Abstract
Despite debates on the real impact of plant invasion on native biodiversity, there remain many situations where exotic invasive plants must be managed and habitats restored. Restoration practices that build on plant community assembly principles could be useful to delay or prevent re-invasion after control, but there are still few syntheses of the biodiversity theory, ecological mechanisms and experimental evidence relevant to invasive plant management, possibly delaying applications. To provide such a synthesis, we review current knowledge on three key determinants of invasion success: biotic resistance, abiotic constraints, and propagule pressure. We elaborate on the ecological mechanisms at play for each determinant and emphasize, using case studies, their relevance for invasive plant management and ecological restoration. We find evidence that restoring a plant cover can enhance invasion resistance, but the challenge for both research and field applications is to understand how multiple determinants interact in relation to species traits in the fields. Failure to recognize these interactions and their effect on community assembly processes may explain some of the mixed species responses observed. While we need control and restoration case studies with local species at different sites, the development of a coherent, dynamic and adaptive framework around biotic/ecological resistance will have to go beyond the idiosyncrasy of the many species and systems being tested. Emphasizing the functional diversity of the restored community seems a promising approach when facing potentially multiple invaders and/or fluctuating abiotic conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With worldwide trade intensifying, opportunities for species introduction outside of their native range have increased (Hulme 2009; Levine and D’Antonio 2003). In spite of ongoing debates on the impacts of exotic plant invasion on native biodiversity (Gilbert and Levine 2013; Thomas and Palmer 2015), there remain many situations where invasive plants are considered a nuisance and have to be managed (Simberloff 2005). When diverse native plant communities give ways to dense stands of invasive plant species, habitat quality and biogeochemical processes are often compromised (Blossey 1999; Zedler and Kercher 2004). Invasive plant species can therefore greatly undermine conservation or restoration goals where biodiversity is valued, for instance in nature reserves or ecologically significant habitats.
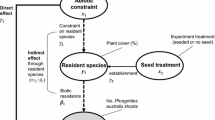
Exotic invasive plants can be controlled by herbicides, mowing, burning or labour-intensive practices like slashing or hand-felling. If control is not selective, the resident plant community is disturbed or has disappeared, and if the invader is still part of the local species pool, the risk of reinvasion can be high. For seed dispersed invasive species, the success of seedlings in the colonization/establishment phase (Fig. 1) will determine the potential to (re)establish a self-sustaining population (Catford et al. 2009; Dietz and Edwards 2006; Van Kleunen and Johnson 2007). This usually represents the most vulnerable stage for many invasive plants (Fraser and Karnezis 2005) and therefore seedling establishment provides a critical window of opportunity for plant invasion management. Restoring a competitive plant cover that can interfere with invasive plant establishment can be effective, but synthesis of biodiversity theory, ecological mechanisms, and experimental evidence relevant to biodiversity restoration is lacking, possibly delaying applications (Esler et al. 2010).
The recruitment of invaders depends on three factors that together determine the outcome of invasion—biotic resistance, abiotic constraints, and propagule pressure (Catford et al. 2009; Miller et al. 2014). Biotic resistance refers to the ability of species in a resident community to resist or limit invasion by plants (Catford et al. 2009; Levine et al. 2004). Various components of a community, including competitors, consumers and/or pathogens, can contribute to biotic resistance to invasion (Levine et al. 2004). The role of biotic interactions has been well-documented in community ecology (Byun et al. 2013; Fox 1987; Fridley et al. 2007; Levine and D’Antonio 1999; Pokorny et al. 2005; Prieur-Richard et al. 2000), and is central to our understanding of how communities at risk of invasion assemble after disturbances (Clark and Johnston 2011).
Abiotic constraints refer to environmental conditions, stressors, or filters that can suppress the recruitment of invaders and/or resident competitors depending on their tolerance (Melbourne et al. 2007; Weiher and Keddy 1995). Limited nutrients, low light level, or unsuitable climatic conditions, for example, constitute abiotic constraints (Davis et al. 2000; Davis and Pelsor 2001). Disturbances may release invaders from abiotic constraints, providing invasion opportunity (Hobbs and Huenneke 1992).
Propagule pressure has also been identified as a key element in determining invasion outcome (Lockwood et al. 2005). Pressure depends on propagule size—the number of individuals arriving at any one time, and frequency of arrivals. Colonization pressure is also used to refer to the number of species released into a single location, a variant of propagule pressure (Lockwood et al. 2009). An obviously important and initially underestimated factor in the invasion process, propagule pressure interacts with other factors (biotic resistance or abiotic constraints) in ways that need to be better understood.
In this paper, we review current knowledge on these three key determinants of invasion success, focusing on a common field situation in which biodiversity needs to be restored after disturbances and plant invaders are part of the species pool. We elaborate on the ecological mechanisms at play for each determinant and emphasize their relevance for ecological restoration and exotic invasive plant management. This synthesis allows us to identify gaps in knowledge to inform future studies as well as opportunities and constraints for field applications aiming at the restoration of invasion-resistant habitats.
Mechanisms of invasion resistance and related ecological concepts
Several mechanisms have been proposed to explain how biotic resistance, abiotic constraints, and propagule pressure each acts during invasion (Fridley et al. 2007; Funk et al. 2008; MacDougall et al. 2009; Sax et al. 2007; Shea and Chesson 2002). These mechanisms are not necessarily mutually exclusive, since several processes may work synergistically or in alternation, depending on context and scale (Fridley et al. 2007; Pauchard and Shea 2006). Moreover, concepts and mechanisms are often related through scale, from individual species to community to ecosystems.
