Abstract
Objective
Microglia/macrophage activation is previously reported to be involved in various ocular diseases. However, the separate role of M1/M2 phenotype microglia/macrophage in the pathological process of oxygen-induced retinopathy (OIR) remains unknown. In this research, we explored the role and regulatory mechanism of M1/M2 microglia/macrophage in OIR in C57BL/6J mice. Furthermore, we demonstrated the time phase of M1/M2 shifting of microglia/macrophage during the natural process of OIR, which is very essential for further investigations.
Materials and methods
C57BL/6j pups were exposed to hyperoxia environment from postnatal 7(P7) to P12 then returned to normoxia. The mice were then euthanized, and the eyes were harvested at a series of time points for further investigation. The M1/M2 phenotype microglia/macrophage activity was presented by immunofluorescent staining and real-time quantitative polymerase chain reaction (qPCR). The NF-κb-STAT3 signaling and IL-4-STAT6-PPAR-γ signaling pathway activity was examined by western blot analysis.
Results
The microglia/macrophage were activated when the OIR model was set up after P12. The M1 microglia/macrophage activation was found in neovascularization (NV) tufts in both central and peripheral retina, which started from P12 when the mice were returned to normoxia environment and peaked at P17. During this period of time, the NF-κb-STAT3 signaling pathway was activated, resulting in the upregulated M1 phenotype microglia/macrophage polarization, along with the enhanced inflammatory cytokine expression including tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and IL-1β. Consequently, the NV tufts were observed from P12 and the volume continued to increase until P17. However, the M2 phenotype microglia/macrophage activity took over during the late phase of OIR started from P17. The IL-4-STAT6-PPAR-γ signaling activity was upregulated from P17 and peaked at P20, inducing M2 phenotype microglia polarization, which consequently led to the inhibition of inflammatory cytokines and spontaneous regression of NV tufts.
Conclusions
Microglia/macrophage participate actively in the natural process of OIR in mice, and two phenotypes exert different functions. Treatment modulating microglia/macrophage polarize toward M2 phenotype might be a novel and promising method for ocular neovascular diseases such as retinopathy of prematurity (ROP), wet age-related macular degeneration (wAMD), and diabetic retinopathy (DR).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The mouse model of OIR was originally described by Smith in 1994 and is widely applied in numerous studies concerning ocular diseases featured by retinal NV [1]. In short, the mice are exposed to hyperoxia environment during the early stage of retinal growth, which leads to the dysplasia of normal retinal vascular. When the animals return to the normoxia environment, they are in a relative hypoxic situation and the retina becomes relatively ischemia and is not adequate to support the neural tissue in normoxia conditions. This ischemic situation results in unregulated, abnormal vascular growth. The pathological process of OIR perfectly mimics the pathogenic mechanism of retinopathy of ROP, making the OIR models very useful for studying various ischemic retinopathies including ROP, wet age-related retinopathy, and diabetic retinopathy [2].
Microglia are resident immune cells located in the central nervous system (CNS), and played important role in the maintenance of the neuro-retinal microenvironment [3]. In the retina, microglia/macrophage are reported to not only keep the retina under immunological surveillance but also exert multiple functions such as synaptic pruning, and regulation of neurogenesis and axonal growth [4].
Microglia/macrophage are involved in various retinal diseases, including pathological retinal angiogenesis [5], retinal detachment [6], retinal degeneration [7], and autoimmune uveitis [8], However, activated microglia/macrophage in ocular have variable functions, including phagocytosis, antigen presentation, and production of inflammatory factors and it can be either beneficial or harmful to the affected tissue [5, 9].
The polarization of macrophages is resulted from the integration of the intracellular signals stimulated by their microenvironment [10]. Similar to macrophages, microglia/macrophage can be polarized to dual function phenotypes that range from M1 (pro-inflammatory) to M2 (anti-inflammatory) phenotype [11]. M1, or classically activated microglia/macrophage, produce inflammatory cytokines such as TNF-α, IL-12, IL-23, IL-1β, IL-6 and are involved in the inflammatory response. On the contrast, alternatively activated microglia/macrophage (M2) secrete high levels of arginase-1 (Arg-1) and IL-10 but low levels of IL-12 and IL-23 and this phenotype is usually induced by the anti-inflammatory cytokines such as IL-4 and IL-13 [12].
