Abstract
Short shelf-life of banana at ambient temperature is major concern in tropical regions because the temperature elevates the ripening related changes such as tissue softening, senescence, spotting on peel, off-odour and fungal disease mainly anthracnose. Here, this work is focused to evaluate the potential effect of Aloe gel (AG), lemon peel extract (LPE) and their combinations as edible coating treatment on postharvest quality and shelf-life of banana during storage at 23 ± 1 °C. Effectiveness of coating on banana was assessed by performing variety of physiochemical parameters namely weight loss, decay, disease severity, pH, acidity, soluble solids, sugars and ascorbic acid. Moreover, the antifungal efficacy of lemon peel extract was examined on the most disastrous fungus Colletotrichum musae causing anthracnose in banana. The treated fruits showed reduction in the rate of weight loss and decay of the fruits during storage. The breakdown of organic acids and accumulation of soluble solids were slower in bananas protected with edible coatings. Furthermore, the amount of ascorbic acid in banana was maintained in AG (50%) + LPE (15%) coated fruits during storage. Besides, the application of edible coating extends the banana shelf life up to 9 days with no disease incident. Thus, it indicates that the Aloe gel (AG) and lemon peel extract (LPE) constituted edible coating is an effective alternative to extend the shelf life and reduce the quality losses in banana rather than the use of hazardous fungicides and chemical preservatives.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Banana (Musa sp. L.) is a very delicate and nutritive tropical fruit with a unique flavor. Bananas are very popular in mostly all the regions of the world and one of the commercially important crops. However, the pathogenic infection in banana causes remarkable yield loss after harvest [1]. Though India has commercial and agronomic potential to cultivate different varieties of bananas, the inevitable postharvest losses due to different diseases can severely reduce the marketable yield [1]. The control of postharvest diseases via expensive methods such as modified atmosphere storage (MAS) is not feasible in India because small-scale growers (farmers) are largely involved in the production of bananas. Colletotrichum musae, a genus causing disease in more than 300 species of commercial crop plants, causing anthracnose in banana [2]. Modern agricultural techniques to control the pathogenic infection in banana involve dipping in or spraying fungicides shortly after harvest [3]. However, the resistance to fungicides over time require farmers to apply fungicides in higher dosage. However, the public concern limits the application of chemical fungicides as it causes hazardous effects on human health. Hence, the preference for chemical-free fruits and the use of plant-derived biocontrol agents [4] in fruit preservation has become an emerging trend.
Edible coating application is an effective tool for the postharvest preservation of fruits from pathogens and to avoid quality loss. The correct combination of suitable coating material with antimicrobial compounds would give surprising results in reducing postharvest losses. The reports have been stated that the use of natural edible coatings such as Aloe gel, Cactus mucilage and gum arabic increase the shelf life and reduce the qualitative losses of various fruits, including banana [1, 5]. The parenchymatous cells in the fresh leaves of the commercially important plant Aloe barbadensis Mill. popular as Aloe vera L. is the source of colorless mucilaginous gel. The use of Aloe gel as a resource of functional food is increased interestingly in the ice-cream and beverages [6]. The Aloe gel reduced the growth of fungal mycelia by 22–38% in Colletotrichum, Rhizoctonia and Fusarium [7], and reduced viability of fungal spore by 15–20% in Botrytis, Penicillium and Alternaria [7]. The determination towards the disease control in postharvest storage of banana could be achieved by applying Aloe gel as edible coating. Though the Aloe gel affects the mycelial growth and spore survival of certain fungus, the potential could increase by the addition of a natural antimicrobial agents. Plants develop various bioactive compounds as a byproduct of development or in response to the pathogen infection or stress [8]. Keeping this in mind, the antimicrobial agent included in the present study from the following source.
Lemon (Citrus limon L.), a member of the Rutaceae family, is a rich source of active compounds that possess antimicrobial and antioxidant activity [9]. The lemon fruit residues like its peel are considered waste and discarded in the environment, but they can be potential resources full of nutraceuticals [10]. Fruit flavedo or epicarp or peel of the lemon contains beneficial metabolites such as flavonoids, essential oils, pectins, pigments and various other biomolecules. [9]. Flavonoids are a class of secondary metabolites with high antioxidant activity, and essential oils have shown potent antimicrobial activity against mould growth. They are non-toxic to mammals and environmentally safe to use [11, 12]. These compounds are biodegradable and given the status of Generally Recognized as Safe (GRAS) [13] and has no phytotoxic effect on humans and a small concentration is enough to create a significant effect on microorganisms [8, 14]. Thus, the use of plant derived biodegradable compounds is increasing to control pests and diseases in postharvest fresh produces and their use in postharvest treatments has been considered safe; therefore, no regulatory issues [15].
The work presented in this manuscript outlined the application and effectiveness of the edible coating formulated from Aloe gel in combination with crude extract of lemon epicarp (peel) on the banana fruits to improve their shelf life and marketability. Based on the literature review, there were no reports available on Aloe gel with lemon peel extract as an edible coating to prolong the shelf life and maintain the quality of banana fruit. Moreover, the in vitro antifungal assay can establish the potentiality of crude extract of C. limon epicarp against the fungal organisms such as Colletotrichum musae, Aspergillus niger, Fusarium oxysporum, Mycorrhizae macrophomina, which are responsible for remarkable postharvest losses of banana. The Aloe gel and crude lemon peel extract are also convenient for the farmers to prepare who do not have scientific knowledge and ability to handle sophisticated instruments.
Materials and methods
Materials and experimental procedure
Physiologically matured banana fruits were sourced from the Anand district of Gujarat, India. The fruits selected in this work were free from any visible defect including mechanical injury and diseases and were at a similar stage of ripening and appearance i.e., skin colour. Prior to the edible coating treatments, the fruit hands were washed with running tap water to remove soil particles and debris and then surface sanitized using sodium hypochlorite (10 mL L−1) for 40 s.
