were bought from Dakewe BioMart (Shenzhen, China).
Mice
Male C57/B6 mice were acquired from the Beijing Experimental Animal Center in Beijing, China. B6.129S6(Cg)-Kdm5atm1Kael/J mice, Tg (Nes-cre)2Wme/J mice and MafBCre mice were brought from Jackson Laboratory (Bar Harbor, ME). B6.129S6 (Cg)-Kdm5atm1Kael/J mice were crossed with Tg (Nes-cre)2Wme/J mice to obtain the Kdm5af/f mice. Kdm5af/fMafBCre mice were developed in-house by crossing Kdm5af/f mice with MafBCre mice. The mice were used in experiments after 5 generations. The Cre-loxP system in mice was activated by feeding them with tamoxifen (75 mg/Kg in 0.3 ml corn oil) daily for 5 days prior to the experiment. Mice were kept in an environment that was free of pathogens. They were granted free access to food and water. The animal experiment protocol was approved by the Animal Ethics Committee at Shanxi Medical University (Approval#A2023006). All animal experiments were carried out in accordance with ARRIVAL’s guidelines.
Preparation of LgDNA, SaDNA and EpiDNA
DNA was extracted from Lactobacillus rhamnosus (LGG) or Staphylococcus aureus or mouse epithelial cells (isolated from the mouse lungs) using a DNA extraction kit (Qiagen) following the protocol provided by the manufacturer. The DNA was designated as LgDNA (from LGG) or SaDNA (from S. aureus). The DNA was quantified using a ThermoFisher Qubit fluorescence spectrometer.
Establishment of an airway allergy mouse model
Mice were sensitized by subcutaneous injection with dust mite extracts (DME; 0.1 mg/mouse in 0.1 ml alum) on the back skin on day 1 and day7, respectively. From day 9 to day 22, mice received nasal instillation (20 µl/nostril, containing 5 mg DME/ml) daily. On day 23, mice received nasal instillation (20 µl/nostril, containing 50 mg DME/ml). After cervical dislocation, 1 ml of saline was introduced into the lung through the trachea, or the nasal cavity was rinsed with 1 ml of saline. Instantly, the lavage fluid was retrieved and used as either bronchoalveolar lavage fluid (BALF) or nasal lavage fluid (NLF). BALF or NLF were analyzed using ELISA to determine the quantity of Th2 cytokines (IL-4, IL-5, IL-9, and IL-13).
Treating mice with LgDNA
Naïve mice, or AA mice (one day after the completion of sensitization) were treated with LgDNA through nasal instillation (20 µl/nostril, containing LgDNA 1 mg/ml) daily in the period of sensitization. Mice were used for further experiments.
Flow cytometry
Cells were stained with fluorescence labeled Abs of interest (Ab types are detailed in figures) or isotype IgG for 30 min at 4 °C. If staining intracellular molecules, cells were fixed with 1% paraformaldehyde (containing 0.05% Triton X-100) first, then stained with Abs. Using a flow cytometer (BD FACSCanto II), the number of positively stained cells was counted. The data was processed using Flowjo software package. The data generated by isotype IgG staining were used as gating references.
Preparation of single cells from the airway tissues
The lungs were excised from mice upon the sacrifice and cut into small pieces. Collagenase IV (0.5 mg/ml) was used to incubate the samples at 37 °C for 30 min with mild agitation to separate cells from the tissues. Single cells were passed through a cell strainer, and collected by centrifugation at 500 ×g for 5 min. The cells were used in further experiments.
Isolation of immune cells
Single cells were prepared from the airway tissues as described above, and labeled with fluorescence-conjugated Abs. Cells of interest (detailed in figures) were purified by flow cytometry cell sorting. The cell purity was assessed by flow cytometry. If purity did not reach or exceed 90%, the purification procedures were repeated.
Cell culture
RPMI1640 medium was used to culture immune cells. Antibiotics (penicillin and streptomycin), L-glutamine and fetal calf serum. The Trypan blue exclusion assay showed that the cell viability was 98–99%.