Biotic resistance
Niche and fitness difference
Each species has acquired through natural selection a set of traits (canopy height, specific leaf area, type of reproduction, timing of germination, seed size, etc.) that determines its fitness in a particular environment and the level of resources (nutrient, light, moisture, etc.) that it needs or can tolerate. The niche of a species describes the habitat requirements and ‘behaviors’ (for a plant, examples could be dormancy or foraging through deep roots) that may allow for persistence and reproduction. A functional group framework associates species in terms of their traits, and consequently of their niche and fitness similarity (Drenovsky et al. 2012; Eisenhauer et al. 2013; Funk et al. 2008). Simply put, the way in which a species interacts with another species depends on their respective traits and niche requirements and how similar or dissimilar these traits or niches are.
Biotic resistance can be explained by mechanisms of niche difference and fitness difference between resident and invading species. ‘Limiting similarity’, from classical competition theory (Macarthur and Levins 1967; Weltzin et al. 2003), postulates that there is a limit to the similarity in niche overlapping or resource use between resident species and invading species. According to this theory, an invading species will not establish where a resident species occupies a similar niche or has similar traits. A functional group interpretation of the concept of limiting similarity can also be considered: the related ‘Fox’s assembly rule’ hypothesizes that the lack of a certain functional group in a resident community will make that community more susceptible to invasion by species from that particular functional group (Fox 1987; Von Holle and Simberloff 2004) until niches become saturated.
When niches overlap, the fitness difference between the resident species and the invader determines which one will be competitively excluded (MacDougall et al. 2009). Performance traits such as plant height (Gaudet and Keddy 1988), biomass (Gaudet and Keddy 1988; Lulow 2006; Rinella et al. 2007), plant cover (Gerhardt and Collinge 2003) or plant size (Schamp and Aarssen 2010) can be indicative of potential for biotic resistance. Biomass of resident communities, in particular, has been identified as a good indicator of competitive ability (Gaudet and Keddy 1988) and level of biotic resistance (Lulow 2006). High biomass of resident species could reflect the way in which available resources are utilized and, consequently, whether resources are made (or not) available for potential invaders. Moreover, difference in the timing of life events can contribute to differential plant fitness and temporal partitioning of resources. Early-season emergence, for instance, can contribute to fitness (Verdú and Traveset 2005) and biotic resistance at the critical establishment stage (Firn et al. 2010). Species that establish early and grow fast benefit from a so-called ‘priority effect’, eventually inhibiting a subsequent invader from establishing (Mwangi et al. 2007). Priority effect is expressed as ‘first come, first served’ in terms of available resources (Byun et al. 2013; Fukami et al. 2005; Stuble and Souza 2016b; Young 2001).
In the context of manipulating biotic resistance for restoration purposes, there is experimental evidence that establishing native species functionally similar to potential invaders, or establishing fast growing species early, may be successful in limiting exotic plant invasion. Fargione et al. (2003), introducing species (both native and exotic) into 147 experimental prairie-grasslands plots at Cedar Creek Natural History, showed that established species most strongly inhibited introduced species from the same functional group. In a prairie restoration experiment, Larson et al. (2013) found that early robust establishment of native species increased biotic resistance to Cirsium arvense (more so than functional similarity). Leffler et al. (2014) examined the ability of monocultures and assemblages of prairie species (native or non-invasive exotic) to resist invasion by two exotic invasive species, Bromus tectorum (cheatgrass; an annual grass) and Isatis tinctoria (dyer’s woad, a biennal forb). They found that monocultures of a single growth form were in general more resistant to invasion by invasive plants of the same growth form. It must be noted that applying the limiting similarity concept to ecological restoration assumes that the identity of the potential invader is known. To increase biological resistance to several known potential invaders or to undetermine invaders, the concept must be extended to include a diversity of established native species, as we discuss below.
Diversity effect
At community level, the diversity-resistance hypothesis predicts a positive relationship between species diversity and biotic resistance (Levine and D’Antonio 1999). Based on the mechanisms previously mentioned, diverse communities can have fewer unused niche spaces and more efficient resource use through niche partitioning than species-poor communities, thus resisting invasion (Funk et al. 2008; MacDougall et al. 2009). Additionally, the more species in a resident community, the more likely a resident species’ niche will overlap with that of invaders, increasing competition through limiting similarity. Niche partitioning can be demonstrated empirically by increasing the number of neighboring plants (Kennedy et al. 2002), canopy complexity (Lindig-Cisneros and Zedler 2002) or resource uptake partitioning in a community (e.g., soil nitrogen forms) (Ashton et al. 2010; Booth et al. 2003; Frankow-Lindberg 2012). When resident species are functionally very different from each other, functional diversity, through trait complementarity, can help maintain both community stability and resistance (Fargione and Tilman 2005; Funk et al. 2008).