Plant collection and extract preparation
Fresh and mature lemon (Citrus limon L.) fruits were collected from the lemon trees cultivated in an agriculture farm near the Bakrol village, Anand, Gujarat, India. Lemon fruits used in the study were free from any mechanical injury or pathogenic infection. The lemons were washed thoroughly in tap water followed by washing with distilled water.
The lemon peel contains oil glands in pits filled with essential oil which can be used as a defense shield against the pathogen attack on various fruits. The leathery rind or exocarp of lemon fruit was peeled carefully and cut into small pieces. These pieces of lemon exocarp (1 g) were immersed with 10 mL of hydro alcohol (70% methanol) and placed in a rotary shaker at 100 rpm for 72 h. Thereafter, it was homogenized and centrifuged at 4500 × g (RCF) for 15 min. The filtrate was collected by filtering them through Whatman No.1 filter paper. The clear filtrate was evaporated to dryness at 40 °C in a laboratory oven. The dried residues of lemon peel extract (LPE) were collected and used for antifungal assay as well as edible coating treatment [16].
Isolation and collection of fungal organisms
The antifungal assay lies in the center of this experiment due to the potential effect of antimicrobial compound-lemon peel extract against the pathogens present on the fruit surface. For this reason, some of the fungal organisms responsible for the spoilage of bananas during the storage were isolated from infected banana to evaluate the effect of crude lemon peel extract on them.
Colletotrichum musae, the primary causal organism of anthracnose disease on banana was isolated from the banana field on potato dextrose agar (PDA) and re-cultured to obtain pure cultures. The culture was identified using a combination of cultural and morphological characteristics. Moreover, Aspergillus niger, Fusarium oxysporum, Mycorrhizae macrophomina were obtained from the Department of plant pathology, Anand Agriculture University, Anand, Gujarat, India and maintained on the PDA plates [9].
Preparation of coating treatments
Aloe gel was prepared from fresh and matured leaves of Aloe vera L. The leaves were washed in water followed with 2% hypochlorite solution. The outer green cortex of leaves was separated from mesophyll pulp, and the colorless thick parenchyma matrix was ground in a blender. This blend was filtered to remove fibrous mass and the resultant liquid is fresh and pure Aloe gel. The gel was pasteurized at a temperature of 70 °C. It was then cooled and stabilized by adding ascorbic acid (2.0 g L−1) and citric acid (4.5 g L−1) and pH adjusted to 4.0. A commercial gelling agent was added to the pure Aloe gel to improve its coating efficiency as well as the viscosity and stability during the experiment. The Aloe gel was diluted 1:1 for durable and better film formation on the fruit surface [17]. The crude lemon peel extract (0.75%) was dissolved in Aloe gel to make the edible coating.
Experimental design
Banana, without any physical damage, were sorted into small and even hands of 5 fingers with cautious handling to avoid injury. A completely randomized experimental design involved 16 lots (fruits of similar size) of 15 fruits each were assembled. One lot was kept for evaluating the biochemical and nutritional values of banana when storage period started and the remaining were divided into five different groups. Every single lot was considered as one replica and every treatment contain three replicas for assessment. Five treatments of Aloe gel and lemon peel extract (LPE) used in the experiment were as T1-(Aloe gel 50%), T2-(Aloe gel 50% + LPE 5%), T3-(Aloe gel 50% + LPE 10%), T4-(Aloe gel 50% + LPE 15%) and C-(Control, treated with water). Glycerol (10 g L−1) was added in every single treatment except control as a plasticizer. The sorted replicas of fruits were treated with the respective treatment. The fruits were protected with the above-mentioned edible coating treatments by immersed them in coating solution for 5 min. After that, the protected fruits were allowed to drain the excess liquid of the coating solution to avoid water lesions on the surface. Prior to storage, the fruits were kept at room temperature for 1 h to allow the drying of edible coating completely and making uniform film on the fruit surface. Afterward, the fruits were packed into well aerated zip lock bags to avoid anaerobic respiration. The storage temperature and relative humidity were maintained at 23 ± 1 °C and 78%, respectively, in storage chamber. A range of quality parameters such as weight loss percentage, disease severity, pH, total soluble solid, titratable acidity, reducing sugars, non-reducing sugars, total sugar and ascorbic acid were assessed at regular intervals to monitor the changes in the nutritional qualities of fruit during storage [18].
Antifungal activity
The in vitro antifungal activity of Lemon peel extract (LPE) against Colletotrichum musae, Aspergillus niger, Fusarium oxysporum, Mycorrhizae macrophomina was determined by agar diffusion method by the previously described method of Sales et al. [19] with slight modification. The dried residues (1 g) of lemon peel extract (LPE) was dissolved in 10 mL of distilled water. The agar cup method was followed to evaluate the potential antifungal activity of the Lemon peel extract (LPE). The fungal disc of 1 cm diameter from the six-day grown fungal culture was placed in the center of the Petri plate containing PDA. The wells of approximately 8 mm in diameter and 3.5 mm deep were made in the completely solidified PDA plate by the sterile cork-borer with 3 cm distance from the fungal disc. The lemon peel extract (75 μL) was inoculated into the well, and one plate was inoculated with solvent blank (DMSO). The plates were incubated at 25 °C for 24 h. After incubation, the zone of inhibition was measured and recorded.
