Abstract
Objective
Toll-like receptors (TLRs) pathway has been demonstrated to play an important role in periodontitis. However, the regulatory mechanism of microRNAs (miRNAs) on TLRs pathway is still unclear. Hence, this study is to explore the function of miRNA-146a in inflammatory reaction induced by Porphyromonas gingivalis lipopolysaccharide (LPS) in human periodontal ligament cells (hPDLCs).
Methods
Cells were treated with 1 or 10 μg/ml P. gingivalis LPS. The expression of TLR2, TLR4 and miRNA-146a were measured by real-time polymerase chain reaction (PCR). Enzyme-linked immunosorbent assay (ELISA) was applied to detect nuclear factor (NF)-κ B p65 nuclear activity, interleukin-1β (IL-1β), IL-6, IL-8 and tumor necrosis factor-α (TNF-α). To examine the underlying mechanisms, cells were exposed to anti-TLR2/4 mAb or miRNA-146a inhibitor/mimic and evaluated by real-time PCR and ELISA.
Results
10 μg/ml P. gingivalis LPS increased the expressions of TLR2 (3.79 ± 0.31), TLR4 (2.21 ± 0.31), and miRNA-146a (4.91 ± 0.87), NF-κ B p65 nuclear activity (6.51 ± 0.77 fold) (p < 0.05). 1 μg/ml P. gingivalis LPS induced TLR2 (3.05 ± 0.23), miRNA-146a (3.66 ± 0.83) and NF-κ B p65 nuclear activity (4.06 ± 0.78 fold) (p < 0.05), except TLR4 (1.11 ± 0.30, p > 0.05). Also, cytokines production increased (p < 0.05). The up-regulation of miRNA-146a could be blocked by anti-TLR2/4 mAb (p < 0.05). After the blockage of miRNA-146a, TLR2, TLR4, NF-κ B p65 nuclear activity and proinflammatory cytokines increased. However, after application of miRNA-146a mimic, the levels of these indexes decreased obviously (p < 0.05).
Conclusion
MiRNA-146a functions as a negative feedback regulator via down-regulating proinflammatory cytokine secretion and blocking TLRs signaling pathway in hPDLCs after P. gingivalis LPS stimulation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
MicroRNAs (miRNAs) are non-protein-coding RNA molecules (20–25 nucleotides), acting as post-transcriptional regulators via binding to the 3′ untranslated regions (3′-UTRs) of target messenger RNAs (mRNAs) and usually resulting in gene silence [1]. MiRNAs are involved in innate immunity by regulating toll-like receptors (TLRs) signaling pathway and then ensuing inflammatory cytokine response [2]. In mammals, TLR2 and TLR4 play important roles in innate immunity through recognizing the components of gram-negative bacteria and inducing the secretion of proinflammatory cytokines and chemokines, which might trigger the occurrence and progression of periodontitis [3–8]. Also, nuclear factor (NF)-κ B plays a significant role in immune responses, differentiation, inflammation, apoptosis and tumorigenesis [9, 10]. MiRNA-146a is up-regulated by the NF-κ B signaling pathway which is activated by ligands of toll-like receptors (TLRs) such as interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) [11]. This study indicates that miRNA-146a plays an important role in NF-κ B signaling pathway in inflammatory pathogenesis.
Human periodontal ligament cells (hPDLCs), as one of important cells in periodontal tissue, not only are involved in the repair and regeneration of periodontal tissue, but also act as immunological cells to secret cytokines in periodontal inflammation [7]. As known, Porphyromonas gingivalis (P. gingivalis) have been considered to be one of the important pathogenic microorganisms in periodontitis [12]. The cell-wall components of this pathogenic microorganism, especially lipopolysaccharide (LPS), might stimulate host cells to trigger inflammatory responses and induce the destruction of periodontal tissues [13]. It has been demonstrated that hPDLCs could secret IL-1β, IL-6, IL-8, IL-10 and TNF-α after being stimulated by P. gingivalis LPS [7, 14, 15].
Recent studies have shown different miRNAs expression profiles between healthy and inflammatory gingiva, which implies that miRNAs may be involved in periodontal disease [16, 17]. Although it has been reported that miRNA-146a increased pronouncedly in human gingival fibroblasts, not in hPDLCs after Escherichia coli (E. coli) LPS challenge [18], the function of miRNA-146a in hPDLCs after P. gingivalis LPS stimulation is still unclear. In this study, we explored the gene expression changes of miRNA-146a in hPDLCs stimulated with P. gingivalis LPS or/and TLR 2, 4 monoclonal antibody (mAb), and the expression changes of TLR2, 4 in hPDLCs stimulated with P. gingivalis LPS, miRNA-146a inhibitor or mimic by real-time polymerase chain reaction (PCR). In addition, NF-κ B p65 nuclear activity and proinflammatory cytokines secreted by hPDLCs stimulated with P. gingivalis LPS, miRNA-146a inhibitor or mimic were analyzed by enzyme-linked immunosorbent assay (ELISA).