Enzyme-linked immunosorbent assay (ELISA)
Cytokines in BALF, culture supernatant, and cell extracts were measured by ELISA using commercial kits following the manufacturer’s protocol.
Chromatin immunoprecipitation (ChIP)
Cells were fixed with 1% formalin for 15 min. The cells were lysed with a lysis buffer, and then the DNA was sheared into small pieces by sonication. Protein G agarose beads were used to clear the pre-existing immune complexes in the samples. The beads were discarded. The supernatants were incubated overnight with Abs of interest (detailed in figures). Immune complexes in samples were adsorbed by protein G agarose beads, which were eluted using an eluting buffer. DNA was extracted from the samples using a DNA recovery kit (Qiagen) and analyzed by qPCR in the presence of a pair of primers for the Il10 promoter (ccggggagtgtacccctctaca and tcagtttgggtgggaagaac). ELISA was used to quantify proteins in the samples. The results are presented as fold changes compared to the input.
Real-time quantitative RT-PCR (RT-qPCR)
Cells collected in relevant experiments were used to extract RNA samples. Using a reverse transcription kit (Qiagen), cDNA was synthesized from the RNA samples according to the manufacturer’s protocol. The cDNA samples were amplified in a qPCR device (Bio Rad CFX96) using a SYBR green master mix kit in the presence of primers of Kdm5a (cctggcagtaggagcaaaag and cgaccacaaaacatgcaaac) or Il10 (ccaagccttatcggaaatga and ttttcacaggggagaaatcg). We used a formula (2−ΔΔCt) to process the results, which were expressed as RE (relative expression) against Actb (agccatgtacgtagccatcc and ctctcagctgtggtggtgaa), the housekeeping gene.
Il10 gene promoter demethylation analysis by methylation-specific PCR
DNA (1 µg/sample) extracted from M2 cells was used for bisulfite treatment using an EZ DNA Methylation kit (Zymo Research, Orange, CA) based on the manufacturer’s protocol.
Histology of the lung tissues
A piece of the lung tissues was excised from mice upon the sacrifice. The tissue was fixed in 4% formalin overnight, and processed for paraffin sections. The sections were stained with hematoxylin and eosin, and observed using a light microscope.
Modulation of the expression of IL-10 and KDM5A in airway M2 cells
Mice received nasal instillations (containing 10 µg/ml LPS) daily for 5 days. Single cells were prepared from airway tissues, and analyzed by flow cytometry. Furthermore, M2 cells were isolated from the airway single cells. The cellular extracts of M2 cells were analyzed by RT-qPCR and ELISA. On the other hand, Raw264.7 cells were exposed to LPS (10 µg/ml) or IL-4 (0.1 µg/ml) in culture for 24 h. RNA was extracted from the cells and analyzed by RT-qPCR to quantify the Il10 mRNA in the cells.
Statistical analysis
Student’s t-test was used to determine the difference between data collected from two groups. ANOVA followed by Bonferroni test or Dunnett’s test was used to determine differences among multiple groups. p < 0.05 was the significant criterion.
Results
Low levels of KDM5A in airway M2 cells of mice with airway Th2 polarization
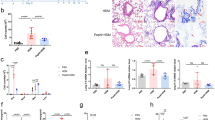
An airway allergy (AA) mouse model was established. Bronchoalveolar lavage fluid (BALF) was collected from mice, which showed a feature of Th2 polarization (Fig. 1A-D). BALF’s cellular components were examined using flow cytometry. Approximately 30% of tall cells in BALF were occupied by macrophages (Fig. 1E-H). M2 cells were more abundant in the BALF of AA mice than in naive control (NC) mice (Fig. 1I-J). NC M2 cells showed a stronger staining of IL-10 compared to AA M2 cells (Fig. 1K). M2 cells were isolated. Protein extracts of M2 cells were analyzed by ELISA. We found that the amounts of KDM5A were much less in the AA group than in the NC group (Fig. 1L). The results indicate that airway M2 cells of AA mice express a reduced amount of KDM5A, which may be associated with the pathogenesis of AA.