Physiological loss of weight
The physiological loss of weight (PLW) was determined by weighing a replica before and after storage period and expressed as a percentage of initial weight [20]:
Decay and shelf life
Decay percentage was calculated by counting the no. of diseased or decayed fruit on every interval during storage period and expressed as a percentage of initial no. of fruits at the time of storage [21]:
Disease severity
Disease severity defines the area covered by the disease in infected fruit and was determined by the previously described method of Sivakumar et al. [22]. The infected area was measured by visual observation. Severity of disease in stored fruit was scored using the scale as follow: 1 = 0% of the fruit surface rotten due to disease; 2 = 1- 25%; 3 = 26- 50%; 4 = 51- 75% and 5 = 76- 100%.
pH, titratable acidity and total soluble solids
The pH of the aqueous extract of fruit pulp (4 g) was determined by the digital pH meter (Model: EUTECH Instruments, Singapore). Titratable acidity was determined according to the previously described method of Horwitz and Latimer [20] and the total acidity was expressed as percentage of malic acid. Total soluble solid from fruit pulp was determined using digital refractometer (Pal-1, Atago Co., Tokyo, Japan) and expressed in °Brix.
Reducing and non-reducing sugars
A previously described method of Thimmaiah [23] and Sadasivam and Manickam [24] was followed to determine the reducing sugars and the non-reducing sugars. 1 g of fruit tissue was homogenized with 10 mL ethanol (80%) to extract the sugars from fruit. The blend was centrifuge at 5000 rpm for 15 min. The supernatant was poured into Petri plates to evaporate the ethanol. The dried residues were dissolved again in distilled water for further estimation.
For reducing sugars, 100 μL of extract was diluted to 3 mL with distilled water and then added with 3 mL DNS (3,5-Dinitrosalicylic acid) reagent. This mixture was incubated in a boiling water bath till development of light brown color. The reaction of sugars with DNS reagent was terminated using 1 mL Rochelle salt (40%). The absorbance for reducing sugars was recorded at 510 nm against the reagent blank. Evaluation of sugar level was configured from the curve recorded using D- glucose as standard. The concentration of sugar was expressed in mg g−1.
For non-reducing sugars, 100 μL of extract was hydrolyzed with the same volume of 0.5 M H2SO4 for 30 min at 49 °C. The mixture was then cooled, 1–2 drop of methyl red was added, neutralized by adding 1 N NaOH and makeup to 3 mL with distilled water. DNS reagent (3 mL) was added and this mixture was incubated in a water bath until light brown colour developed. Then, 1 mL Rochelle salt (40%) was added to stop the reaction. Absorbance was recorded at 510 nm against reagent blank. The sugar content of fruit was analyzed from the standard curve of D-glucose and expressed as mg g−1.
Total sugar
Total sugar of banana fruit was estimated using the previously described method of Thimmaiah [23] and Sadasivam and Manickam [24]. Fruit tissue (1 g) was hydrolyzed with 2.5 M HCl for 3 h. After that, it was neutralized with Na2CO3 till the effervescence ceases and the volume was made up to 10 mL. The resulting homogenate was centrifuged at 5000 rpm for 15 min. For estimation, a clear supernatant (10 μL) was diluted to 1 mL with distilled water. 1 mL of crystalline phenol (5%) followed by 5 mL of H2SO4 (96%) was added to this extract and agitated for uniform distribution. This mixture was incubated at 25–30 °C for 20 min. Absorbance was recorded at 490 nm against reagent blank and evaluated from the standard curve of D-glucose. The concentration was expressed as mg g−1.
Ascorbic acid
The ascorbic acid content of banana was determined following the method of Kapur et al. [25]. Fruit tissue (1 g) was homogenized with 5% m-phosphoric acid and glacial acetic acid (1:1). The blend of fruit tissue was centrifuged and used to quantify ascorbic acid. 100 μL of extracted fruit sample was made up to 1 mL with 5% m-phosphoric acid. Thereafter, 2% DNPH (2,4-Dinitrophenyl hydrazine) reagent (2 mL) and 10% thiourea (25 μL) was added. This mixture was incubated for 3 h at 37 °C. The reaction was terminated by adding 85% H2SO4 (5 mL). The absorbance was recorded at 540 nm in Vis-Spectrophotometer. Standard curve of L-ascorbic acid was configured to examine the change in the ascorbic acid content of fruit and expressed as μg g−1.
Statistical analysis
One-way analysis of variance (ANOVA) was performed using SPSS statistical software (SPSS v 22, Chicago, IL) to determine the significant difference between the treated and control fruits on the same day of storage. The entire experiment was conducted based on completely randomized design. Multiple comparisons were detected using the Duncan test at p ≤ 0.05 [26].
Results and discussion
Antifungal assay of lemon peel extract (LPE)
The natural antifungal compounds are widely used in food industries because of the abandoning of chemically contaminated food by public concerns and awareness. This experiment attempted to evaluate the potential effect of lemon peel extract (LPE) on the common pathogenic fungi of fruits during storage. The effects of LPE on the fungal organisms is summarized as zones of inhibition in Table 1. The antifungal activity of LPE recorded in the present study represents the inhibition of radial growth of the fungal pathogen on solid agar medium. The results revealed that the LPE possesses the potential antifungal activity on the C. musae, A. niger, F. oxysporum, M. macrophomina. The zones of inhibition obtained for the tested fungus were 32.03 mm against C. musae, 23.93 mm against A. niger, 21.07 mm against F. oxysporum, 16.97 mm against M. macrophomina. The activity of the lemon peel extract was due to the presence of different aromatic compounds such as limonene, α-pinene, β-pinene, p-cymene, β-myrcene and terpinolene in the flavedo (peel) of the lemon fruit [9]. Ammad et al. [9] reported that the essential oil extracted from Citrus limon L. prevented the radial growth of some fungal disease of grapevine wood. Moreover, they noted that the Citrus essential oil has potential antifungal activity against Eutypa sp., F. mediterranea, and B. dothidea. Ramírez-Pelayo et al. [27] reported that the coumarins from the peel of citrus significantly inhibited the mycelial growth of Colletotrichum sp. It was also suggested that the potential effect of citrus essential oil increases with increase in the concentration or dosages [9, 27]. During the present study, the correlation of the lemon peel extract (LPE) with the visual observation of fruits in terms of disease severity showed that the LPE could effectively retard and reduce the germination and growth of the fungal pathogen on the fruit surface. The quality of banana in treated fruits at the end of storage period suggested that the higher concentration of LPE in the edible coating treatment such as Aloe gel 50% and LPE 15% retard the infection and protect the fruits from the deteriorative effect of the pathogenic fungi. In the current study, the lesser disease incidences, particularly anthracnose (the devastating disease of banana caused by C. musae), might due to a synergy between the constituents of Aloe gel and LPE because both the component of edible coating possesses antifungal activity.