Methods
Culture of human periodontal ligament cells
Approval for human tissue specimens was obtained from the Committee of Ethics in Tianjin Medical University. With informed consent from each patient prior to orthodontic treatment, the explants of periodontal ligaments were obtained from the extracted 20 teeth without inflammation (10 healthy individuals, aged from 17 to 26, male 5, female 5). Clinical periodontal measurements included plaque index (PLI), gingival index (GI), probing depth (PD), clinical attachment loss (CAL) and bleeding on probing (BOP). These data were recorded by probing at six sites (mesio-buccal, buccal, distal-buccal, mesio-lingual, lingual and distal-lingual) per tooth. The data for PLI, GI, and PD represented as mean ± standard deviation (SD) (Table 1).
HPDLCs were isolated and cultured as described previously [7, 19, 20]. In brief, after extraction, the teeth were washed with phosphate buffered saline (PBS) and antibiotics (100 U/ml penicillin G and 100 mg/ml streptomycin sulfate) (Gibco BRL, MD, USA). To avoid contamination with the cells from the gingiva, the periodontal ligaments were scraped off from the middle third of the roots from all the donors. Then, the explants were cut into small pieces, and cultured in Dulbecco’s modified Eagle’s medium (DMEM) with 15 % fetal bovine serum (Gibco BRL, MD, USA) in humidified air containing 5 % CO2 at 37 °C. After confluence, the cells were passaged. Before experimental use, the cells were characterized by analyzing the known hPDLCs markers as described previously [21]. After phenotyping, the cells from all the donors were pooled and used at the third-passage for all the following tests.
Treatments
The cells were treated as following. (A) hPDLCs were stimulated with 1 or 10 μg/ml P. gingivalis LPS (Invivogen, CA, USA) separately in the absence of antibodies, inhibitors or mimic; (B) hPDLCs were incubated with the same stimuli in the presence of TLR2 mAb (1:100) or IgG control (Imgenex, CA, USA); (C) hPDLCs were incubated with the same stimuli in the presence of TLR4 mAb (1:100) or IgG control (Imgenex, CA, USA); (D) hPDLCs were incubated with the same stimuli in the presence of miRNA-146a inhibitor or negative control (Applied Biosystems, CA, USA); (E) hPDLCs were incubated with the same stimuli in the presence of miRNA-146a mimic or negative control (Applied Biosystems, CA, USA). The cells as above were stimulated with LPS for 24 h. As Escherichia coli (E. coli) LPS is a specific agonist of TLR4, hPDLCs were stimulated with 1 μg/ml E. coli LPS (Sigma, CA, USA) without antibodies, inhibitor or mimic, which was used to be positive control of TLR4 expression. The non-LPS-treated cells were taken as the blank group. The cells treated with negative control or IgG control were taken as the control groups.
MiRNA inhibitor or mimic transfection
HPDLCs were transfected with miRNA-146a inhibitor, mimic or negative controls (Applied Biosystems, CA, USA) at the concentration of 10 nM according to our previous study [22]. In brief, the medium was changed to Opti-MEM (Invitrogen, CA, USA) after hPDLCs were cultured overnight. Then, the cells were transfected with miRNA-146a inhibitor, mimic or negative control using Lipofectamine 2000 (Invitrogen, CA, USA). After 6 h, the medium was replaced with DMEM containing 10 % FBS.
RNA quantification
After hPDLCs were stimulated as above, total RNA, containing miRNA, was extracted with TRIzol reagent (Invitrogen, CA, USA) following the manufacturer’s instructions. The cDNA reverse transcription was performed using GoldScript cDNA synthesis system (Invitrogen, CA, USA). The qPCR SuperMix-UDG ROX kit (Invitrogen, CA, USA) was used to detect TLR2, TLR4 and β-actin as the manufacture’s instruction on Applied Biosystems 7900HT Sequence Detection System (Applied Biosystems, CA, USA). The real-time PCRs were performed in triplicates. For TLR2, TLR4, and β-actin mRNA analysis, the primers were described previously [7]. For TLR2, the primers were: 5′-GCCAAAGTCTTGATTGATTGG-3′ (forward) and 5′-TTGAAGTTCTCCAGCTCCTG-3′ (reverse); for TLR4, the primers were: 5′-AGGATGAGGACTGGGTAAGGA-3′ (forward) and 5′-CCTGTACGCCAACACAGTGC-3′ (forward) and 5′-ATACTCCTGCTTGCTGATCC-3′ (reverse); for β-actin, the primers were: 5′-CCTGTACGCCAACACAGTGC-3′ (forward) and 5′-ATACTCCTGCTTGCTGATCC-3′ (reverse); data were normalized by the level of β-actin expression in each sample by using 2−ΔΔCT method [16].