Airway M2 cells of mice with AA show low amounts of IL-10 and KDM5A. An airway allergy (AA) mouse model was established. (A-D), bars show Th2 cytokine amounts in BALF. E-K, BALF cells were analyzed by flow cytometry. E, dead cells were gated out. (F), adhesive cells were gated out. (G-H), flow cytometry plots show macrophages; bars show macrophage counts. (I-J), gated cells are M2 cell proportion in macrophages; bars indicate M2 cell counts. (K), bars show IL-10 MFI in M2 cells. L, M2 cells were isolated. Protein extracts of M2 cells were analyzed by ELISA. Bars show KDM5A protein in M2 cell extracts. NC: Naïve control. ViD: An amine active fluorescence dye used to stain dead cells. MFI: Median fluorescence intensity
Inhibition of KDM5A impairs the induction of IL-10 in macrophages
Kdm5af/fMafBCre mice and control mice (Kdm5af/f) were employed in the present study. The deficiency of Kdm5a expression in airway macrophages was verified by RT-qPCR (Fig. 2A). The mice were treated with LPS-containing nasal instillations daily for 5 days. Single cells were prepared from the excised lungs after the sacrifice. As assessed by flow cytometry, macrophages from NC and Kdm5af/f mice showed more IL-10+ macrophages than those from Kdm5af/fMafBCre mice (Fig. 2B-C). The results were verified using RAW264.7 cell line. The expression of Kdm5a was knocked down in the cells (Fig. 2D). The cells were stimulated using LPS or IL-4 in culture. We found that the knockdown of Kdm5a resulted in lower expression of IL-10 in the cells (Fig. 2E-F). The results indicate that KDM5A plays an important role in the induction of IL-10 in airway macrophages.
Assessment of the role of KDM5A in the induction of IL-10 in airway M2 cells. (A), bars show the amount of Kdm5a mRNA in M2 cells isolated from Kdm5af/fMafBCre mice (c) and Kdm5af/f mice (d; control mice). (B-C), mice were treated with LPS-containing nasal instillations daily for 5 days. M2 cells were isolated from the lungs. Gated plots indicate IL-10+ M2 cells. Bars show the counts of IL-10+ M2 cells. D-F, bars show amounts of Kdm5a mRNA (D) and Il10 (E, F) in Raw264.7 cells. The data of bars are presented as mean ± SD. Each dot in bars presents one sample. Statistics: Student’s t-test (A) and ANOVA followed by Bonferroni test. **p < 0.01; ***p < 0.001; ****p < 0.0001. NC: Naïve control. RNAi: Kdm5a RNA interference. cRNAi: Control RNAi. LPS: 10 µg/ml in nasal instillation or in culture medium. IL-4: 0.1 µg/ml in culture medium
Inhibition of KDM5A in macrophages results in Th2 polarization in the airways
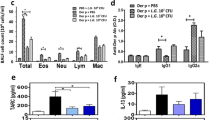
Kdm5af/fMafBCre mice were employed in the present study. High quantity of Th2 cytokines and low quantity of IL-10 were detected in BALF of Kdm5af/fMafBCre mice as compared to those of Kdm5af/f mice (Fig. 3A-E). A negative correlation was detected between IL-10 and Th2 cytokines in BALF (Fig. 3F-I). The results indicate that depletion of the Kdm5a expression in macrophages can decrease IL-10 and induce Th2 polarization in the airways.