Weight loss in banana
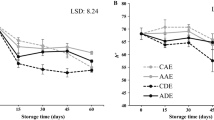
Weight loss is referring as the loss of water and solutes from fresh produces after harvest which declines the quality and marketable value of fruits and vegetables. Application of the edible coating treatment on the fruit significantly reduces the water loss and extend the shelf life. The weight loss in the present study of banana was noticed in both treated and control fruits (Fig. 1). However, weight loss was significantly more in control compared to treated fruits. The weight loss in control fruit was 0.49% on 3rd day and it peaked to 5.45% on 9th day which was noted as the highest compared to treated fruits. The weight loss was observed less in two coating treatments among the coated and control fruits on 9th day, and it was 3.77% in T2 and 3.87% in T4. The minimum weight loss in T4 treatment indicates the semipermeable nature of Aloe gel and LPE coating which effectively reduce the moisture and mass transfer from fruit to environment. The weight loss of fresh fruits during storage is considered as the result of respiration and transpiration processes, and the later one through the lenticels present in the pericarp of fruits due to the vapour pressure gradient driven by surrounding atmosphere [28,29,30]. Silva et al. [31] explained that the coating treatment create a barrier on the fruit surface which reduce the loss of moisture from fresh produce and thus maintain the quality and fresh appearance of fruits. Suseno et al. [28] noted the less weight loss in ‘Cavendish’ banana coated with chitosan due to partial blockage of gases, water and solute transfer [32]. Similarly, the reduction of weight loss in banana and papaya during storage life was also reported by Maqbool et al. [33]. The water as an important role player in maintaining shelf life, quality and market price of fruits [31], less loss of water during storage is critical to maintain and our findings showed that the Aloe gel and LPE coating effectively reduced the rate of water loss from banana during storage.
Effect of edible coating treatments on the quality of weight loss percentage of Banana fruit during storage at 23 ± 1 °C. C control, T1 Aloe vera 50%, T2 Aloe vera 50% + Lemon peel extract 5%, T3 Aloe vera 50% + Lemon peel extract 10%, T4 Aloe vera 50% + Lemon peel extract 15%. Different letters over the bars represent significant differences between the treatments on the same storage day at P ≤ 0.05 according to DMRT Duncan’s Multiple Range Test
Decay rate and shelf life of banana
The Shelf life of fruits depends on the rate of decay by different pathological and mechanical means. After 3 days of storage, fruits started showing decaying characteristics (Table 2). All the fruits of control set (100%) were decayed on 3rd day of storage whereas the treated fruits were fresh and there was no sign of decay and pathogen infection except the fruits of Aloe gel 50% + LPE 5% (T1) treatment which showed 20% decay on 3rd day. Therefore, the control fruits lost their market acceptance hence not analyzed further. As expected, the fruit decay rate was comparatively higher in control than treated fruits. The decay percentage was 20% in both T3 and T4 on 9th day which was least compared to control and other treatments such as T1 (100%) and T2 (80%), showed maximum decay on 9th day among treated fruits. Among the treated banana, the T1 and T2 showed reduced firmness, black spots on the surface and fungal infection on the joints of the banana hands from 3rd day of storage. However, the fruits treated with higher concentration of LPE showed slower ripening and remained fresh for entire storage period with minimum loss and decay. The pathogen infection became very damaging for the stored banana and shortened their shelf life during this experiment. The infectious disease and fungal spore germination on banana surface were controlled by incorporation of antimicrobial agent extracted from lemon peel into the edible coating and this view was supported by Murmu and Mishra [32]. They noted that the addition of antimicrobial compound with edible coating reduced the decay of fruit by preventing the fungal growth. Sivakumar and Bautista-Baños [15] stated that the antimicrobial agent could have protective effect on fruits against invading pathogens and delayed senescence when incorporated with coating treatments. Similarly, Dong and Wang [34] reported lower decay percentage and subsequently extended the shelf life of sweet cherry coated with guar gum and ginseng extract.