For miRNA analysis, real-time PCR was performed as previously described [16]. Briefly, total RNA was transcribed reversely into cDNA using gene-specific reverse transcription (RT) primers designed according to miRNA sequences in the Sanger miRBase. The U6 small nuclear RNA (NR_003027) was used as an internal control. For hsa-miRNA-146a, the RT primer is: GTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTGCACTGGATACG ACaaccca; Quantitative PCR (q-PCR) primers were as follows: hsa-miR-146a: 5′-GGGTGAGAACTGAATTCCA-3′ (forward); 5′-CAGTGCGTGTCGTGGAGT-3′ (reverse). The forward and reverse primers for U6 small nuclear RNA was 5′-GCTTCGGCAGCACATATACTAAAAT-3′ (forward) and 5′-CGCTTCACGAATTTGCGTGTCAT-3′ (reverse). The relative expression level of miRNAs was normalized to that of internal control U6 by using 2−ΔΔCT method [16].
NF-κ B nuclear activity assay
After treatment with P. gingivalis LPS in the absence or presence of miRNA-146a inhibitor, mimic or negative control, nuclear fraction was collected from hPDLCs using nuclear extract kit (Active Motif, CA, USA). In brief, after being washed with ice-cold PBS/phosphatase inhibitors, the cells were transferred to a pre-chilled tube and centrifuged. After incubation with hypotonic buffer on ice, cell pellet was added detergent. The mixture was centrifuged to obtain the nuclear pellet. The nuclear pellet was resuspended in lysis buffer with vortex at highest speed, and then incubated on ice. After centrifugation, the supernatant was aliquoted and stored at −80 °C.
NF-κB p65 DNA binding activity in the nuclear extracts of hPDLCs was determined using the non-radioactive TransAM transcription factor assay (Active Motif, CA, USA) according to the manufacturer’s instruction. Briefly, the nuclear extracts were added to bind the NF-κB p65 consensus sequence in wells. After reaction with primary and secondary antibodies serially, the nuclear extracts incubated with the developing solution at room temperature without direct light. Then, the wells were read in Microplate Reader (Bio-tek, VT, USA) at 450 nm. NF-κ B p65 nuclear activity was described as the x-fold expression over the untreated cells.
Cytokine detection
HPDLCs were stimulated with P. gingivalis LPS in the absence or presence of miRNA-146a inhibitor, mimic or negative control for 24 h at 37 °C. The cell-free supernatants were harvested and stored at −20 °C for cytokine assays (R&D, MN, USA). The levels of IL-1β, IL-6, IL-8 and TNF-α in the culture supernatants were measured by ELISA according to the manufacturer’s instruction. The plates were read in Microplate Reader (Bio-tek, VT, USA) at 450 nm.
Statistical analysis
All experiments were repeated three times. The results were represented as mean ± SD. The differences in the data were tested for statistical significance using analysis of variance (ANOVA) and Student–Newman–Keuls test. The level of significance was set at p < 0.05.
Results
Expression of TLR2, TLR4 and miRNA-146a in hPDLCs stimulated with P. gingivalis LPS
Compared with the untreated cells, the level of TLR2 mRNA increased significantly (3.05 ± 0.23, 3.78 ± 0.31, respectively, p < 0.05) in the cells treated by 1 and 10 μg/ml P. gingivalis LPS in concentration-dependent manner (Fig. 1a), while its level was unchanged in the cells stimulated by E. coli LPS (1.03 ± 0.14, p > 0.05). The data indicated that P. gingivalis LPS activated different TLR from E. coli LPS. Meanwhile, the expression of TLR4 mRNA increased significantly in the cells treated by 10 μg/ml P. gingivalis LPS (2.21 ± 0.31, p < 0.05) compared with the untreated cells, which was much lower than 1 μg/ml E. coli LPS (6.04 ± 0.35, p < 0.05). Whereas TLR4 level did not increase in 1 μg/ml P. gingivalis LPS treated-group (1.11 ± 0.30, p > 0.05) compared with the non-LPS treated group (Fig. 1b).
Expression of TLR2, TLR4 and miRNA-146a in human periodontal ligament cells. a Expression of TLR2 in cells stimulated with P. gingivalis LPS or E. coli LPS; b expression of TLR4 in cells stimulated with P. gingivalis LPS or E. coli LPS;c expression of miRNA-146a in cells stimulated with P. gingivalis LPS or E. coli LPS. The observation was based on the pooled cells from all the donors. Values are expressed as mean ± SD from three independent experiments. *p < 0.05, compared to the blank group. Blank no LPS treatment
Also, there was a significant increase in the expression of miRNA-146a in the hPDLCs stimulated with 1, 10 μg/ml P. gingivalis LPS and 1 μg/ml E. coli LPS (3.66 ± 0.83, 4.91 ± 0.87, 5.65 ± 0.57, respectively, p < 0.05) compared with the blank group (Fig. 1c).