Depletion of Kdm5a expression results in airway Th2 polarization. BALF was collected from NC mice, Kdm5af/fMafBCre mice and Kdm5af/f mice, and analyzed using ELISA. (A-E), Bars show mean ± SD of the amounts of Th2 cytokine amounts and IL-10 from 6 mice per group. (F-I), correlation between the amounts of IL-10 and Th2 cytokine amounts in BALF. Statistics: ANOVA followed by Bonferroni test (A-E) and Pearson correlation coefficient test (F-I). *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001. Each dot in bars presents one sample
LgDNA increases the expression of KDM5A in airway M2 cells
Mice received LgDNA (or SaDNA)-containing nasal instillation daily for 5 days. After the sacrifice, single cells were prepared from the lung tissues. The cells were analyzed using flow cytometry. The frequency of KDM5A M2 cells was significantly enhanced after LgDNA treatment, but not after SaDNA treatment (Fig. 4A-D). The administration of inhibitors of either TLR9 (TLR9-IN-1) or MyD88 (ST 2825) abolished the effects of LgDNA on enhancing KDM5A in M2 cells (Fig. 4A-D). We then isolated M2 cells from the single cells. The cells were analyzed by RT-qPCR and ELISA. The results also showed that the expression of KDM5A was upregulated by the treatment with LgDNA (Fig. 4E-F). The data were verified by treating RAW264.7 cells (ATCC) with LgDNA in culture. LgDNA increased the expression of KDM5A in the cells in a dose-dependent manner (Fig. 4G-H). The results demonstrate that LgDNA can enhance the expression of KDM5A in M2 cells via activating the TLR9-MyD88 signaling pathway.
LgDNA induces the expression of Kdm5a in airway M2 cells. Mice received LgDNA-containing nasal instillations daily for 5 days (the treatment is denoted on panel D). Single cells were prepared from the lungs and analyzed using flow cytometry. (A), macrophages were gated. (B), M2 cells were gated from total macrophages, from which KDM5A+ M2 cells were counted (C, D, E), M2 cells were isolated and analyzed by RT-qPCR. Bars show the amount of Kdm5a mRNA in M2 cells. The group labels of (D) and (E) are the same as C. (G-H), RAW264.7 cells were exposed to LgDNA in culture for 48 h. The lines show the amounts of Kdm5a mRNA (G) and KDM5A protein (H) in RAW264.7 cells. The data of bars are presented as mean ± SD. Each dot in bars presents one sample. Statistics: ANOVA followed by Bonferroni test. **p < 0.01; ****p < 0.0001. SaDNA: DNA extracted from S. aureus. EpiDNA: DNA was extracted from mouse airway epithelial cells. TLR9-IN-1: An inhibitor of TLR9 (10 nM). ST 2825: An inhibitor of MyD88 (10 µM)
KDM5A mediates the effects of LgDNA on increasing IL-10 expression in airway M2 cells
Mice received nasal instillations (containing LgDNA) daily for 5 days. M2 cells were isolated from the airway tissues, and analyzed by ChIP, RT-qPCR, and ELISA. We found that KDM5A was localized in the Il10 promoter locus, which was significantly increased following the exposure to LgDNA (Fig. 5A). The amount of H3K9me3 in the Il10 promoter was down regulated by LgDNA (Fig. 5B). Elevated demethylation was observed in the Il10 promoter (Fig. 5C). LgDNA led to an increase in RNA polymerase II (Pol II), which is an indicator of gene transcription, in the Il10 promoter (Fig. 5D). Exposure to LgDNA resulted in an increase in the amounts of Il10 mRNA and IL-10 protein in M2 cells (Fig. 5E-F). On the other hand, ablation of the Kdm5a expression in macrophages (Fig. 5G) abolished LgDNA-induced demethylation of Il10 promoter, thereby facilitating the transcription and expression of Il10 in M2 cells (Fig. 5C-F). The results indicate KDM5A is associated with the LgDNA-increased IL-10 production in airway M2 cells. Additionally, we also found that exposure to LgDNA in culture did not apparently enhance the mRNA levels of Kdm5b, Kdm5c, and Kdm5d in macrophages (Fig. 5H-K).