The related hypothesis of ‘insurance effect’ states that species diversity increases community-wide stability under a fluctuating or heterogeneous environment (Ives et al. 2000; Loreau et al. 2001; Tilman et al. 2006), with a potential scale effect in the field (Balvanera et al. 2006; Fridley et al. 2007). Trait or functional diversity allows species with suitable traits to fill available niches when conditions change, preventing invaders from taking advantage of fluctuating resource availability (Davis et al. 2000). In this regard, functional group richness (or functional diversity based on trait complementarity) could be a more direct indicator of invasion resistance than species diversity alone (Symstad 2000; Pokorny et al. 2005; but see also Prieur-Richard et al. 2000). All types of functional groups can be of equal importance (Pokorny et al. 2005; Rinella et al. 2007), but others predict that functional group composition (i.e., ratio of each functional group) can be equally important (Prieur-Richard et al. 2000; Tilman et al. 1997).
For any particular community, diversity effect can be divided into ‘selection effect’ and ‘complementarity effect’ (Loreau and Hector 2001). The effect in question can be measured in terms of any process of interest such as productivity, resistance to invasion, etc. A selection effect is significant if certain dominant species in a community mainly influence diversity effects (Emery and Gross 2007; van Ruijven et al. 2003). Complementarity effect is significant when resources partitioning or positive interaction between species contribute most to diversity effect (Kennedy et al. 2002; Levine et al. 2004; Levine and D’Antonio 1999). Empirical evidence indicates that both complementarity and selection effects contribute to a net diversity effect on invasion resistance (Fargione and Tilman 2005; Frankow-Lindberg et al. 2009), although their relative contribution is not always quantified.
For restoration purposes, ecological theory and empirical evidence suggest establishing high native plant functional diversity after disturbance can increase resistance to invasion by exotic invasive species. For example, Phragmites australis seed germination is sensitive to light level and competition, and seedling recruitment through seed dispersal contributes to invasion of disturbed habitats (Albert et al. 2015). To test mechanisms of biotic resistance to P. australis seed invasion in freshwater wetland (Byun et al. 2013, 2015) first classified 35 wetland plants from the regional pool of native species within four functional groups based on trait similarity. An additive competition design was conducted with P. australis and different mixtures of native plants and invasion success (P. australis seedlings) was measured. The functional group characterised by fast growing annual plants was most resistant to early invasion by P. australis, supporting priority effect, followed by the functional group of tall long lived perennials with rhizomes. Mixtures of four species were more resistant than monoculture due to complementarity diversity effect. Comparing resistance to invasion from three exotic invasive species of North American grasslands (Cirsium vulgare, Melilotus officinalis, Bromus inermis), in plots seeded with low and high richness seed mixtures (15 and 97 species respectively), high richness plots were more efficient at resisting invasion (Nemec et al. 2013). Sheley and Half (2006) found that forb mixtures were significantly more successful against the invasive Centaurea maculosa than forb monocultures. A garden experiment by Ammondt and Litton (2012) showed that combination of two or three Hawaiian native species had a greater negative impact than monocultures on biomass and fecundity of the invasive grass Megathysus maximus, also suggesting that increased functional diversity may improve restoration success. Experimental studies conducted in the context of restoration, however, do not always show diversity effect. In an experiment with different planting methods and seed species richness using seed mixtures commonly used by practitioners, Larson et al. (2011) found that greater species richness did not translate into greater resistance to exotic invasion. Similarly, Leffler et al. (2014) found that a diverse mixture of grasses was no less, but also not more resistant to invasion than a monoculture, In such cases, higher plant functional diversity may still be advisable if it brings other benefits (e.g., enhance habitat quality) than biotic resistance alone, or other possibly abiotic constraints on resistance must be evaluated.
Enemy release hypothesis
Pathogens or herbivory by native organisms may facilitate exotic plant invasion. For example, the overabundance of deer in North America facilitates the success of invasive Alliaria petiolata and Microstegus vimineum (Knight et al. 2009). When pathogens or herbivores are viewed as important determinants of plant invasion however, it is more often within the framework of the Enemy release hypothesis (ERH). ERH stipulates that species invading new regions gain competitive advantage over resident species as they leave behind their herbivores, pathogens, parasites or predators (Keane and Crawley 2002). Support for this hypothesis comes mostly from (1) biogeographical studies showing that damages from enemies are fewer on invasive plant species in the invaded range than on plants from the same species in the native range (Colautti et al. 2004), (2) from fewer damages on invasive compared to native species within the same area (Cappuccino and Carpenter 2005), and (3) successful biological control programs (Mitchell and Power 2003). While ERH may be determinant for some exotic introduction, it is not universal and several studies failed to find patterns consistent with this hypothesis (Parker and Gilbert 2007).
ERH frequently serves as justification for biological control of exotic species using herbivore insects or pathogenic fungi specific to their plant host and originating from its native area. While biological control rarely eliminates invasive plants, it may reduce their population size, productivity or fertility, thereby increasing biotic resistance of restored native plant communities (Van Driesche et al. 2010). One invasive plant species that has attracted much attention regarding the effect of biological control using imported exotic insect herbivores is Lythrum salicaria (purple loosestrife) and its biological agents Galerucella calmariensis and Galerucella pusilla (leaf beetles) (Yeates et al. 2012). In a survey of 36 Lythrum stands in Central New York 10 years after exposures to the biological control agents, Grevstad (2006) found that plants were shorter and showed various signs of beetle damage, but that stem density and coverage remained unaffected, a pattern consistent with other surveys. Biological control for the invasive Ageratina riparia (mist flower) in New Zealand, on the other hand, was very successful. Within five years of the introduction of the Entyloma ageratinae (white smut fungus) and Procecidochares alani (gall fly) as biological agents, mean percent cover of mist flower in heavy infested sites declined from 81 to 1.5% (Barton et al. 2007). Biological control of invasive species can be a valuable tool because it may be potentially self-sustaining, non-polluting and relatively inexpensive (Culliney 2005). While this field of research is actively increasing, biological control agents, however, exist for only a few species within a specific area, and development for additional species is slowed by the risk of damages to non-target native species. Consequently, it is still very rarely applied in restoration projects.