According to previous studies, microglia/macrophage are activated throughout the natural history of OIR, including both the hyperoxic period and hypoxia phase [13]. Also, during our pervious investigation concerning OIR, we found a close correlation between the pathological process of OIR mouse model and the differentiation of microglia/macrophage cells which has never been reported before [14, 15]. Moreover, the actual role and mechanism of M1/M2 microglia/macrophage in regulating retinal neovascularization remain unclear. Thus, we think it is necessary to conduct this research to reveal the role of these two phenotypes of microglia/macrophage during the whole process of OIR and hopefully inspire novel treatment method for ocular neovascular diseases.
Materials and methods
Reagents
Primary antibody against Ionized calcium binding adaptor molecule-1 (Iba1), Isolectin-B4 (IB4), CD16, CD206, and Trizol were purchased from Thermo Fisher Scientific (Waltham, MA). IScript cDNA Synthesis Kit and SsoFast EvaGreen Supermix was purchased from Bio-Rad Laboratories (Hercules, CA). Primers were purchased from TaKaRa (Japan). Antibody against NF-κb, p-NF-κb, STAT3, p-STAT3, STAT6, p-STAT6, PPAR-γ, and secondary antibody against mouse and rabbit were purchased from Cell Signaling Technology (Danvers, MA). Phosphate buffer saline (PBS), 4% paraformaldehyde, TritonX-100, and bovine serum albumin (BSA) were purchased from Beyotime Biothechnology (Dalian, China).
Mouse model of OIR
OIR was induced in C57BL/6J wild-type (WT) mice. All the animals used in the study were approved by the Institutional Animal Care and Use Committee of Zhongshan Ophthalmic Center. In this model, 7-day-old pups were exposed to 75% oxygen until postnatal day (P)12, which induced vascular dysplasia of the central retina. At P12, the mice were returned to room air, and the retina became hypoxic and the expression of angiogenic factors was upregulated, consequently leading to retinal neovascular growth.
Immunofluorescent staining of whole retina flat mounts
BL57/6 J WT were subjected to the OIR model to assess pathological hypoxia-driven angiogenesis and euthanized on P5, 8, 12, 14, 17, 20, 22, 26, 30. The eyes were enucleated and fixed in 4% paraformaldehyde for 30 min at room temperature. On the next day, the retinas were carefully removed and permeabilized overnight at 4 °C (PBS with 1% bovine serum albumin, 0.5% triton X-100) and then incubated with Iba1 (1:100), isolectin B4 (1:200), CD16 (1:100), CD206 (1:100) at 4 °C overnight, followed by incubation with a secondary antibody. After flat mounting, the retinas were imaged with an Axiovert 200 inverted fluorescence microscope (Carl Zeiss, Oberkochen, Germany) and Zen 2 (blue edition; Carl Zeiss) image-processing software.
Real-time quantitative PCR
Total RNA from retinas was extracted with Trizol and cDNA was synthesized with an iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, CA), and mRNA expression levels for each gene were quantified by RT-qPCR using SsoFast EvaGreen Supermix (Bio-Rad Laboratories) and a Bio-Rad Laboratories cycler system and gene-specific primers (TaKaRa) and normalization to Tbp (TATA-box-binding protein) mRNA. Primer sequences are included in Table 1.
Protein extraction and western blot analysis
Pooled retinas were lysed in ice-cold extraction buffer and equivalent amounts of protein were resolved on a 10% SDS–polyacrylamide gel and transferred by electrophoresis to PVDF membrane. The protein bands were incubated with antibody against NF-κb (1:1000), p-NF-κb (1:1000), STAT3 (1:2000), p-STAT3 (1:1000), STAT6 (1:1000), p-STAT6 antibody, PPAR-γ (1:1000), followed by anti-mouse horseradish peroxidase-conjugated secondary antibody (1:5000). Protein bands were visualized and quantified as described previously [14].
Statistical analysis
Results are presented as the mean ± SEM format. Statistical significance was determined by the Student’s t test for comparison between two groups or one-way ANOVA for multiple-group comparison (Prism; GraphPad, San Diego, CA). p < 0.05 was considered statistically significant in all statistical analyses.