KDM5A mediates the effects of LgDNA on enhancing Il10 expression in airway M2 cells. Mice were treated with LgDNA-containing nasal instillations daily for 5 days. M2 cells were isolated from the lung tissues, and analyzed by ChIP, RT-qPCR, and ELISA. (A), the amount of KDM5A in the Il10 promoter. (B), the amount of H3k9me3 in the Il10 promoter. (C), the changes of Il10 promoter demethylation. D, the amount of Pol II in the Il10 promoter. E-F, the amounts of Il10 mRNA (E) and IL-10 protein (F) in extracts of M2 cells. (G), Kdm5a mRNA amount in M2 cells of indicated mice. H-K, macrophages were isolated from the airway tissues, and exposed to LgDNA (10 µg/ml) in culture for 24 h. The bars show the mRNA levels of indicated molecules in macrophages. The data of bars are presented as mean ± SD. Each dot in bars presents one sample. Statistics: ANOVA followed by Bonferroni test (A-G) or Student’s t-test (H-K). **p < 0.01; ***p < 0.001; ****p < 0.0001; ns: Not significant. NC: Naïve control mice (C57/B6). KO: Kdm5af/fMafBCre mice. cKO: Kdm5af/f mice (control mice)
Administration of LgDNA mitigates experimental airway allergy
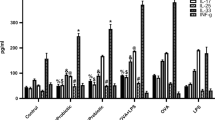
A mouse model of airway allergy (AA) was established. Inflammation in the lungs (Fig. 6A-C), clinical signs of allergic rhinitis (Fig. 6D-E), infiltration of inflammatory cells in the airway (Fig. 6F-H), elevated amounts of specific IgE (Fig. 6I), allergic mediators (EPX and Mcpt1, Fig. 6J-K), and Th2 cytokines (IL-4, IL-5, and IL-13, Fig. 6L-N) in nasal lavage fluid (NLF) and bronchoalveolar lavage fluid (BALF) were observed in AA mice. Administration of LgDNA through nasal instillations in the period of sensitization significantly suppressed the AA response. The results demonstrate that LgDNA can efficiently suppress the AA response.
Administration of LgDNA mitigates airway allergy (AA). Mice (Mouse types are denoted on the X axis) were treated with LgDNA through nasal instillations daily for 5 days. BALF was collected from each mouse, and analyzed using ELISA. (A-C), representative histology images (×200) of the lung from AA mice. The bar graphs of panels (D-N) show mean ± SD of indicated items in Y axis from 6 mice per group. Each dot in bars presents one sample. Statistics: ANOVA followed by Bonferroni test. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001. NC: Naïve control mice (C57/B6). AA: Mice with airway allergy. !: Mice receiving LgDNA-containing nasal instillations in the period of sensitization. NLF: Nasal lavage fluid. BALF: Bronchoalveolar lavage fluid
Discussion
The present data show that administration of LgDNA can reconcile airway Th2 polarization by upregulating KDM5A in airway M2 cells. KDM5A enhances histone demethylation in the Il10 promoter, and promotes the IL-10 production in M2 cells in the airways. Experimental AA can be mitigated by administering LgDNA.
The data indicate that LgDNA has the ability to control the skewed Th2 polarization in the airways. LgDNA is extracted from a probiotic strain, Lactobacillus rhamnosus GG (LGG). This probiotic strain has been used to regulate immune response in the body. LGG administration has been reported to prevent the establishment of AA with birch pollen [16]. LGG can stimulate the production of non-specific IgG, IgA, and IgM, and maintain the intestinal epithelial barrier functions [17]. These data indicate that the immune regulatory functions of this probiotic strain have been recognized. The mediators of this probiotic strain are yet to be fully understood.
According to published data, DNA plays a role in immune regulation [18, 19]. One example is CpG. By binding to Toll-like receptor 9 (TLR9), CpG activates the MyD88-IRF7-NF-κB signaling pathway to initiate immune responses [20]. CpG has been used in immunotherapy for immune diseases [21]. In general, probiotics are administered through oral ingestion. There is a chance that some of them, if not all, may die in the digestive tract. The bacterial DNA can be released from the dead probiotics. By interaction with TLR9, DNA can regulate targeting cell activity [18, 19]. Our data show that exposure to LgDNA can regulate the immune response by increasing the expression of KDM5A in airway M2 cells.