Disease severity in banana
Spreading of pathogenic organisms is the major concern in the postharvest preservation of the fruits because disease caused by these organism spoils tonnes of fruits which become inadequate for human consumption. Disease control during storage of fruits is a primary need to ensure the wide availability of fruits for the consumers. The fruits included in different treatments of Aloe gel and LPE were of better appearance and with no or minimum pathogenic lesions on the surface compared to control fruits (Table 3). The control fruits showed the highest score of disease severity i.e., 4.6 and considered decayed on 3rd day. On the same day, the fruits treated with only Aloe gel 50% showed the score of 1.2 and the combination of Aloe gel and LPE treated fruit observed with least score of disease severity (i.e. 1). On the 6th day, the minimum severity of disease (i.e. 1) was observed in the fruits treated with Aloe gel 50% + LPE 10%, and Aloe gel 50% + LPE 15%. On 9th day, fruits of both of these treatments (Aloe gel 50% and LPE 10%, Aloe gel 50% and LPE 15%) showed least disease incidences and the score was 1.2, whereas the Aloe gel treated fruits scored 4.4 and Aloe gel 50% + LPE 5% treated banana scored 3. Aloe gel and LPE treated fruits showed less severity of the disease on the fruit surface during the entire storage period of banana. The lower score of disease severity in the Aloe gel and LPE coated bananas was correlated with the antifungal activity of the Aloe gel and LPE due to the existence of secondary metabolites in the peel. Potential of Aloe gel with plant extracts as well as essential oil to prevent fungal diseases have been established by the previous studies on the quality and disease incidences in table grapes [5], pomegranate arils [7], papaya [35] and nectarine [36].
pH, titratable acidity and total soluble solids (TSS) in banana
pH and titratable acids measure the quantity of organic acids and their salts present in the fruit. pH of coated and control banana fruit pulp decreased continuously during storage. However, this decline in pH was slower in coated fruits compared to control fruits from harvest to the end of storage (Fig. 2). On day 0, the pH was 6.47 in coated and control banana. The minimum pH was 4.33 in control on 3rd day, whereas on the same day the maximum pH i.e., 5.24 was recorded in Aloe gel 50% + LPE 5% treated fruit. At the end of storage (9th day), the minimum pH among treated fruits was observed in Aloe gel 50% + LPE 15% treatment i.e., 5.12. The fall in pH during storage indicates the increase in the malic acid and citric peak (citric acid plus certain phosphate) during the storage period which was suggested by Wyman and Palmer [37]. The minimal changes in the pH of coated fruits might due to the regulated diffusion of O2 through edible coating for respiratory processes of fruit [38]. Moreover, the variation in total acidity of fruit pulp is accompanied with the concentration of the organic acids during ripening. The major organic acid in banana is malic acid [39].
Effect of edible coating treatments on the quality of (A) pH, (B) Titratable acidity, (C) Total soluble solids of Banana fruit during storage at 23 ± 1 °C. C Control, T1 Aloe vera 50%, T2 Aloe vera 50% + Lemon peel extract 5%, T3 Aloe vera 50% + Lemon peel extract 10%, T4 Aloe vera 50% + Lemon peel extract 15%. Different letters over the bars represent significant differences between the treatments on the same storage day at P ≤ 0.05 according to DMRT Duncan’s Multiple Range Test
Here, the acidity in control and coated fruits increased during first 3 days which declined steadily with the period of storage (Fig. 2), but the changes were slower in coated fruits compared to the control. The titratable acidity was 11.52% on the 0 day. It increased to 20.48% in control, whereas the minimum acidity among the treated fruits was observed 14.29% in fruit treated with Aloe gel 50% + LPE 15% on 3rd day. Then after, the acidity level in Aloe gel 50% + LPE 15% was dropped to 10.24% which was found least among treated banana on 9th day. It is well known and also expected that the coating reduced the rate of respiration and metabolism in fruits because they form barrier on the fruit’s surface which helps to reduce the availability of oxygen for respiration during storage. Due to less availability of oxygen, the rate of metabolic activity goes down [38]. The increase in the acidity of banana during the initial stage of storage period has been ascribed to the increasing activity of phosphoenolpyruvate and malate synthase enzymes [40].
Soluble solids content (SSC) are good indicators of ripening because of hydrolyzation of starch, soluble pectin, organic and amino acids. Our results showed that the soluble solids decreased in the coated and control banana during storage. The concentration of soluble solids was 11.33°Brix on 0 day. On 3rd day, the maximum soluble solids were 10.33°Brix in control and AG 50% treated fruits. However, it dropped rapidly in AG 50% + LPE 15% and observed 7.67°Brix on 3rd day. In the present study, the soluble solids decreased continuously from the day of experiment till the end of storage which might be due to the higher activity of hydrolyzing enzymes. Similar results were observed by Ayala-Zavala et al. [41] in strawberry that the high depletion of the TSS was observed only in the strawberry stored at 10 °C rather than 0 or 5 °C, and it was explained that the higher depletion of the TSS could be due to increase in temperature and thus increase in respiration rate and ripening. Alencar et al., [42] also found the decrease in TSS of banana during storage which was attributed to the senescence of fruit, but in the present study, the banana fruits are not senescing during the storage.
Reducing sugars and non-reducing sugars in banana
Banana is a rich source of the sugar or carbohydrates, which is pre-dominated by starch during unripe condition and turn into sucrose, glucose and fructose after the onset of ripening. The sugar concentration is increasing rapidly in banana during ripening period. The banana in the present study showed increasing profile of the reducing and non-reducing sugars concentration during storage. Comparatively, control fruits showed rapid increase in reducing sugars than coated banana fruits (Table 4). On 0 day, reducing sugars concentration was 8.75 mg/g of fruit. The reducing sugars level was increased to 15.16 mg/g in control fruits on 3rd day whereas the T3 (9.58 mg/g) and T4 (9.46 mg/g) showed least increase in reducing sugars on the same day of storage. On 6th day, the reducing sugars content of fruits treated with T1, T3 and T4 was increased till 6th day and started decreasing towards the end of storage period whereas the T2 treated fruit showed decreasing trend of reducing sugars from 6th day. On 9th day, the reducing sugars contents were 8.0 mg/g, 8.76 mg/g and 8.73 mg/g for T2, T3 and T4 treated banana, respectively.