Results
Microglia/macrophage cells activated in the retina of OIR mice
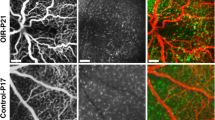
To investigate whether microglia/macrophage are involved in the pathological process of OIR, pups of different days of age (P5, P8, P12, P14, P17, P20, P22, P26, P30) were euthanized and their eyes were harvested for qPCR and whole retina flat mount analysis. A significantly increased level of Iba-1 mRNA expression was observed at P14, and peaked at P17, (as shown in Fig. 1a) which was very consistent with previous report [13] (p < 0.01). Similarly, an immediate activation of microglia/macrophage cells labeled by Iba-1 antibody was observed in the NV tufts in both central and peripheral retina as soon as the pups were taken out of hyperoxic environment at P12 and peaked at P17 (Fig. 1b–j). These results confirmed that microglia/macrophage cells were involved in the pathological process of OIR.
Microglia/macrophage activation after OIR onset. a Microglia/macrophage activation peaked at P17 and gradually declined until P30; b–d microglia/macrophage marked with iba1 in normal retina at P17; e–g the density of microglia/macrophage in central area of retina is significantly higher than control group; h–j the density of microglia/macrophage in periphery area of retina is significantly higher than control group. Data are presented as mean ± SEM. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001; ns not significant
Time course of mRNA levels of inflammatory factors expressed by M1 phenotype microglia/macrophage cells in the retina of OIR mice
To investigate the role of M1 polarization of microglia/macrophage in the natural history of OIR, pro-inflammatory cytokines expressed by M1 phenotype microglia/macrophage cells including TNF-α, IL-6, and IL-1β were evaluated by RT-qPCR. As shown in Fig. 2, mRNA levels of these pro-inflammatory cytokines were significantly higher when the pups were taken out of hyperoxic environment and peaked at P14 followed by a gradual decline until P30 (p < 0.0001).
The mRNA level of TNF-α, IL-6, IL-1β at different time points was measured by RT-qPCR. a TNF-α mRNA level peaked at P14, then gradually declined until P30; b the mRNA level of IL-6 peaked at P14 and declined until P30; c the mRNA level of IL-1β peaked at P14 and declined until P30. Data are presented as mean ± SEM. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001; ns not significant
Time course of mRNA levels of anti-inflammatory and pro-angiogenetic factors expressed by M2 phenotype microglia/macrophage cells in the retina of OIR mice
To investigate the role of M2 polarization of microglia/macrophage in the natural history of OIR, specific markers expressed by M2 phenotype microglia/macrophage cells including Arg-1, YM1, and IL-4 were evaluated by qPCR. As shown in Fig. 3, mRNA levels of these markers rose gradually from P14 and peaked at P20, indicating a rising trend of M2 microglia/macrophage functioning at this period of OIR (p < 0.001).
The mRNA level of Arg-1, YM-1, IL-4 at different time points was measured by RT-qPCR. a Arg-1 mRNA level rose from P14 and peaked at P20, then gradually declined until P30; b MRNA level of YM-1 rose from P14 and peaked at P20 and declined until P30; c MRNA level of IL-4 rose from P14 peaked at P20 and declined until P30. Data are presented as mean ± SEM. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001; ns not significant
Differentiation of M1 phenotype microglia/macrophage cells in the retina peaked at the onset of OIR followed by a gradual reduction.
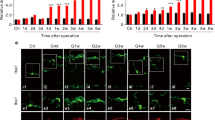
To confirm these results, whole retina flat mounts of P14 and P20 pups were proceeded and M1 microglia/macrophage cells were marked by CD16 antibody co-stained with iba1 (as shown in Fig. 4a). The M1-polarized microglia/macrophage presented a ramified phenotype with numerous long, thin tentacles and small cell body. The proportion of CD16 positive cells against iba1 was significantly higher at P14 than P20, indicating a dominant role of M1 phenotype microglia/macrophage/macrophage at the early onset of OIR in mice (p < 0.01).