Abiotic constraints
Abiotic filtering and fluctuating resources
Studies describing the variation in the levels of invasion among habitats in different regions and on different continents have found similar patterns of invasion for similar habitats. The most and the least invaded habitats remain the same, despite being in different regions (Chytrý et al. 2008; Pysek and Chytry 2014), emphasizing the importance of abiotic conditions (Kalusová et al. 2015). Harsh abiotic constraints suppress intolerant species, including invaders (Melbourne et al. 2007; Parepa et al. 2013; Weiher and Keddy 1995). Invasion levels therefore tend to be low in harsh climatic conditions and nutrient-poor habitats (Chytrý et al. 2008). Field studies have shown that abiotic constraints play a significant role in determining invasibility in relation to flooding (Collinge et al. 2011; Gerhardt and Collinge 2003), sediment salinity (Dethier and Hacker 2005), soil nutrients (Goldstein and Suding 2013) overstory tree composition (Von Holle 2005), or extreme climatic events (Collinge et al. 2011; Goldstein and Suding 2013). A strong environmental filtering process may lead to trait underdispersion (traits are more similar to each other than what would be expected by chance) and phylogenetic clustering during community reassembly (Adler et al. 2013; Procheş et al. 2008).
The ‘fluctuating resource availability’ hypothesis states that plant communities become more vulnerable to invasion when the amount of unused community resources (i.e., resource availability) increases (Davis et al. 2000). A combination of abiotic and biotic factors determines resource availability. When the supply of resources (abiotic factor) is controlled, or if resource acquisition by resident communities (biotic factor) increases, invasion will occur less frequently. The notion that competition is less important in recently disturbed environments where resident plants are not sequestering all available resources underlies this hypothesis (Davis et al. 1998; Grime 1998), which has been the subject of much debate and is supported by some empirical evidence (Davis and Pelsor 2001; Frankow-Lindberg 2012; Iannone and Galatowitsch 2008). Any disturbance event that changes abiotic constraints to increase resource availability can trigger an invasion.
One of the most common application of a change in abiotic conditions in the context of restoration to manage plant invasion concerns the lowering of nitrogen availability. N enrichment was found to stimulate exotic invasive plant species in many environments. Addition of carbon in the form of sawdust or sucrose reduces N availability, thus presumably favouring native species at the expense of fast-growing exotic species. An example where this approach was successful is reported by Prober et al. (2005), who showed that repeated sucrose applications in grassy woodlands in a temperate region of Australia significantly increased the abundance of native perennial grasses at the expense of exotic annuals. Creating a crop plant cover on bare soil (thereby lowering light availability) controlled 89% of invasion by P. arundinacea, but also suppressed desired species (restoration target communities) by 57% (Iannone and Galatowitsch 2008). In the same experiment, applying sawdust (high C:N ratio) lowered available nitrogen in soil and decreased invasion by 59%,while not affecting the desired target communities. Alternatively, sawdust addition brought no significant benefits to native plants of California grassland over a 2-year period (Corbin and D’Antonio 2004). Despite the mixed results from the literature, carbon addition or other modification of the abiotic environment may still hold promise as a tool for restoration, depending on habitats, but requires a good understanding of the response of the invading species involved.
Propagule pressure
The role of propagule pressure in the invasion process is relatively straightforward: ‘the more you introduce, the more you get’ (Lockwood et al. 2009). For example, propagule pressure alone explained 56% of the variance in exotic species richness in a study (Lonsdale 1999). In a restoration experiment, Phalaris arundinacea (reed canary grass) was sown in wetland mesocosms at different density (0, 10, 50, 100, or 500 seeds/m2) with a mixture of native species (Reinhardt Adams and Galatowitsch 2008). Increasing the seed density of the invader increased invasion success (more shoots, more biomass of P. arundinacea). There is, however, considerable interest in the relationship between propagule numbers and invasion success (the dose–response curve) (Lockwood et al. 2005). It is uncertain whether there are always increasing invasion benefits from increasing propagule pressure, or whether saturation occurs beyond a certain threshold (Byun et al. 2015; Lockwood et al. 2005). If so, it is not entirely clear what ultimately determines that threshold. For Hieracium lepidulum, an invasive perennial herb in New Zealand, recruitment varied not only with propagule pressure, but also with the biotic and abiotic characteristics of habitats including resident plant cover and plant richness (Miller et al. 2014). This suggests taking into account complex interactions when evaluating the role of propagule pressure and that the effects of propagule pressure are likely to be exacerbated in areas displaying poor biotic resistance.
Managing the propagule pools of invasive species early in the restoration process could significantly reduce invasion risk but also poses challenges. Eliminating on-site seed bank often requires destructive or costly approaches (chemical control, tarping). Moreover, managing invasive species populations from the surrounding matrix that most contribute to seed rain in the restored site (closest populations, those facing predominant wind, etc.) is not always possible, and so nearby propagule sources are commonly ignored in restoration projects. Evidence suggests, however, that restoring a plant cover will reduce the effect of propagule pressure on invasion success.