As per the data, LgDNA enhances the expression of KDM5A in airway M2 cells. KDM5A is a demethylase. It is involved in the gene transcription of many genes in cancer [22, 23]. Published data indicate low expression of IL-10 in airway M2 cells [24]. These macrophages are incompetent in suppressing other immune cell activities [11]. Restoration of the expression of IL-10 can restore the immune regulatory ability of M2 cells [11]. The expression of KDM5A can be enhanced by LgDNA, which in turn increases the expression of IL-10 in airway M2 cells, according to current data. These data indicate that LgDNA can be a useful agent to be used in immunotherapy for diseases with dysfunctional immune regulation.
Restoring immune regulatory functions is a promising remedy for the treatment of allergic diseases. By up-regulating functions in immune regulatory cells, skewed Th2 polarization can be resolved. It is recognized that IL-10 is the canonical immune regulatory cytokine [25, 26]. The production of IL-10 by M2 cells is crucial for their immune regulatory function [27]. The amount of IL-10 in airway macrophages is negatively correlated with the allergic response in the airways [11]. Restoration of the expression of IL-10 in M2 cells can migrate the allergic inflammation in the airways [11]. Thus, our data demonstrate that LgDNA has the translation potential to be developed into a drug to be used in the treatment of airway allergy.
The demethylase KDM5 family has several members. Our data show that exposure to LgDNA mainly increases the expression of KDM5A, but not KDM5B, KDM5C, or KDM1B, in macrophages. There are many more demethylases have been identified, which play a variety of roles in regulation of a variety of organs, cells, or biochemical reactions [28]. It would be beneficial to investigate if LgDNA also influences the expression of other demethylases in future studies.
The data show that administration of LgDNA through nasal instillations resulted in upregulating the expression of IL-10 in M2 cells. This event reveals that after reaching the airway tissues, LgDNA contacts macrophages to regulate their activities. In fact, LgDNA also contacts other cells in the airway tissues. Whether LgDNA also modulates other immune cell activities, or whether it influences other cells’ activities to indirectly modulate macrophage activities is an interesting topic to be further investigated.
Probiotics are generally given through oral ingestion. LGG has been used as a supplement in the treatment of AA in animal studies [29] and patients with AA [30]. The use of probiotic DNA in nasal instillations requires more manufacturer processes and higher costs than oral ingestion. There is reports to introduce probiotic bacteria through nasal instillations to alleviate experimental AA [16]. It is necessary to conduct more research to compare the therapeutic effects and possible harmful risks of employing probiotic DNA and live probiotics via nasal instillations.
The data indicate that exposure to LgDNA improves the expression of IL-10 in M2 cells. As a consequence, the M2 cells help regulate inflammation, repair tissue, remodel tissues, and maintain homeostasis [31]. However, it is also reported that IL-10 facilitates the development of inflammation related to Th2 polarity. In such an instance, IL-10 inhibits IFN-γ and therefore promotes Th2 polarization [32]. It seems that IL-10 can suppress IFN-γ [32] or IL-4 depending on some unknown factors. The latter needs to be further investigated.
In summary, probiotic Lactobacillus rhamnosus-derived LgDNA can reconcile skewed Th2 polarization in the airways by up regulation of KDM5A and IL-10 expression in M2 cells.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- M2 cells:
-
M2 macrophages
- AA:
-
Airway allergy
- TLR9:
-
Toll like receptor 9
- KDM5A:
-
Lysine demethylase 5 A
- LGG:
-
Lactobacillus rhamnosus
- DME:
-
Dust mite extracts
- BALF:
-
Bronchoalveolar lavage fluid
- NLF:
-
Nasal lavage fluid
- ELISA:
-
Enzyme-linked immunosorbent assay
- ChIP:
-
Chromatin immunoprecipitation
- RT-qPCR:
-
Real-time quantitative RT-PCR
- NC:
-
Naive control
- Pol II:
-
RNA polymerase II
References
Bousquet J, Anto JM, Bachert C, Baiardini I, Bosnic-Anticevich S, Walter Canonica G, et al. Allergic rhinitis. Nat Rev Dis Primers. 2020;6:95.