M1-polarized microglia/macrophage at P14 and P20, stained with CD16 and iba1. a The proportion of CD16 positive microglia/macrophage in OIR mice at P14 is significantly higher than P20; b statistical analysis of a. Data are presented as mean ± SEM. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001; ns not significant
Differentiation of M2 phenotype microglia/macrophage cells in the retina opposite to M1 phenotype microglia/macrophage cells
To investigate the M2-polarized microglia/macrophage/macrophage polarization during the progress of OIR, the M2 microglia/macrophage cells in retina flat mounts at P14 and P20 were marked by CD206 antibody, co-stained with iba1. Unlike the M1 phenotype, the M2-polarized microglia/macrophage/macrophage presented an ameboid shape featuring fewer tentacles and a larger cell body. As shown in Fig. 5, a significantly higher proportion of CD206 positive microglia/macrophage/macrophage cells was observed at P20 than P14 (p < 0.01). These results indicated that the M2 phenotype microglia/macrophage/macrophage exert important functions in the late phase of OIR in mice.
M2-polarized microglia/macrophage/macrophage at P14 and P20. a The proportion of M2 phenotype microglia/macrophage/macrophage marked with CD206 in OIR mice at P20 is significantly higher than P14. b statistical analysis. Data are presented as mean ± SEM. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001; ns not significant
The NF-κb-STAT3 signaling and IL-4-STAT6- PPAR-γ signaling regulated M1/M2 polarization in the retina of OIR mice
To investigate the regulatory mechanism of M1/M2 microglia/macrophage cells, eyes of P14 and P20 pups were harvested and retina was isolated for western blot analysis. As shown in Fig. 6a–c, p-NF-κb and p-STAT3 were significantly higher in OIR group than the control group at P14 (p < 0.0001), while at P20, the NF-κb and STAT3 signaling activation declined dramatically (p < 0.001). Meanwhile, the IL-4-STAT6-PPAR-γ signaling took over and polarized microglia/macrophage toward M2 phenotype. At P20, the protein level of PPAR-γ and p-STAT6 of OIR group was significantly higher than the control group, (p < 0.0001, p < 0.001, respectively), while at P14, these differences were absent, and the PPAR-γ was even lower in OIR group than the control group. The time course of the STAT6-PPAR-γ pathway activation was closely related to the time points of M1/M2 shifting of microglia/macrophage. These results indicate a protective role of M2 phenotype microglia/macrophage cells in the late phase of OIR pathological process.
Activity of NF-κb-STAT3 pathway and STAT6-PPAR-γ pathway at P14 and P20 was investigated using western blot analysis. a The protein level of p-NF-κb and p-STAT3 is significantly higher in the retina of OIR mice than control group at P14, while at P20, the trend is reversed; b and c statistical analysis of a; d the protein level of PPAR-γ and p-STAT6 in the retina of OIR mice is significantly higher than control group at P20, while the trend is opposite at P14. Data are presented as mean ± SEM. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001; ns not significant
Discussion
The microglia are the resident macrophage in the CNS and are the predominant macrophage cell population in normal conditions. However, distinguishing these two populations is often challenging, owing to a paucity of universally accepted and reliable markers [16]. Also, these two cell populations share some similarities in the appearance and function, so when investigating the microglia/macrophage polarization in different pathogenic processes, researchers still tend to consider microglia and macrophage to be homogeneous [17]. The microglia/macrophage constantly maintain the integrity of the retina by constantly monitoring the local environment for potential assault [15, 18]. However, the role of microglia/macrophage in the pathological process of OIR has not been extensively investigated since fewer studies specifically examining microglia/macrophage in the retina than elsewhere in the CNS despite the fact that the retina represents an advantageous and unique system for studying microglia/macrophage biology. Previous studies demonstrated that microglia/macrophage activation involves in various ocular diseases, including glaucoma, retinitis pigmentosa, and age-related macular degeneration [19,20,21]. It is reported that the products of microglia/macrophage activation can potentiate RGC degeneration via proinflammatory and oxidative stress pathways and can induce proliferation and neovascularization as well [22, 23]. Based on these results, microglia/macrophage ablation has already been suggested as a potential treatment method for ocular inflammatory diseases [24]. However, studies investigating phenotypic changes in macrophages/microglia/macrophage in peripheral inflammation and immunity have shown that microglia/macrophage can be differentiated into two main phenotypes. One is the classic or M1 activation, characterized by high capacity to produce high levels of nitric oxide (NO), reactive oxygen species (ROS), and inflammatory cytokines such as TNF-α, IL-1β, and IL-6, while the second phenotype (M2) is reported to be more related to a fine tuning of inflammation, which induced scavenging of debris, tissue remodeling and repair [25, 26]. Considering that, maintaining the balance between M1 and M2 might be even more crucial for the healing and remodeling of damaged tissues, rather than mere microglia/macrophage ablation.