The expression of miRNA-146a was measured at different time point. The miRNA-146a level increased after stimulation with 1 and 10 μg/ml P. gingivalis LPS (p < 0.05) (Fig. 2a), which was in time-dependent manner. The pre-treatment with miRNA-146a inhibitor could downregulate the increased level of miRNA-146a in hPDLCs after 1 and 10 μg/ml P. gingivalis LPS stimulation compared with the control group (p < 0.05) (Fig. 2b–d).
miRNA-146a level in human periodontal ligament cells after stimulated with 1 and 10 μg/ml P. gingivalis LPS. The observation was based on the pooled cells from all the donors. Values are expressed as mean ± SD from three independent experiments. *p < 0.05, compared to the beginning time point or the control group. Blank no LPS treatment, medium no miRNA-146a inhibitor or negative control treatment, control negative control treatment
Involvement of miRNA-146a in TLR2, 4 pathway in hPDLCs stimulated with P. gingivalis LPS
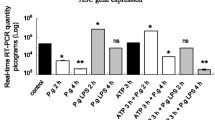
To detect the role of miRNA-146a in TLR2, 4 pathway in hPDLCs stimulated with P. gingivalis LPS, anti-TLR2 mAb or anti-TLR4 mAb was used to block the sensing of LPS by TLR2 or TLR4 in hPDLCs. After the pretreatment with anti-TLR2 mAb, there was a significant decrease in the expression of miRNA-146a in hPDLCs stimulated with 1 and 10 μg/ml P. gingivalis LPS (1.34 ± 0.71, 1.90 ± 0.68, respectively, p < 0.05) (Fig. 3a). In the presence of anti-TLR4 mAb, there was an obvious decrease in the expression of miRNA-146a in hPDLCs stimulated with 10 μg/ml P. gingivalis LPS (2.91 ± 0.86, p < 0.05). However, it did not increase obviously in hPDLCs stimulated with 1 μg/ml P. gingivalis LPS (3.32 ± 0.72, p > 0.05) (Fig. 3b). These data indicate that miRNA-146a is involved in TLRs pathway, especially TLR2.
Expression of miRNA-146a in human periodontal ligament cells stimulated with P. gingivalis LPS after pretreatment with anti-TLR2 mAb or anti-TLR4 mAb. a Expression of miRNA-146a after pretreatment with anti-TLR2 mAb; b expression of miRNA-146a after pretreatment with anti-TLR4 mAb. Cells pooled from all the donors were observed. Values are expressed as mean ± SD from three independent experiments. *p < 0.05, compared to the control group. Blank no LPS treatment, medium no antibody and negative control treatment, control negative control treatment
Furthermore, the miRNA-146a inhibitor or mimic was used to test whether miRNA-146a could regulate TLR2, 4 expressions or not. The results showed that TLR2 increased significantly in hPDLCs after pretreatment with miRNA-146a inhibitor and stimulation with 1 and 10 μg/ml P. gingivalis LPS (3.76 ± 0.74, 5.28 ± 0.62, respectively, p < 0.05) (Table 2), while TLR4 increased at 10 μg/ml P. gingivalis LPS (3.64 ± 0.36, p < 0.05), not at 1 μg/ml P. gingivalis LPS (1.02 ± 0.32, p > 0.05) (Table 2). The miRNA-146a mimic could inhibit the expression of TLR2 (TLR2: 1.36 ± 0.42, 1.87 ± 0.61 respectively, p < 0.05) at different concentration of P. gingivalis LPS (Table 3). Also, the miRNA-146a mimic could inhibit the expression of TLR4 (1.02 ± 0.23, p < 0.05) in hPDLCs stimulated with 10 μg/ml P. gingivalis LPS (Table 3). From these data, it is showed that miRNA-146a negatively regulates the levels of TLR2 and TLR4.
Activation of NF-κ B p65 nuclear activity in hPDLCs stimulated with P. gingivalis LPS
The results indicated a significant increase in the nuclear activity of NF-κ B p65 in hPDLCs after stimulation with 1 and 10 μg/ml P. gingivalis LPS (4.06 ± 0.78 fold, 6.51 ± 0.77 fold, respectively, p < 0.05) (Fig. 4).