Similarly, non-reducing sugars are also increased in coated and control fruits during the storage of banana (Table4). The concentration of the non-reducing sugars in banana was 13.12 mg/g on the day of storage (0 day). Subsequently, it increased drastically and became almost doubled in control (27.56 mg/g) and T1 (23.52 mg/g) treated fruit, whereas this increase was relatively slower in the T2 (20.41 mg/g), T3 (17.17 mg/g) and T4 (14.76 mg/g) treated banana fruits on 3rd day. The non-reducing sugars were noted least in T4 (Aloe gel 50% + LPE 15%). Non-reducing sugars were started decreasing after 6th day of storage. On the 9th day, minimum amount of non-reducing sugars was found in T2 (9.80 mg/g) and T3 (10.42 mg/g) compared to T4 (12.14 mg/g). The lower amount of reducing and non-reducing sugars in the T3 and T4 treatments is attributed to the delay in the conversion of starch into such sugars due to lesser activity of starch degrading enzyme (mainly alpha-amylase). It might due to the semi-anaerobic atmosphere created by surface coating as higher level of O2 is fatal for fruit quality during postharvest storage [43].
Almost all the carbohydrates present in banana is in the form of starch at a pre-climacteric stage which breaks down subsequently at the climacteric stage with the commencement of respiration and produces reducing and non-reducing sugars [2]. In the present study, the sugars concentration increased in the fruits just before they started decaying during storage, indicates the higher senescence activity in fruits that could be correlated with the increase in starch hydrolysis by extreme enzymatic activity and rapid respiration, which are obviously higher in senescing fruits [44]. Similarly, Maqbool et al. [30] reported that the reducing sugars increased in the gum arabic and chitosan coated banana fruit during cold storage. Moreover, it was noted that the increase in reducing and non-reducing sugars during storage is a result of the starch breakdown with the advancement in the ripening process [30].
Total sugar in banana
The total sugar content of fruits is considered as a good indicator for tracking the ripening stage and also estimating the storage life. Starch reserves of the fruits are considered as a prime factor contributing to the sugar content of the fruit during ripening [45]. Banana as a popular fruit contains higher amount of sugar. The sugar is initially in the form of starchy compounds (complex carbohydrates) in unripe fruits which later hydrolyzed and produced simple sugar during ripening that provides sweetness to the fruit. In current experiment, the sugar content of the stored banana fruit increased with the advancement of storage period in both control and coated fruits. However, the coated fruits delayed the increasing rate of the sugar content compared to control banana (Table 4). The sugar concentration of banana was 21.87 mg/g on 0 day. Control fruits showed maximum amount of sugar on 3rd day i.e., 42.01 mg/g. On the same day, treated fruits displayed the sugar content ranging from 23.64 to 34.47 mg/g, which was lower in T4 (Aloe gel 50% + LPE 15%) compared to T1 (Aloe gel 50% + LPE 5%). On 9th day, total sugar was observed least in T2 (17.19 mg/g) followed by T3 (18.61 mg/g) and T4 (21.51 mg/g) compared to other treated fruits. In this study, the total sugar was increased on 3rd day and then subsequently decreased with further storage. The unripe banana contains 70–80% of its carbohydrates in the form of starch which decreased to less than 1% with onset of ripening and produced sucrose, glucose and fructose and also maltose in some amount [46]. Similarly, Oiram Filho et al. [47] reported that the total sugar content increased during the storage of banana due to the ripening related changes which include the breakdown of starch and biosynthesis of the sugar. Moreover, Thakur et al. [43] reported that the rise alpha-amylase activity increases the accumulation of sugars in the surface coated banana with starch edible coating. In the present study, the delayed and lesser accumulation of the total sugar in the T3 and T4 compared to other treated banana fruits attributed to the reduced exposure of the alpha-amylase to the atmospheric oxygen due to its sensitivity to the oxygen [43]. Aloe Gel coating along with LPE might suppress the respiration rate which in turn slow down the synthesis and use of metabolites, and reduces the conversion of complex carbohydrates to sugar [48].
Vitamin C or ascorbic acid content in banana
Vitamin C is an important component in antioxidant capacity of fruits and in human nutrition which plays significant role in plant defense and human health as an immunity booster. The nutrition requirement of vitamin C or ascorbic acid in the human diet mostly comes from fruits itself. The present experiment evaluated the changes in vitamin C profile during the storage of the banana. The vitamin C concentration in pulp was increased in all the treated bananas from starting, while it decreased from the day of storage in the control fruits (Table 5). On 0 day, the concentration of vitamin C was 90.16 μg g−1. The level of vitamin C was increased in all the treatment but decreased in control and noted 62.04 μg g−1 on 3rd day. On the same day, the concentration of vitamin C was noted highest in Aloe gel 50% and LPE 15% (164.90 μg g−1). Thereafter, the concentration of vitamin C was reduced continuously till the end of the storage period. On 9th day, the maximum of vitamin C was recorded in Aloe gel 50% and LPE 10% (116.73 μg g−1) whereas minimum in Aloe gel 50% and LPE 15% (88.98 μg g−1) and Aloe gel 50% and LPE 5% (89.80 μg g−1). The increase in the ascorbic acid content of banana during ripening is due to the activity of the enzyme ascorbic acid oxidase. Coating such as gum arabic and Aloe gel decreases the decomposition rate of ascorbic acid significantly by reducing the internal O2 availability and thus, decreasing the activity of cytochrome oxidase [49, 50]. Generally, the plant responds rapidly to the elevated level of reactive oxygen species (ROSs) produced by changes in storage temperature (abiotic stress) [51]. These changes in storage temperature trigger enhanced activity of cellular antioxidants, and that induces the activity of natural antioxidants [52]. Moreover, it has been reported that the chitosan coating application (1% or 2%) raised the ascorbic acid level in mango [53] and banana [25]. Similarly, our previous study on strawberry showed that the vitamin C content increased in strawberry coated with the combinations of guar gum, gum acacia and clove oil during initial phase of storage, and then after decreased gradually towards the end of storage [18].