Interplay between determinants of biological invasion
Depending on their focus, studies are not always consistent regarding the importance of each of the main determinants of biological invasion, but it is also unlikely that a single mechanism will govern the entire invasion process in all types of habitats and for all invaders. Rather, biotic resistance, abiotic constraint, and propagule pressure interact with each other in ways that depend on habitat conditions and the species involved (Berg et al. 2016; Perelman et al. 2007; Warren et al. 2012). The intensity of abiotic constraints will determine the relative importance of abiotic versus biotic factors in invasion resistance (Gerhardt and Collinge 2003). In stressful and harsh environments, abiotic constraints may entirely determine the fate of invaders (Chytrý et al. 2008; Dethier and Hacker 2005; Wang et al. 2006b). In benign or intermediate conditions, in contrast, biotic resistance can become as, or more important than abiotic constraints (Gerhardt and Collinge 2003; Naeem et al. 2000; Perelman et al. 2007; Thomsen et al. 2006a, b). Abiotic constraints can modulate biotic resistance in direct as well as indirect manners (Byun et al. 2015; Collinge et al. 2011; Perelman et al. 2007). Similarly, environmental heterogeneity and biotic resistance can modify invasion outcome from propagule pressure alone (Eschtruth and Battles 2011; Miller et al. 2014). Understanding and manipulating invasion resistance therefore requires a synthetic approach: evaluating not only the individual effect of each factor, but also disentangling their interaction effects (Table 1).
The importance of considering simultaneously different mechanisms of biological invasion for management can be illustrated using a well-known macrophyte invader of wetlands of North America, Phragmites australis. Experiments showed that abiotic constraints can modulate biotic resistance through direct and indirect effects (Byun et al. 2015). High water level always directly affected invasion by P. australis by reducing recruitment success, but also indirectly affected invader recruitment through the resident plant cover, reducing or increasing their ability to resist invasion. For example, Typha latifolia increased its biotic resistance to invasion with increased flooding, whereas Lolium multiflorum suffered from flooding, decreasing its biotic resistance effect. The presence of a dense resident plant cover also lowered the threshold at which invasion success occurred even when propagule supply increased and biotic resistance was most effective under low propagule pressure of the invader. These results mirror those of Miller et al. (2014) for Hieracium lepidulum. We know from the manipulation of species and functional group richness and composition in resident communities that they all have a significant impact on biotic resistance (Byun et al. 2013). Increasing biotic resistance through the restoration of a resident plant cover, however, will be most efficient when considering abiotic constraints and propagule pressure.
Enhancing ecological resistance
Restoring a native plant cover after control can be an effective measure to prevent or delay invasion. A plant cover can be self-regenerative, so repeated control interventions become less necessary although monitoring is recommended. Table 2 summarizes the results of research projects focusing on plant cover to control invasive plants. This approach, however, raises many practical issues, including how to select and combine species and how to determine the appropriate conditions where ecological resistance will be enhanced. Invasive plant control programs usually do not evaluate the role of native revegetation following removal (Kettenring and Adams 2011) and, to date, empirical tests have shown mixed results in terms of the effectiveness of plant restoration for controlling invasion. This may be because these tests are more often based on available species than on the community assembly principles that determine species associations. The selection and combination of plants is crucial as just randomly adding species may increase diversity but not necessarily enhance resistance, or worse may result in imbalanced competition amongst certain plant types, which may actually increase the susceptibility to invasion. Failure to detect the effect of a plant cover on resistance may also result from the fact that other modulating factors such as the ones explored in this study (abiotic constraints, propagule pressure) are not considered, while in some cases they may override biotic resistance effect. In any case, it is unlikely that biotic resistance alone will completely suppress invasion without complementary preventive measures such as reducing propagule pressure from nearby sources or pathways and address, when possible, site conditions for abiotic filtering.
As the ultimate test of ecological theories (Ewel 1987), the control of invasion requires a thorough and operational understanding of invasion mechanisms that could apply to a range of environments and taxa. The complex nature of the invasion process where abiotic and biotic factors interact in ways that are not yet fully understood makes not only field applications, but also research experiments rather daunting. Since rarely a single mechanism (or factor) governs the entire invasion process, multiple drivers must be addressed simultaneously. Even though we know more about resistance mechanisms and functional traits of plant species, most restoration studies have tested the effect of specific local species on an invader instead of specific resistance mechanisms. The latter are often inferred a posteriori. Yet, testing the interaction between species traits (both of the resident species and the invaders), abiotic condition, and propagule pressure could lead to useful generalisation. While we need control and restoration case studies with local species at different sites, the development of a coherent, dynamic and adaptive framework around biotic/ecological resistance will require going beyond the idiosyncrasy of the many species and systems being tested. Developing such a coherent framework would be beneficial as many current managerial frameworks quickly become obsolete or are applied incorrectly or out of context. This will require identifying and sharing emerging principles at the crossroad between community ecology, invasion ecology, and ecological restoration. Given the evidence uncovered, emphasizing the functional diversity of the restored community seems a promising approach when facing potentially multiple invaders and/or fluctuating abiotic conditions and could inform species selection for restoration.