In the OIR mouse model, the normal retinal vascular development is suspended when the mice are placed in hyperoxia environment from P7 to P12. When the mice are returned to room air at P12, the central retina becomes relatively hypoxic due to a lack of sufficient capillary perfusion, consequently induced neovascularization. However, according to previous investigation, the NV tufts started to regress spontaneously at P17, leading to a morphologically normal retinal vascular system around P25 [27, 28]. Our previous research reported that, when the OIR was induced, microglia/macrophage in the retina are activated, along with the upregulated expression of several inflammatory factors [14]. However, whether M2-polarized microglia/macrophage are involved in the pathological process of OIR and their regulatory mechanism remains unclear. In this very study, we found that the M2 microglia/macrophage are also involved in the natural process of OIR and exert anti-inflammation effect. The M2 polarization of microglia/macrophage stated to increase from P17 when the M1 polarization started to decrease gradually until P30. More importantly, the time phase of M1/M2 shifting is perfectly corresponded with the time phase of spontaneous regression of neovascularization tufts. These results suggest that M1/M2 shifting of microglia/macrophage played an important role in the pathological process of OIR, consequently inhibiting neovascularization and promoting vascular normalization in the OIR mouse model.
Growing evidences suggested that many nuclear factors were implicated in the M1/M2 phenotype transformation [29, 30]. The NF-κB signaling and STAT3 signaling have both been recognized as very much responsible for inflammation response since they both induce the expression of various inflammatory mediators [31, 32]. And it is reported that STAT3 could interact with NF-κb at several levels as several inflammatory factors encoded by NF-κb target genes, most notably IL-6, are also important STAT3 stimulators [33,34,35,36]. Also, it is demonstrated that NF-κb and STAT3 activation could trigger the M1 polarization of microglia/macrophage and induce pro-inflammation effects [37]. During our investigation, we found similar phenomenon in the pathological process of OIR. The M1 polarization of microglia/macrophage started at P12, when the NF-κb and STAT3 signaling is activated right after the mice were taken out from the oxygen chamber. The M1-polarized microglia/macrophage consequently express high levels of inflammatory cytokines, inducing increased permeability of the vessel, proliferation and finally, neovascularization.
In the contrast, IL-4, a pleiotropic cytokine involved in the regulation of diverse immune and inflammatory responses, is reported to enhance M2 polarization and exert anti-inflammation and neuroprotection effects [38, 39]. It is demonstrated that IL-4 has the ability to polarize microglia/macrophage toward M2 phenotype. Consequently, M2 microglia/macrophage induced the resolution of inflammation via increased trophic input and upregulated phagocytosis and proteolysis of dead, diseased cells, ultimately inducing tissue normalization [10, 40]. Moreover, it has been reported that activation of the IL-4 receptor by IL-4 itself leads to activation of the PPAR-γ through STAT6 activation [41, 42]. And the PPAR-γ and its agonist are found to modulate M2 microglia/macrophage polarization and inhibit inflammation [43, 44]. Our experiment revealed similar results in OIR: the M2 polarization of microglia/macrophage started from P17, when the neovascularization started to regress and peaked at P20, along with upregulated expression of p-STAT6 and PPAR-γ and inhibited NF-κb and STAT3 activity.
This study provides new insight into the role of microglia/macrophage in the pathological process of OIR. First, our results confirmed that microglia/macrophage cells exert various regulatory function during the whole procedure of OIR in mice. Second, in the early stage of OIR, microglia/macrophage cells gathered in the NV tufts and tend to be M1 polarized. The NF-κb and STAT3 activation triggered M1 phenotype microglia/macrophage polarization and upregulated the expression of pro-inflammation cytokines. Such inflammatory response started at P12 and peaks at P17 then gradually declined until P30. On contrast, M2 polarization of microglia/macrophage, which resulted from IL-4-STAT6-PPAR-γ pathway activation, significantly inhibited inflammation at P20, and consequently inhibited proliferation and neovascularization. These results demonstrated that M1/M2 shifting of microglia/macrophage is crucial for the recession of NV tufts and promoted vascular normalization during the natural process of OIR, which also suggests modulating microglia/macrophage polarization towards M2 phenotype is a promising method for ocular inflammatory diseases.