Activation of NF-κ B p65 nuclear activity in human periodontal ligament cells stimulated with P. gingivalis LPS in presence of miRNA-146a inhibitor or mimic. a NF-κ B p65 nuclear activity in human periodontal ligament cells stimulated with P. gingivalis LPS in presence of miRNA-146a inhibitor; b NF-κ B p65 nuclear activity in human periodontal ligament cells stimulated with P. gingivalis LPS in presence of miRNA-146a mimic. Cells pooled from all the donors were observed. Values are expressed as mean ± SD from three independent experiments. *p < 0.05, compared to the control group. Blank no LPS treatment, medium no miRNA-146a inhibitor, mimic or negative control treatment, control negative control treatment
After the pretreatment with miRNA-146a inhibitor, the nuclear activity of NF-κ B p65 increased significantly in 1 μg/ml P. gingivalis LPS-treated cells (5.71 ± 0.86 fold, p < 0.05). Although the nuclear activity of NF-κ B p65 increased (7.82 ± 1.03 fold) in miRNA-146a inhibitor + 10 μg/ml P. gingivalis LPS-treated cells, there was no significant difference while compared with only 10 μg/ml P. gingivalis LPS-treated cells (p > 0.05) (Fig. 4a). The miRNA-146a mimic was used to further confirm the inhibitory effect of miRNA-146a. After miRNA-146a mimic pretreatment, the nuclear activity of NF-κ B p65 decreased obviously (2.01 ± 0.22 fold, 2.61 ± 0.84 fold, p < 0.05) (Fig. 4b), which indicates that miRNA-146a can block the signaling pathway of inflammation in hPDLCs.
Cytokines production in hPDLCs stimulated with P. gingivalis LPS in presence of miRNA-146a mimic or inhibitor
Cells were incubated with P. gingivalis LPS at different concentration in the absence or presence of miRNA-146a mimic, inhibitor or negative control. The production of IL-1β, IL-6, IL-8, and TNF-α in the culture supernatants was quantified by ELISA after 24 h of incubation. The production of IL-1β (202.26 ± 25.36, 275.31 ± 33.22 pg/ml), IL-6 (102.32 ± 26.32, 146.45 ± 30.13 pg/ml), IL-8 (426.45 ± 68.38, 614.35 ± 90.52 pg/ml) and TNF-α (152.35 ± 18.37, 212.38 ± 46.68 pg/ml) increased significantly after 1 and 10 μg/ml P. gingivalis LPS treatment (p < 0.05) (Tables 4, 5), which was in the concentration-dependent manner. In the presence of miRNA-146a inhibitor, these cytokines increased after 1 μg/ml P. gingivalis LPS treatment (IL-1β: 285.19 ± 27.33; IL-6: 168.47 ± 27.24; IL-8: 586.22 ± 70.32; TNF-α: 267.33 ± 26.47 pg/ml) (p < 0.05) (Table 4). Although IL-1β (326.54 ± 34.35 pg/ml), IL-6 (195.32 ± 28.34 pg/ml), IL-8 (726.32 ± 95.13 pg/ml), and TNF-α (309.90 ± 50.10 pg/ml) levels had the increasing tendency after the inhibition of miRNA-146a and treatment with 10 μg/ml P. gingivalis LPS (Table 4), there was no statistical significance compared with only 10 μg/ml P. gingivalis LPS treatment (p > 0.05), possibly due to the culture condition and cell secretion ability up to the limit. Furthermore, after the pretreatment with miRNA-146a mimic, there was a significant decrease in the production of these cytokines induced by 1 and 10 μg/ml P. gingivalis LPS treatment (IL-1β: 72.32 ± 26.51, 100.36 ± 33.68; IL-6: 46.78 ± 24.13, 65.126 ± 29.32; IL-8: 168.33 ± 62.47, 189.34 ± 82.44; TNF-α: 68.26 ± 23.57, 115.91 ± 44.76 pg/ml, respectively) (p < 0.05) (Table 5).
Discussion
In this study, we have identified that miRNA-146a was involved in TLRs-NF-κB signaling pathway, functioning as a negative feedback regulator in P. gingivalis LPS-induced inflammatory reaction in hPDLCs.
Periodontitis is an infectious disease, which is mainly caused by bacterial biofilm and host immune response. Inflammation in periodontal ligament contributes to the absorption of alveolar bone and the formation of deep periodontal pockets, resulting in the development of periodontitis. As the dominant cells in periodontal ligament, hPDLCs have been demonstrated to be involved in the inflammatory response and secret inflammatory cytokines [7], which is consistent with the results in this study.
It has been known that P. gingivalis is one of the main periodontopathic bacteria. Its LPS has been implicated as one of major virulent factors in the process of periodontitis. It has been found that P. gingivalis LPS stimulated macrophages to produce cytokines [23]. In our study, P. gingivalis LPS could induce hPDLCs to secret inflammatory cytokines, which is consistent with Sun et al. [7] study.