References
Adler PB, Fajardo A, Kleinhesselink AR, Kraft NJ (2013) Trait-based tests of coexistence mechanisms. Ecol Lett 16:1294–1306. doi:10.1111/ele.12157
Albert A, Brisson J, Dubé J, Lavoie C (2013) Do woody plants prevent the establishment of common reed along highways? Insights from Southern Quebec. Invas Plant Sci Manag 6:585–592. doi:10.1614/IPSM-D-13-00025.1
Albert A, Brisson J, Belzile F, Turgeon J, Lavoie C (2015) Strategies for a successful plant invasion: the reproduction of Phragmites australis in north-eastern North America. J Ecol 103:1529–1537
Ammondt SA, Litton CM (2012) Competition between native Hawaiian plants and the invasive grass Megathyrsus maximus: implications of functional diversity for ecological restoration. Restor Ecol 20:638–646
Ashton IW, Miller AE, Bowman WD, Suding KN (2010) Niche complementarity due to plasticity in resource use: plant partitioning of chemical N forms. Ecology 91:3252–3260. doi:10.1890/09-1849.1
Balvanera P, Pfisterer AB, Buchmann N, He JS, Nakashizuka T, Raffaelli D, Schmid B (2006) Quantifying the evidence for biodiversity effects on ecosystem functioning and services. Ecol Lett 9:1146–1156. doi:10.1111/j.1461-0248.2006.00963.x
Barton J, Fowler SV, Gianotti AF, Winks CJ, De Beurs M, Arnold GC, Forrester G (2007) Successful biological control of mist flower (Ageratina riparia) in New Zealand: agent establishment, impact and benefits to the native flora. Biol Control 40:370–385
Berg JA, Meyer GA, Young EB (2016) Propagule pressure and environmental conditions interact to determine establishment success of an invasive plant species, glossy buckthorn (Frangula alnus), across five different wetland habitat types. Biol Invasions 18:1363–1373
Blossey B (1999) Before, during and after: the need for long-term monitering in invasive plant species management. Biol Invasions 1:301–311
Booth MS, Caldwell MM, Stark JM (2003) Overlapping resource use in three great basin species: implications for community invasibility and vegetation dynamics. J Ecol 91:36–48
Byun C, de Blois S, Brisson J (2013) Plant functional group identity and diversity determine biotic resistance to invasion by an exotic grass. J Ecol 101:128–139. doi:10.1111/1365-2745.12016
Byun C, de Blois S, Brisson J (2015) Interactions between abiotic constraint, propagule pressure, and biotic resistance regulate plant invasion. Oecologia 178:285–296. doi:10.1007/s00442-014-3188-z
Cappuccino N, Carpenter D (2005) Invasive exotic plants suffer less herbivory than non-invasive exotic plants. Biol Lett 1:435–438
Catford JA, Jansson R, Nilsson C (2009) Reducing redundancy in invasion ecology by integrating hypotheses into a single theoretical framework. Divers Distrib 15:22–40. doi:10.1111/j.1472-4642.2008.00521.x
Chytrý M, Maskell LC, Pino J, Pyšek P, Vilà M, Font X, Smart SM (2008) Habitat invasions by alien plants: a quantitative comparison among Mediterranean, subcontinental and oceanic regions of Europe. J Appl Ecol 45:448–458
Clark GF, Johnston EL (2011) Temporal change in the diversity–invasibility relationship in the presence of a disturbance regime. Ecol Lett 14:52–57. doi:10.1111/j.1461-0248.2010.01550.x
Colautti IR, Ricciardi A, Grigorovich IA, MacIsaac HJ (2004) Is invasion success explained by the enemy release hypothesis? Ecol Lett 7:721–733
Collinge SK, Ray C, Gerhardt F (2011) Long-term dynamics of biotic and abiotic resistance to exotic species invasion in restored vernal pool plant communities. Ecol Appl 21:2105–2118. doi:10.1890/10-1094.1
Connell JH, Slatyer RO (1977) Mechanisms of succession in natural communities and their role in community stability and organization. Am Nat 111:1119–1144. doi:10.1086/283241
Corbin JD, D’Antonio CM (2004) Can carbon addition increase competitiveness of native grasses? A case study from California. Restor Ecol 12:36–43
Culliney TW (2005) Benefits of classical biological control for managing invasive plants Crit Rev. Plant Sci 24:131–150
Cutting KJ, Hough-Goldstein J (2013) Integration of biological control and native seeding to restore invaded plant communities. Restor Ecol 21:648–655. doi:10.1111/j.1526-100X.2012.00936.x
Davis MA, Pelsor M (2001) Experimental support for a resource-based mechanistic model of invasibility. Ecol Lett 4:421–428. doi:10.1046/j.1461-0248.2001.00246.x
Davis MA, Wrage KJ, Reich PB (1998) Competition between tree seedlings and herbaceous vegetation: support for a theory of resource supply and demand. J Ecol 86:652–661
Davis MA, Grime JP, Thompson K (2000) Fluctuating resources in plant communities: a general theory of invasibility. J Ecol 88:528–534
Dethier MN, Hacker SD (2005) Physical factors vs. biotic resistance in controlling the invasion of an estuarine marsh grass. Ecol Appl 15:1273–1283. doi:10.1890/04-0505