Conclusion
The study ensures the efficiency and synergic effect of the Aloe gel as a film-forming agent and lemon peel extract (LPE) as an antimicrobial agent to extend the shelf life and maintain the quality of stored banana. The edible coating application significantly lowered the respiration of fruits, delayed the ripening and retarded appearance of the visual indications that associated with quality loss, which make them unacceptable for consumers. The coated banana presents less weight loss and good firmness with no severe infection which is the ultimate factor that determines the storage life and quality of the banana during storage compared to control. The biomolecules like sugar, organic acid and ascorbic acid were greatly preserved in coated banana fruits than that of control. Without refrigerated storage, the 9 days of shelf life was extended with quite good acceptability in the market than that of control, which lasted 3 days only. Thus, the potential of Aloe gel and LPE edible coating to extend the shelf life and maintain the quality of the banana fruit is incredibly beneficial for the small-scale producers and retailers, to store and transport the banana at long distances. However, further work is required for confirmation and establishment of the edible coating in relation to its effects on sensory attributes as well as consumer acceptance.
Data availability
The dataset(s) supporting the conclusions of this article is (are) included in the article. The conclusions are based on the data generated from the current study. The author can be contacted for any additional supporting data required by the journal.
Abbreviations
- AG:
-
Aloe Gel
- LPE:
-
Lemon peel extract
References
A.A. Alali, M.A. Awad, A.D. Al-Qurashi, S.A. Mohamed, Sci. Hortic. 237, 51–58 (2018)
W.J. da Silva Junior, R.M. Falcão, L.C. de Sousa-Paula, N. Sbaraini, W.A. dos Santos Vieira, W.G. Lima, S.L. Paiva Junior, C.C. Staats, A. Schrank, A.M. Benko-Iseppon, V. de Queiroz Balbino, M.P.S. Cậmara, Data Brief 17, 256–260 (2018)
K. Sripong, P. Jitareerat, S. Tsuyumu, A. Uthairatanakij, V. Srilaong, C. Wongs-Aree, G. Ma, L. Zhang, M. Kato, Crop Prot. 77, 1–8 (2015)
R. Vilaplana, G. Hurtado, S. Valencia-Chamorro, LWT-Food Sci. Technol. 95, 247–254 (2018)
S. Castillo, D. Navarro, P.J. Zapata, F. Guillén, D. Valero, M. Serrano, D. Martínez-Romero, Postharvest Biol. Technol. 57(3), 183–188 (2010)
K. Eshun, Q. He, Crit. Rev. Food Sci. Nutr. 44, 91–96 (2004)
A. Nabigol, A. Asghari, Int. J. Agro. Plant Prod. 4(4), 833–838 (2013)
N.N. Van Long, C. Joly, P. Dantigny, Int. J. Food Microbiol. 220, 73–90 (2016)
F. Ammad, O. Moumen, A. Gasem, S. Othmane, K.N. Hisashi, B. Zebib, O. Merah, C.R. Biol. 341(2), 97–101 (2018)
S. Rafiq, R. Kaul, S.A. Sofi, N. Bashir, F. Nazir, G.A. Nayik, J. Saudi Soc. Agric. Sci. 17(4), 351–358 (2018)
V.H. Campos-Requena, B.L. Rivas, M.A. Pérez, C.R. Figueroa, N.E. Figueroa, E.A. Sanfuentes, Postharvest Biol. Technol. 129, 29–36 (2017)
K. Munhuweyi, O.J. Caleb, C.L. Lennox, A.J. van Reenen, U.L. Opara, Postharvest Biol. Technol. 129, 9–22 (2017)
FDA, Code of federal regulations title 21. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=182.20. Accessed 26, Mar 2014
S. Burt, Int. J. Food Microbiol. 94, 223–253 (2004)
D. Sivakumar, S. Bautista-Baños, Crop. Prot. 64, 27–37 (2014)
H.A. Hemeg, I.M. Moussa, S. Ibrahim, T.M. Dawoud, J.H. Alhaji, A.S. Mubarak, S.A. Kabli, R.A. Alsubki, A.M. Tawfik, S.A. Marouf, Saudi J. Biol. Sci. 27(12), 3221–3227 (2020)
Q. He, L. Changhong, E. Kojo, Z. Tian, Food Control 16(2), 95–104 (2005)
K.A. Jodhani, M. Nataraj, Environ. Exp. Biol. 17, 123–135 (2019)
M.D.C. Sales, H.B. Costa, P.M.B. Fernandes, J.A. Ventura, D.D. Meira, Asian Pac. J. Trop. Biomed. 6(1), 26–31 (2016)