However, whether P. gingivalis LPS was capable of stimulating TLR2 and/or TLR4 is still controversial [24]. It has been reported that P. gingivalis LPS induced the production of cytokines through TLR2 in macrophages [23]. Meanwhile, knockdown of TLR2 can downregulate the secretion of IL-6 and IL-8 in hPDLCs stimulated by P. gingivalis LPS in vitro [25]. In another report, P. gingivalis LPS could increase TLR2 expression, however, induce TLR4 expression only at high concentration in hPDLCs [7], which is further confirmed by our data. However, NF-κ B dependent CD25 level increased in TLR2 deficient ovary cells after P. gingivalis LPS stimulation, and its level did not change in TLR4 deficient ovary cells [26]. This report indicated that LPS from P. gingivalis is agonist for TLR4. All these data implied that cells from different tissues might sense P. gingivalis LPS differently or express different primary TLRs. In our study, the comparative experiments were done with P. gingivalis LPS and E. coli LPS. Our results showed that E. coli LPS exhibited TLR4-agonistic activity alone, which is consistent with Savitri et al. [27] research. These researches demonstrated that P. gingivalis LPS had the unique activities, which were different from E. coli LPS. More researches are needed to explore the mechanism of signal transduction induced by P. gingivalis LPS.
It has been demonstrated that miRNA-146 was involved in periodontal inflammation after compared healthy tissues with inflammatory tissues from periodontitis [18]. Also, in our previous study, we demonstrated that miRNA-146 increased in periodontal-diseased gingiva [17]. In addition, miRNA-146 expression increased in human gingival fibroblasts after stimulation with P. gingivalis LPS [17]. However, the expression and function of miRNA-146a in hPDLCs is still unclear.
In this study, miRNA-146a was induced by P. gingivalis LPS in hPDLCs. Meanwhile, miRNA-146a decreased after TLR2, 4 mAb application, and miRNA-146a inhibitor increased the mRNA level of TLR2, 4 and NF-κ B p65 nuclear activity. Furthermore, miRNA-146a mimic decreased these levels. Taken together, miRNA-146a was involved in TLRs-NF-κ B pathway induced by P. gingivalis LPS and functioned as a negative feedback regulator through regulating the expression of TLR2, 4 and NF-κ B p65 nuclear activity. Also, it has been demonstrated that miRNA-146 was involved in TLR signaling pathways, which is consistent with other researches [11, 28].
IL-1β, IL-6, IL-8, and TNF-α as pro-inflammatory cytokines could activate inflammation. In addition, IL-1β, IL-6 and TNF-α could cause bone resorption through activating osteoclast. The levels of IL-1β, IL-6, IL-8 and TNF-α increased in periodontitis [29, 30]. Therefore, these cytokines are closely related to the occurrence and progression of periodontitis. It has been shown that hPDLCs function as one of the regulatory cells of cytokine network and produce inflammatory cytokines in response to P. gingivalis LPS [7, 31]. Also, from our data, hPDLCs secreted IL-1β, IL-6, IL-8, and TNF-α after stimulation with P. gingivalis LPS. In this present study, we further explored the possible role of miRNA-146a in the secretion of these cytokines. The levels of IL-1β, IL-6, IL-8 and TNF-α increased after the inhibition of miRNA-146a, while their levels decreased after the application of miRNA-146a mimic. These data indicate that miRNA-146a negatively regulates the secretion of pro-inflammatory cytokines and prevents aggressive inflammation. Also, it has been demonstrated that miRNA-146 was induced by IL-1β and TNF-α in the synovial tissues of patients with rheumatoid arthritis [28]. These data implies that miRNA-146 and cytokines such as IL-1β and TNF-α form mutual regulatory network. We guess that miRNA-146a could be directly induced due to an increase in IL-1β and TNF-α after stimulation with P. gingivalis LPS. However, further confirmation is necessary.
Cells pooled from the different donors were used in this study, which is consistent with other researches [7, 32, 33]. Previous researches have demonstrated that hPDLCs from extracted teeth for orthodontic reason in young and healthy individuals showed identical morphology and functional characteristics in spite of the different donors [34–36]. Meanwhile, some researches have showed that the numeric data were not very similar among the donors, but the pattern of cell responses to external stimuli was identical [18, 25]. Thus, although it is a limitation in our study that individual differences exist among the cells from different donors, the data reveal, to some extent, the regulatory mechanism of miRNA-146a in hPDLCs.
In summary, miRNA-146a would regulate the immune response in P. gingivalis LPS-stimulated hPDLCs, functioning as a negative regulator in TLRs-NF-κ B pathway in inflammation response. Therefore, it is critical to keep appropriate expression levels of TLR2, 4 and miRNA-146a to maintain homeostasis in periodontal tissues during periodontal infection. Also, miRNA-146a plays a protective role in periodontal inflammation.
References
Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–97.