Dietz H, Edwards PJ (2006) Recognition that causal processes change during plant invasion helps explain conflicts in evidence. Ecology 87:1359–1367. doi:10.1890/0012-9658(2006)87[1359:RTCPCD]2.0.CO;2
Drenovsky RE, Grewell BJ, D’Antonio CM, Funk JL, James JJ, Molinari N, Parker IM, Richards CL (2012) A functional trait perspective on plant invasion. Ann Bot 110:141–153. doi:10.1093/aob/mcs100
Eisenhauer N, Schulz W, Scheu S, Jousset A (2013) Niche dimensionality links biodiversity and invasibility of microbial communities. Funct Ecol 27:282–288. doi:10.1111/j.1365-2435.2012.02060.x
Elton CS (1958) The ecology of invasions by animals and plants. The University of Chicago Press, London
Emery SM, Gross KL (2007) Dominant species identity, not community evenness, regulates invasion in experimental grassland plant communities. Ecology 88:954–964. doi:10.1890/06-0568
Eschtruth AK, Battles JJ (2011) The importance of quantifying propagule pressure to understand invasion: an examination of riparian forest invasibility. Ecology 92:1314–1322. doi:10.1890/10-0857.1
Esler K, Prozesky H, Sharma G, McGeoch M (2010) How wide is the “knowing-doing” gap in invasion biology? Biol Invasions 12:4065–4075. doi:10.1007/s10530-010-9812-x
Ewel JJ (1987) Restoration is the ultimate test of ecological theory. Restoration ecology. Cambridge University Press, Cambridge
Fargione JE, Tilman D (2005) Diversity decreases invasion via both sampling and complementarity effects. Ecol Lett 8:604–611. doi:10.1111/j.1461-0248.2005.00753.x
Fargione J, Brown CS, Tilman D (2003) Community assembly and invasion: an experimental test of neutral versus niche processes. Proc Natl Acad Sci USA 100:8916–8920. doi:10.1073/pnas.1033107100
Firn J, MacDougall A, Schmidt S, Buckley Y (2010) Early emergence and resource availability can competitively favour natives over a functionally similar invader. Oecologia 163:775–784. doi:10.1007/s00442-010-1583-7
Fox BJ (1987) Species assembly and the evolution of community structure. Evol Ecol 1:201–213. doi:10.1007/bf02067551
Frankow-Lindberg B (2012) Grassland plant species diversity decreases invasion by increasing resource use. Oecologia 169:793–802. doi:10.1007/s00442-011-2230-7
Frankow-Lindberg BE, Brophy C, Collins RP, Connolly J (2009) Biodiversity effects on yield and unsown species invasion in a temperate forage ecosystem. Ann Bot 103:913–921. doi:10.1093/aob/mcp008
Fraser LH, Karnezis JP (2005) A comparative assessment of seedling survival and biomass accumulation for fourteen wetland plant species grown under minor water-depth differences. Wetlands 25:520–530. doi:10.1672/0277-5212(2005)025[0520:ACAOSS]2.0.CO;2
Fridley JD, Stachowicz JJ, Naeem S, Sax DF, Seabloom EW, Smith MD, Stohlgren TJ, Tilman D, Holle BV (2007) The invasion paradox: reconciling pattern and process in species invasions. Ecology 88:3–17
Fukami T, Bezemer TM, Mortimer SR, van der Putten WH (2005) Species divergence and trait convergence in experimental plant community assembly. Ecol Lett 8:1283–1290. doi:10.1111/j.1461-0248.2005.00829.x
Funk JL, Cleland EE, Suding KN, Zavaleta ES (2008) Restoration through reassembly: plant traits and invasion resistance. Trends Ecol Evol 23:695–703
Gaudet CL, Keddy PA (1988) A comparative approach to predicting competitive ability from plant traits. Nature 334:242–243
Gerhardt F, Collinge SK (2003) Exotic plant invasions of vernal pools in the Central Valley of California, USA. J Biogeogr 30:1043–1052. doi:10.1046/j.1365-2699.2003.00911.x
Gilbert B, Levine JM (2013) Plant invasions and extinction debts. Proc Natl Acad Sci USA 110:1744–1749. doi:10.1073/pnas.1212375110
Gleason HA (1926) The individualistic concept of the plant association. Bull Torrey Bot Club 53:7. doi:10.2307/2479933
Godoy O, Levine JM (2013) Phenology effects on invasion success: insights from coupling field experiments to coexistence theory. Ecology. doi:10.1890/13-1157.1
Goldstein L, Suding K (2013) Applying competition theory to invasion: resource impacts indicate invasion mechanisms in California shrublands. Biol Invasions. doi:10.1007/s10530-013-0513-0
Grevstad FS (2006) Ten-year impacts of the biological control agents Galerucella pusilla and G. calmariensis (Coleoptera: Chrysomelidae) on purple loosestrife (Lythrum salicaria) in Central New York State. Biol Control 39:1–8
Grime JP (1998) Benefits of plant diversity to ecosystems: immediate, filter and founder effects. J Ecol 86:902–910. doi:10.1046/j.1365-2745.1998.00306.x
Hobbs RJ, Huenneke LF (1992) Disturbance diversity, and invasion: implications for conservation. Conserv Biol 6:324–337
Holle BV, Simberloff D (2005) Ecological resistance to biological invasion overwhelmed by propagule pressure. Ecology 86:3212–3218. doi:10.1890/05-0427