Conclusions
Microglia/macrophage participate actively in the natural process of OIR in mice, and two phenotypes exert different functions. These results indicate that ocular neovascular diseases might be attenuated by inhibiting M1 polarization or promoting M2 polarization of microglia/macrophage, but further investigation is still needed to confirm such therapeutic effect.
References
Smith L, Wesolowski E, McLellan A, Kostyk S, D’Amato R, Sullivan R, et al. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35(1):101–11.
Villacampa P, Menger K, Abelleira L, Ribeiro J, Duran Y, Smith A, et al. Accelerated oxygen-induced retinopathy is a reliable model of ischemia-induced retinal neovascularization. PLoS ONE. 2017;12(6):e0179759. https://doi.org/10.1371/journal.pone.0179759.
Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science (New York, NY). 2010;330(6005):841–5. https://doi.org/10.1126/science.1194637.
Rathnasamy G, Foulds W, Ling E, Kaur C. Retinal microglia—a key player in healthy and diseased retina. Prog Neurobiol. 2019;173:18–40. https://doi.org/10.1016/j.pneurobio.2018.05.006.
Connor K, SanGiovanni J, Lofqvist C, Aderman C, Chen J, Higuchi A, et al. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat Med. 2007;13(7):868–73. https://doi.org/10.1038/nm1591.
Okunuki Y, Mukai R, Pearsall E, Klokman G, Husain D, Park D, et al. Microglia inhibit photoreceptor cell death and regulate immune cell infiltration in response to retinal detachment. Proc Natl Acad Sci USA. 2018;115(27):E6264–73. https://doi.org/10.1073/pnas.1719601115.
Ma W, Silverman S, Zhao L, Villasmil R, Campos M, Amaral J, et al. Absence of TGFβ signaling in retinal microglia induces retinal degeneration and exacerbates choroidal neovascularization. eLife. 2019. https://doi.org/10.7554/eLife.42049.
Dagkalis A, Wallace C, Hing B, Liversidge J, Crane I. CX3CR1-deficiency is associated with increased severity of disease in experimental autoimmune uveitis. Immunology. 2009;128(1):25–33. https://doi.org/10.1111/j.1365-2567.2009.03046.x.
Dong H, Zhang X, Wang Y, Zhou X, Qian Y, Zhang S. Suppression of brain mast cells degranulation inhibits microglial activation and central nervous system inflammation. Mol Neurobiol. 2017;54(2):997–1007. https://doi.org/10.1007/s12035-016-9720-x.
Mantovani A, Biswas S, Galdiero M, Sica A, Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol. 2013;229(2):176–85. https://doi.org/10.1002/path.4133.
Szabo M, Gulya K. Development of the microglial phenotype in culture. Neuroscience. 2013;241:280–95. https://doi.org/10.1016/j.neuroscience.2013.03.033.
Martinez F, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–83. https://doi.org/10.1146/annurev.immunol.021908.132532.
Fischer F, Martin G, Agostini H. Activation of retinal microglia rather than microglial cell density correlates with retinal neovascularization in the mouse model of oxygen-induced retinopathy. J Neuroinflamm. 2011;8:120. https://doi.org/10.1186/1742-2094-8-120.
Xu Y, Lu X, Hu Y, Yang B, Tsui C, Yu S, et al. Melatonin attenuated retinal neovascularization and neuroglial dysfunction by inhibition of HIF-1α-VEGF pathway in oxygen-induced retinopathy mice. J Pineal Res. 2018;64(4):e12473. https://doi.org/10.1111/jpi.12473.
Yang B, Xu Y, Yu S, Huang Y, Lu L, Liang X. Anti-angiogenic and anti-inflammatory effect of Magnolol in the oxygen-induced retinopathy model. Inflamm Res. 2016;65(1):81–93. https://doi.org/10.1007/s00011-015-0894-x.