Sonkoly E, Ståhle M, Pivarcsi A. MicroRNAs and immunity: novel players in the regulation of normal immune function and inflammation. Semin Cancer Biol. 2008;18(2):131–40.
Matera G, Muto V, Vinci M, Zicca E, Abdollahi-Roodsaz S, van de Veerdonk FL, Kullberg BJ, Liberto MC, van der Meer JW, Focà A, Netea MG, Joosten LA. Receptor recognition of and immune intracellular pathways for Veillonella parvula lipopolysaccharide. Clin Vaccine Immunol. 2009;16(12):1804–9.
Harokopakis E, Hajishengallis G. Integrin activation by bacterial fimbriae through a pathway involving CD14, toll-like receptor 2, and phosphatidylinositol-3-kinase. Eur J Immunol. 2005;35(4):1201–10.
Mahanonda R, Sa-Ard-Iam N, Montreekachon P, Pimkhaoham A, Yongvanichit K, Fukuda MM, Pichyangkul S. IL-8 and IDO expression by human gingival fibroblasts via TLRs. J Immunol. 2007;178(2):1151–7.
Gaddis DE, Michalek SM, Katz J. Requirement of TLR4 and CD14 in dendritic cell activation by hemagglutinin B from Porphyromonas gingivalis. Mol Immunol. 2009;46(13):2493–504.
Sun Y, Shu R, Li CL, Zhang MZ. Gram-negative periodontal bacteria induce the activation of toll-like receptors 2 and 4, and cytokine production in human periodontal ligament cells. J Periodontol. 2010;81(10):1488–96.
Uehara A, Takada H. Functional TLRs and NODs in human gingival fibroblasts. J Dent Res. 2007;86(3):249–54.
Gilmore TD. Introduction to NF-kappa B: players, pathways, perspectives. Oncogene. 2006;25(51):6680–4.
Taganov KD, Boldin MP, Baltimore D. MicroRNAs and immunity: tiny players in a big field. Immunity. 2007;26(2):133–7.
Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappa B dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci USA. 2006;103(33):12481–6.
van Winkelhoff AJ, Loos BG, van der Reijden WA, van der Velden U. Porphyromonas gingivalis, Bacteroides forsythus and other putative periodontal pathogens in subjects with and without periodontal destruction. J Clin Periodontol. 2002;29(11):1023–8.
Chiang CY, Fu E, Shen EC, Chiu HC. Effects of CD14 receptors on tissue reactions induced by local injection of two gram-negative bacterial lipopolysaccharides. J Periodontal Res. 2003;38(1):36–43.
Seo T, Cha S, Kim TI, Lee JS, Woo KM. Porphyromonas gingivalis-derived lipopolysaccharide-mediated activation of MAPK signaling regulates inflammatory response and differentiation in human periodontal ligament fibroblasts. J Microbiol. 2012;50(2):311–9.
Kato H, Taguchi Y, Tominaga K, Umeda M, Tanaka A. Porphyromonas gingivalis LPS inhibits osteoblastic differentiation and promotes pro-inflammatory cytokine production in human periodontal ligament stem cells. Arch Oral Biol. 2014;59(2):167–75.
Xie YF, Shu R, Jiang SY, Liu DL, Zhang XL. Comparison of microRNA profiles of human periodontal diseased and healthy gingival tissues. Int J Oral Sci. 2011;3(3):125–34.
Lee YH, Na HS, Jeong SY, Jeong SH, Park HR, Chung J. Comparison of inflammatory microRNA expression in healthy and periodontitis tissues. Biocell. 2011;35(2):43–9.
Sipert CR, Morandini AC, Dionísio TJ, Trachtenberg AJ, Kuo WP, Santos CF. MicroRNA-146a and microRNA-155 show tissue-dependent expression in dental pulp, gingival and periodontal ligament fibroblasts in vitro. J Oral Sci. 2014;56(2):157–64.
Tang X, Meng H, Han J, Zhang L, Hou J, Zhang F. Up-regulation of estrogen receptor-beta expression during osteogenic differentiation of human periodontal ligament cells. J Periodontal Res. 2008;43(3):311–21.
Liu K, Meng H, Hou J. Characterization of the autocrine/paracrine function of vitamin D in human gingival fibroblasts and periodontal ligament cells. PLoS One. 2012;7(6):e39878.
San Miguel SM, Opperman LA, Allen EP, Zielinski J, Svoboda KKH. Bioactive polyphenol antioxidants protect oral fibroblasts from ROS-inducing agents. Arch Oral Biol. 2012;57(12):1657–67.
Xie YF, Shu R, Jiang SY, Liu DL, Ni J, Zhang XL. MicroRNA-146 inhibits pro-inflammatory cytokine secretion through IL-1 receptor-associated kinase 1 in human gingival fibroblasts. J Inflamm (Lond). 2013;10(1):20.