Boonpiyathad T, Sözener ZC, Satitsuksanoa P, Akdis CA. Immunologic mechanisms in asthma. Semin Immunol. 2019;46:101333.
Gevaert P, Wong K, Millette LA, Carr TF. The role of IgE in Upper and Lower Airway Disease: more Than just Allergy! Clin Rev Allergy Immunol. 2022;62:200–15.
Lv X, Gao Z, Tang W, Qin J, Wang W, Liu J, et al. Trends of therapy in the treatment of asthma. Ther Adv Respir Dis. 2023;17:17534666231155748.
Zeng X, Xiao X, Hu S, He W, Wu G, Geng X, et al. XBP1 is required in Th2 polarization induction in airway allergy. Theranostics. 2022;12:5337–49.
Yang G, Zeng XH, Geng XR, Liu JQ, Mo LH, Luo XQ, et al. The transcription factor XBP1 in dendritic cells promotes the T(H)2 cell response in airway allergy. Sci Signal. 2023;16:eabm9454.
Ji T, Li H. T-helper cells and their cytokines in pathogenesis and treatment of asthma. Front Immunol. 2023;14:1149203.
Nguyen TV, Piao CH, Fan YJ, Yu ZN, Lee SY, Song CH et al. Artemisia gmelinii Extract alleviates allergic airway inflammation via balancing TH1/TH2 homeostasis and inhibiting mast cell degranulation. Int J Mol Sci 2022; 23.
Jansen K, Cevhertas L, Ma S, Satitsuksanoa P, Akdis M, van de Veen W. Regulatory B cells, a to Z. Allergy. 2021;76:2699–715.
Ferreira LMR, Muller YD, Bluestone JA, Tang Q. Next-generation regulatory T cell therapy. Nat Rev Drug Discov. 2019;18:749–69.
Mo L, Liu Y, Xu X, Wang X, Zhang S, Hu S, et al. Endoplasmic reticulum stress impairs the immune regulation property of macrophages in asthmatic patients. Clin Immunol. 2023;252:109639.
Yan F, Polk DB. Probiotics and immune health. Curr Opin Gastroenterol. 2011;27:496–501.
Hashemi B, Abdollahi M, Abbaspour-Aghdam S, Hazrati A, Malekpour K, Meshgi S, et al. The effect of probiotics on immune responses and their therapeutic application: a new treatment option for multiple sclerosis. Biomed Pharmacother. 2023;159:114195.
Gunawardana T, Ahmed KA, Goonewardene K, Popowich S, Kurukulasuriya S, Karunarathna R, et al. Synthetic CpG-ODN rapidly enriches immune compartments in neonatal chicks to induce protective immunity against bacterial infections. Sci Rep. 2019;9:341.
Yang GJ, Zhu MH, Lu XJ, Liu YJ, Lu JF, Leung CH, et al. The emerging role of KDM5A in human cancer. J Hematol Oncol. 2021;14:30.
Spacova I, Petrova MI, Fremau A, Pollaris L, Vanoirbeek J, Ceuppens JL, et al. Intranasal administration of probiotic Lactobacillus rhamnosus GG prevents birch pollen-induced allergic asthma in a murine model. Allergy. 2019;74:100–10.
Capurso L. Thirty years of Lactobacillus rhamnosus GG: a review. J Clin Gastroenterol. 2019;53(Suppl 1):S1–41.
Lam LKM, Murphy S, Kokkinaki D, Venosa A, Sherrill-Mix S, Casu C, et al. DNA binding to TLR9 expressed by red blood cells promotes innate immune activation and anemia. Sci Transl Med. 2021;13:eabj1008.