W. Horwitz, G.W. Latimer Jr., Association of Analytical Chemists International (AOAC International, Gaythersburg, 2005).
A.M. El-Anany, G.F.A. Hassan, F.R. Ali, J. Food Technol. 7(1), 5–11 (2009)
D. Sivakumar, N.K. Hewarathgamagae, R.W. Wijeratnam, R.L.C. Wijesundera, Phytoparasitica 30(5), 486–492 (2002)
S.K. Thimmaiah, S.K. Thimmaiah, Standard Methods of Biochemical Analysis (Kalyani Publishers, New Delhi, 2004), pp. 51–58
S. Sadasivam, A. Manickam, Biochemical Methods for Agricultural Sciences, 2nd edn. (Wiley, New Delhi, 1992), pp. 6–7
A. Kapur, A. Hasković, A. Čopra-Janićijević, L. Klepo, A. Topčagić, I. Tahirović, E. Sofić, Bull. Chem. Technol. Bosnia Herzegovina 38(4), 39–42 (2012)
S.L.S. Bico, M.F.J. Raposo, R.M.S.C. Morais, A.M.M.B. Morais, Food Contr. 20, 508–514 (2009)
C. Ramírez-Pelayo, J. Martínez-Quiñones, J. Gil, D. Durango, Heliyon 5(6), 01937 (2019)
N. Suseno, E. Savitri, L. Sapei, K.S. Padmawijaya, Procedia Chem. 9, 113–120 (2014)
A.C. Guerreiro, C.M. Gago, M.L. Faleiro, M.G. Miguel, M.D. Antunes, Postharvest Biol. Technol. 110, 51–60 (2015)
M. Maqbool, A. Ali, P.G. Alderson, N. Zahid, Y. Siddiqui, J. Agric. Food Chem. 59, 5474–5482 (2011)
G.M. Cosme Silva, W.B. Silva, D.B. Medeiros, A.R. Salvador, M.H.M. Cordeiro, N.M. da Silva, G.P. Mizobutsi, Food Chem. 237, 372–378 (2017)
S.B. Murmu, H.N. Mishra, Innov. Food Sci. Emerg. Technol. 49, 20–30 (2018)
M. Maqbool, A. Ali, P.G. Alderson, M.T.M. Mohamed, Y. Siddiqui, N. Zahid, Postharvest Biol. Technol. 62, 71–76 (2011)
F. Dong, X. Wang, LWT-Food Sci. Technol. 89, 117–122 (2018)
S.L. Marpudi, L.S.S. Abirami, R. Pushkala, N. Srividya, Indian J. Biotechnol. 10, 83–89 (2011)
D. Navarro, H.M. Díaz-Mula, F. Guillén, P.J. Zapata, S. Castillo, M. Serrano, D. Valero, D. Martínez-Romero, Int. J. Food Microbiol. 151(2), 241–246 (2011)
H. Wyman, J.K. Palmer, Plant Physiol. 39(4), 630 (1964)
E. Bal, J. Agric. Sci. Technol. 15, 1219–1230 (2018)
D.W. Turner, J.A. Fortescue, Crop Post-Harvest: Science & Technology: Perishables (Wiley, Hoboken, 2012), p. 24
P. John, J. Marchal, Bananas and Plantains (Springer, Dordrecht, 1995), pp. 434–467
J.F. Ayala-Zavala, S.Y. Wang, C.Y. Wang, G.A. González-Aguilar, LWT-Food Sci. Technol. 37(7), 687–695 (2004)
E.R.D. Alencar, L.R.D.A. Faroni, M.D.S. Pinto, A.R.D. Costa, T.A.D. Silva, Cienc. Agron. 44(1), 107–114 (2013)
R. Thakur, P. Pristijono, M. Bowyer, S.P. Singh, C.J. Scarlett, C.E. Stathopoulos, Q.V. Vuong, LWT- Food Sci. Technol. 100, 341–347 (2019)
I.S. Arvanitoyannis, A. Mavromatis, Crit. Rev. Food Sci. Nutr. 49(2), 113–135 (2009)
E.J. Souleyre, P.P. Iannetta, H.A. Ross, R.D. Hancock, L.V. Shepherd, R. Viola, M.A. Taylor, H.V. Davies, Physiol. Plant 121(3), 369–376 (2004)
T.A. Anyasi, A.I. Jideani, G.R. Mchau, Compr. Rev. Food Sci. Food Saf. 12(5), 509–522 (2013)
F. Oiram Filho, M.M. de Almeida Lopes, M.L. Matias, T.R. Braga, F.A.S. de Aragão, M.R.S. da Silveira, M.M.M.T. de Oliveira, E. de Oliveira Silva, Sci. Hortic. 251, 267–275 (2019)
D.K. Das, H. Dutta, C.L. Mahanta, LWT-Food Sci. Technol. 50(1), 272–278 (2013)
G. Khaliq, M.T.M. Mohamed, A. Ali, P. Ding, H.M. Ghazali, Scientia Hort. 190, 187–194 (2015)
G. Khaliq, M. Ramzan, A.H. Baloch, Food Chem. 286, 346–353 (2019)
S.Y. Rogiers, G.N. Mohakuman, N.R. Knowles, Ann. Bot. 81, 203–211 (1998)
C. Bowler, M.V. Montague, D. Inze, Annu. Rev. Plant Biol. 43, 83–116 (1992)
P. Jongsri, T. Wangsomboondee, P. Rojsitthisak, K. Seraypheap, LWT- Food Sci. Technol. 73, 28–36 (2016)
Acknowledgements
We are greatly thankful to the Head, P. G. Department of Biosciences, Sardar Patel University, Vallabh Vidyanagar to give us an opportunity and allow us to carry out this research work.
Funding
Not Applicable.
Author information
Authors and Affiliations
Contributions
KJ and MN participated in the design and concept of the study, fruit sets, coating application, analysis of fruit through various parameters and data analysis as well as drafting of the manuscript. MN was involved in conception, design, and coordination of the study and also in preparation of the final draft of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
No Conflict of interest or Competing interests between Dr. Kaushik A. Jodhani and Dr. M. Nataraj.
Ethical approval
Not applicable as the study includes only banana fruits. The authors declare that no animals were sacrificed for this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jodhani, K.A., Nataraj, M. Synergistic effect of Aloe gel (Aloe vera L.) and Lemon (Citrus Limon L.) peel extract edible coating on shelf life and quality of banana (Musa spp.). Food Measure 15, 2318–2328 (2021). https://doi.org/10.1007/s11694-021-00822-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-021-00822-z