Zhou Q, Desta T, Fenton M, Graves DT, Amar S. Cytokine profiling of macrophages exposed to Porphyromonas gingivalis, its lipopolysaccharide, or its Fim A protein. Infect Immun. 2005;73(2):935–43.
Ogawa T, Asai Y, Makimura Y, Tamai R. Chemical structure and immunobiological activity of Porphyromonas gingivalis lipid A. Front Biosci. 2007;12:3795–812.
Morandini AC, Chaves Souza PP, Ramos-Junior ES, Brozoski DT, Sipert CR, Souza Costa CA, Santos CF. Toll-like receptor 2 knockdown modulates interleukin (IL)-6 and IL-8 but not stromal derived factor-1 (SDF-1/CXCL12) in human periodontal ligament and gingival fibroblasts. J Periodontol. 2013;84(4):535–44.
Yoshimura A, Kaneko T, Kato Y, Golenbock DT, Yoshitaka H. Lipopolysaccharides from periodontopathic bacteria Porphyromonas gingivalis and Capnocytophaga ochracea are antagonists for human toll-like receptor 4. Infect Immun. 2002;70(1):218–25.
Savitri IJ, Ouhara K, Fujita T, Kajiya M, Miyagawa T, Kittaka M, Yamakawa M, Shiba H, Kurihara H. Irsogladine maleate inhibits Porphyromonas gingivalis-mediated expression of toll-like receptor 2 and interleukin-8 in human gingival epithelial cells. J Periodontal Res. 2014;. doi:10.1111/jre.12231.
Nakasa T, Miyaki S, Okubo A, Hashimoto M, Nishida K, Ochi M, Asahara H. Expression of microRNA-146 in rheumatoid arthritis synovial tissue. Arthritis Rheum. 2008;58(5):1284–92.
Fentoğlu Ö, Köroğlu BK, Hiçyılmaz H, Sert T, Özdem M, Sütçü R, Tamer MN, Orhan H, Ay ZY, Öztürk Tonguç M, Kırzıoğlu FY. Pro-inflammatory cytokine levels in association between periodontal disease and hyperlipidaemia. J Clin Periodontol. 2011;38(1):8–16.
Noh MK, Jung M, Kim SH, Lee SR, Park KH, Kim DH, Kim HH, Park YG. Assessment of IL-6, IL-8 and TNF-α levels in the gingival tissue of patients with periodontitis. Exp Ther Med. 2013;6(3):847–51.
Zhang Y, Li X. Lipopolysaccharide-regulated production of bone sialoprotein and interleukin-8 in human periodontal ligament fibroblasts: the role of toll-like receptors 2 and 4 and the MAPK pathway. J Periodontal Res. 2014;. doi:10.1111/jre.12193.
Wolf M, Lossdörfer S, Römer P, Craveiro RB, Deschner J, Jäger A. Anabolic properties of high mobility group box protein-1 in human periodontal ligament cells in vitro. Mediator Inflam. 2014;2014:347585. doi:10.1155/2014/347585.
Gölz L, Memmert S, Rath-Deschner B, Jäger A, Baumgarten G, Götz W, Frede S. LPS from P. gingivalisingivalis and hypoxia increases oxidative stress in periodontal ligament fibroblasts and contributes to periodontitis. Mediators Inflam. 2014;2014:986264. doi:10.1155/2014/986264.
Jönsson D, Wahlin A, Idvall I, Johnsson I, Bratthall G, Nilsson BO. Differential effects of estrogen on DNA synthesis in human periodontal ligament and breast cancer cells. J Periodontal Res. 2005;40(5):401–6.
Jönsson D, Nilsson J, Odenlund M, Bratthall G, Broman J, Ekblad E, Lydrup ML, Nilsson BO. Demonstration of mitochondrial oestrogen receptor β and oestrogen-induced attenuation of cytochrome c oxidase subunit I expression in human periodontal ligament cells. Arch Oral Biol. 2007;52(7):669–76.
Nebel D, Svensson D, Arosenius K, Larsson E, Jönsson D, Nilsson BO. 1α, 25-dihydroxyvitamin D3 promotes osteogenic activity and downregulates proinflammatory cytokine expression in human periodontal ligament cells. J Periodontal Res. 2014;. doi:10.1111/jre.12249.
Acknowledgments
This work was supported by Science and Technology Development Project of Tianjin Education Commission (2010134) and National Natural Science Foundation (81100758/H1405).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Bernhard Gibbs.
Rights and permissions
About this article
Cite this article
Jiang, SY., Xue, D., Xie, YF. et al. The negative feedback regulation of microRNA-146a in human periodontal ligament cells after Porphyromonas gingivalis lipopolysaccharide stimulation. Inflamm. Res. 64, 441–451 (2015). https://doi.org/10.1007/s00011-015-0824-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-015-0824-y