Ishii KJ, Akira S. Innate immune recognition of, and regulation by. DNA Trends Immunol. 2006;27:525–32.
Zhu B, Wang T, Wei X, Zhou Y, Li J. CpG DNA-triggered upregulation of TLR9 expression affects apoptosis and immune responses in human plasmacytoid dendritic cells isolated from chronic hepatitis B patients. Arch Physiol Biochem. 2023;129:330–7.
Jacquet A. Nucleic acid vaccines and CpG oligodeoxynucleotides for allergen immunotherapy. Curr Opin Allergy Clin Immunol. 2021;21:569–75.
Hinohara K, Wu HJ, Vigneau S, McDonald TO, Igarashi KJ, Yamamoto KN, et al. KDM5 histone demethylase activity Links Cellular Transcriptomic heterogeneity to Therapeutic Resistance. Cancer Cell. 2018;34:939–e9539.
Garzón-Porras AM, Chory E, Gryder BE. Dynamic opposition of histone modifications. ACS Chem Biol. 2023;18:1027–36.
Zdrenghea MT, Makrinioti H, Muresan A, Johnston SL, Stanciu LA. The role of macrophage IL-10/innate IFN interplay during virus-induced asthma. Rev Med Virol. 2015;25:33–49.
Liu Z, Feng BS, Yang SB, Chen X, Su J, Yang PC. Interleukin (IL)-23 suppresses IL-10 in inflammatory bowel disease. J Biol Chem. 2012;287:3591–7.
Tian GX, Peng KP, Yu Y, Liang CB, Xie HQ, Guo YY, et al. Propionic acid regulates immune tolerant properties in B cells. J Cell Mol Med. 2022;26:2766–76.
Zhu X, Liang R, Lan T, Ding D, Huang S, Shao J et al. Tumor-associated macrophage-specific CD155 contributes to M2-phenotype transition, immunosuppression, and tumor progression in colorectal cancer. J Immunother Cancer 2022; 10.
Greer EL, Shi Y. Histone methylation: a dynamic mark in health, disease and inheritance. Nat Rev Genet. 2012;13:343–57.
Wu CT, Chen PJ, Lee YT, Ko JL, Lue KH. Effects of immunomodulatory supplementation with Lactobacillus rhamnosus on airway inflammation in a mouse asthma model. Journal of microbiology, immunology, and infection = Wei Mian Yu Gan ran Za Zhi 2016; 49:625–35.
Jerzynska J, Stelmach W, Balcerak J, Woicka-Kolejwa K, Rychlik B, Blauz A, et al. Effect of Lactobacillus rhamnosus GG and vitamin D supplementation on the immunologic effectiveness of grass-specific sublingual immunotherapy in children with allergy. Allergy Asthma Proc. 2016;37:324–34.
Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, Mardani F, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233:6425–40.
Webster HC, Gamino V, Andrusaite AT, Ridgewell OJ, McCowan J, Shergold AL, et al. Tissue-based IL-10 signalling in helminth infection limits IFNγ expression and promotes the intestinal Th2 response. Mucosal Immunol. 2022;15:1257–69.
Acknowledgements
This study was supported by research grants from the National Natural Science Foundation of China (81970865, 32090052); Four “Batches” Innovation Project of Invigorating Medical through Science and Technology of Shanxi Province (2022XM06); Research Project Supported by Shanxi Scholarship Council of China (2021 − 172).
Author information
Authors and Affiliations
Contributions
Xue J, Liu Z, Xie B, Dong R, Wu J, Wu Y, Xu Z, Tian Y, Wei Y, Geng Z, Lu L and Liu Y performed experiments, analyzed data, and reviewed manuscript. Yang P and Xie J organized the study and supervised experiments. Yang P designed the project and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Anatolii Kubyshkin
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xue, J., Liu, Z., Xie, B. et al. Probiotic nucleotides increase IL-10 expression in airway macrophages to mitigate airway allergy. Inflamm. Res. (2024). https://doi.org/10.1007/s00011-024-01940-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00011-024-01940-2