Haage V, Semtner M, Vidal R, Hernandez D, Pong W, Chen Z, et al. Comprehensive gene expression meta-analysis identifies signature genes that distinguish microglia from peripheral monocytes/macrophages in health and glioma. Acta Neuropathol Commun. 2019;7(1):20. https://doi.org/10.1186/s40478-019-0665-y.
Xu Y, Cui K, Li J, Tang X, Lin J, Lu X, et al. Melatonin attenuates choroidal neovascularization by regulating macrophage/microglia polarization via inhibition of RhoA/ROCK signaling pathway. J Pineal Res. 2020. https://doi.org/10.1111/jpi.12660.
Ransohoff R, Perry V. Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol. 2009;27:119–45. https://doi.org/10.1146/annurev.immunol.021908.132528.
Bosco A, Steele M, Vetter M. Early microglia activation in a mouse model of chronic glaucoma. J Comp Neurol. 2011;519(4):599–620. https://doi.org/10.1002/cne.22516.
Zhang L, Cui X, Jauregui R, Park K, Justus S, Tsai Y, et al. Genetic rescue reverses microglial activation in preclinical models of retinitis pigmentosa. Mol Ther. 2018;26(8):1953–64. https://doi.org/10.1016/j.ymthe.2018.06.014.
Telegina D, Kozhevnikova O, Kolosova N. Changes in retinal glial cells with age and during development of age-related macular degeneration. Biochemistry. 2018;83(9):1009–17. https://doi.org/10.1134/s000629791809002x.
Gallego B, Salazar J, de Hoz R, Rojas B, Ramírez A, Salinas-Navarro M, et al. IOP induces upregulation of GFAP and MHC-II and microglia reactivity in mice retina contralateral to experimental glaucoma. J Neuroinflamm. 2012;9:92. https://doi.org/10.1186/1742-2094-9-92.
Brandenburg S, Müller A, Turkowski K, Radev Y, Rot S, Schmidt C, et al. Resident microglia rather than peripheral macrophages promote vascularization in brain tumors and are source of alternative pro-angiogenic factors. Acta Neuropathol. 2016;131(3):365–78. https://doi.org/10.1007/s00401-015-1529-6.
Takeda A, Shinozaki Y, Kashiwagi K, Ohno N, Eto K, Wake H, et al. Microglia mediate non-cell-autonomous cell death of retinal ganglion cells. Glia. 2018;66(11):2366–84. https://doi.org/10.1002/glia.23475.
David S, Kroner A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci. 2011;12(7):388–99. https://doi.org/10.1038/nrn3053.
Michelucci A, Heurtaux T, Grandbarbe L, Morga E, Heuschling P. Characterization of the microglial phenotype under specific pro-inflammatory and anti-inflammatory conditions: effects of oligomeric and fibrillar amyloid-beta. J Neuroimmunol. 2009;210:3–12. https://doi.org/10.1016/j.jneuroim.2009.02.003.
Lange C, Ehlken C, Stahl A, Martin G, Hansen L, Agostini H. Kinetics of retinal vaso-obliteration and neovascularisation in the oxygen-induced retinopathy (OIR) mouse model. Graefe’s Arch Clin Exp Ophthal. 2009;247(9):1205–11. https://doi.org/10.1007/s00417-009-1116-4.
Stahl A, Chen J, Sapieha P, Seaward M, Krah N, Dennison R, et al. Postnatal weight gain modifies severity and functional outcome of oxygen-induced proliferative retinopathy. Am J Pathol. 2010;177(6):2715–23. https://doi.org/10.2353/ajpath.2010.100526.
Han R, Xiao J, Zhai H, Hao J. Dimethyl fumarate attenuates experimental autoimmune neuritis through the nuclear factor erythroid-derived 2-related factor 2/hemoxygenase-1 pathway by altering the balance of M1/M2 macrophages. J Neuroinflamm. 2016;13(1):97. https://doi.org/10.1186/s12974-016-0559-x.
Zhao Q, Wu X, Yan S, Xie X, Fan Y, Zhang J, et al. The antidepressant-like effects of pioglitazone in a chronic mild stress mouse model are associated with PPARγ-mediated alteration of microglial activation phenotypes. J Neuroinflamm. 2016;13(1):259. https://doi.org/10.1186/s12974-016-0728-y.
Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9(11):798–809. https://doi.org/10.1038/nrc2734.
Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–44. https://doi.org/10.1038/nature07205.
Bollrath J, Phesse T, von Burstin V, Putoczki T, Bennecke M, Bateman T, et al. gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell. 2009;15(2):91–102. https://doi.org/10.1016/j.ccr.2009.01.002.
Grivennikov S, Karin E, Terzic J, Mucida D, Yu G, Vallabhapurapu S, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15(2):103–13. https://doi.org/10.1016/j.ccr.2009.01.001.
Yin Z, Ma T, Lin Y, Lu X, Zhang C, Chen S, et al. IL-6/STAT3 pathway intermediates M1/M2 macrophage polarization during the development of hepatocellular carcinoma. J Cell Biochem. 2018;119(11):9419–32. https://doi.org/10.1002/jcb.27259.
Zhong Z, Wen Z, Darnell J. Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science (New York, NY). 1994;264(5155):95–8. https://doi.org/10.1126/science.8140422.
Oh H, Park S, Kang M, Kim Y, Lee E, Kim D, et al. Asaronic acid attenuates macrophage activation toward M1 phenotype through inhibition of NF-κB pathway and JAK-STAT signaling in glucose-loaded murine macrophages. J Agric Food Chem. 2019;67(36):10069–78. https://doi.org/10.1021/acs.jafc.9b03926.
Egholm C, Heeb L, Impellizzieri D, Boyman O. The Regulatory effects of interleukin-4 receptor signaling on neutrophils in type 2 immune responses. Front Immunol. 2019;10:2507. https://doi.org/10.3389/fimmu.2019.02507.
Zheng H, Wu X, Wu D, Jiang R, Castillo E, Chock C, et al. Treg expression of CIS suppresses allergic airway inflammation through antagonizing an autonomous TH2 program. Mucosal Immunol. 2020;13(2):293–302. https://doi.org/10.1038/s41385-019-0236-3.
Balce D, Li B, Allan E, Rybicka J, Krohn R, Yates R. Alternative activation of macrophages by IL-4 enhances the proteolytic capacity of their phagosomes through synergistic mechanisms. Blood. 2011;118(15):4199–208. https://doi.org/10.1182/blood-2011-01-328906.
Luo Y, Yin W, Signore A, Zhang F, Hong Z, Wang S, et al. Neuroprotection against focal ischemic brain injury by the peroxisome proliferator-activated receptor-gamma agonist rosiglitazone. J Neurochem. 2006;97(2):435–48. https://doi.org/10.1111/j.1471-4159.2006.03758.x.
Glass C, Natoli G. Molecular control of activation and priming in macrophages. Nat Immunol. 2016;17(1):26–33. https://doi.org/10.1038/ni.3306.
Pisanu A, Lecca D, Mulas G, Wardas J, Simbula G, Spiga S, et al. Dynamic changes in pro- and anti-inflammatory cytokines in microglia after PPAR-γ agonist neuroprotective treatment in the MPTPp mouse model of progressive Parkinson’s disease. Neurobiol Dis. 2014;71:280–91. https://doi.org/10.1016/j.nbd.2014.08.011.
Daniel B, Nagy G, Horvath A, Czimmerer Z, Cuaranta-Monroy I, Poliska S, et al. The IL-4/STAT6/PPARγ signaling axis is driving the expansion of the RXR heterodimer cistrome, providing complex ligand responsiveness in macrophages. Nucleic Acids Res. 2018;46(9):4425–39. https://doi.org/10.1093/nar/gky157.
Acknowledgements
This research is supported by the national natural science foundation of China (No. 81870668), and the Young Scientist Fund of the National Natural Science Foundation of China (No. 81900864).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that none of the authors have any financial or scientific conflicts of interest with regard to the research described in this manuscript.
Additional information
Responsible Editor: John Di Battista.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, J., Yu, S., Lu, X. et al. The phase changes of M1/M2 phenotype of microglia/macrophage following oxygen-induced retinopathy in mice. Inflamm. Res. 70, 183–192 (2021). https://doi.org/10.1007/s00011-020-01427-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-020-01427-w