Hulme PE (2009) Trade, transport and trouble: managing invasive species pathways in an era of globalization. J Appl Ecol 46:10–18
Iannone BV III, Galatowitsch SM (2008) Altering light and soil N to limit phalaris arundinacea reinvasion in sedge meadow restoration. Restor Ecol 16:689–701
Ives AR, Klug JL, Gross K (2000) Stability and species richness in complex communities. Ecol Lett 3:399–411. doi:10.1046/j.1461-0248.2000.00144.x
Kalusová V, Chytrý M, Peet RK, Wentworth TR (2015) Intercontinental comparison of habitat levels of invasion between temperate North America and Europe. Ecology 96:3363–3373
Keane RM, Crawley MJ (2002) Exotic plant invasions and the enemy release hypothesis. Trends Ecol Evol 17:164–170
Kennedy TA, Naeem S, Howe KM, Knops JMH, Tilman D, Reich P (2002) Biodiversity as a barrier to ecological invasion. Nature 417:636–638
Kettenring KM, Adams CR (2011) Lessons learned from invasive plant control experiments: a systematic review and meta-analysis. J Appl Ecol 48:970–979. doi:10.1111/j.1365-2664.2011.01979.x
Knight TM, Dunn JL, Smith LA, Davis J, Kalisz S (2009) Deer facilitate invasive plant success in a Pennsylvania forest understory. Nat Areas J 29:110–116
Kunstler G, Lavergne S, Courbaud B, Thuiller W, Vieilledent G, Zimmermann NE, Kattge J, Coomes DA (2012) Competitive interactions between forest trees are driven by species’ trait hierarchy, not phylogenetic or functional similarity: implications for forest community assembly. Ecol Lett 15:831–840. doi:10.1111/j.1461-0248.2012.01803.x
Larson DL, Bright J, Drobney P, Larson JL, Palaia N, Rabie PA, Vacek S, Wells D (2011) Effects of planting method and seed mix richness on the early stages of tallgrass prairie restoration. Biol Conserv 144:3127–3139
Larson DL, Bright J, Drobney P, Larson JL, Palaia N, Rabie PA, Vacek S, Wells D (2013) Using prairie restoration to curtail invasion of Canada thistle: the importance of limiting similarity and seed mix richness. Biol Invasions 15:2049–2063
Leffler AJ, Leonard ED, James JJ, Monaco TA (2014) Invasion is contingent on species assemblage and invasive species identity in experimental rehabilitation plots. Rangel Ecol Manag 67:657–666
Levine JM (2000) Species diversity and biological invasions: relating local process to community pattern. Science 288:852–854. doi:10.1126/science.288.5467.852
Levine JM, D’Antonio CM (1999) Elton revisited: a review of evidence linking diversity and invasibility. Oikos 87:15–26. doi:10.2307/3546992
Levine JM, D’Antonio CM (2003) Forecasting biological invasions with increasing international trade. Conserv Biol 17:322–326
Levine JM, Adler PB, Yelenik SG (2004) A meta-analysis of biotic resistance to exotic plant invasions. Ecol Lett 7:975–989. doi:10.1111/j.1461-0248.2004.00657.x
Lindig-Cisneros R, Zedler JB (2002) Relationships between canopy complexity and germination microsites for Phalaris arundinacea L. Oecologia 133:159–167. doi:10.1007/s00442-002-1020-7
Lockwood JL, Cassey P, Blackburn T (2005) The role of propagule pressure in explaining species invasions. Trends Ecol Evol 20:223–228. doi:10.1016/j.tree.2005.02.004
Lockwood JL, Cassey P, Blackburn TM (2009) The more you introduce the more you get: the role of colonization pressure and propagule pressure in invasion ecology. Divers Distrib 15:904–910. doi:10.1111/j.1472-4642.2009.00594.x
Lonsdale WM (1999) Global patterns of plant invasions and the concept of invasibility. Ecology 80:1522–1536
Loreau M, Hector A (2001) Partitioning selection and complementarity in biodiversity experiments. Nature 412:72–76
Loreau M, Naeem S, Inchausti P, Bengtsson J, Grime JP, Hector A, Hooper DU, Huston MA, Raffaelli D, Schmid B, Tilman D, Wardle DA (2001) Biodiversity and ecosystem functioning: current knowledge and future challenges. Science 294:804–808. doi:10.1126/science.1064088
Lulow ME (2006) Invasion by non-native annual grasses: the importance of species biomass, composition, and time among California native grasses of the central valley. Restor Ecol 14:616–626. doi:10.1111/j.1526-100X.2006.00173.x
Macarthur R, Levins R (1967) The limiting similarity, convergence, and divergence of coexisting species. Am Nat 101:377–385
MacDougall AS, Gilbert B, Levine JM (2009) Plant invasions and the niche. J Ecol 97:609–615. doi:10.1111/j.1365-2745.2009.01514.x
Meiman P, Redente E, Paschke M (2009) Diffuse knapweed (Centaurea diffusa Lam.) seedling emergence and establishment in a Colorado grassland. Plant Ecol 201:631–638
Melbourne BA, Cornell HV, Davies KF, Dugaw CJ, Elmendorf S, Freestone AL, Hall RJ, Harrison S, Hastings A, Holland M, Holyoak M, Lambrinos J, Moore K, Yokomizo H (2007) Invasion in a heterogeneous world: resistance, coexistence or hostile takeover? Ecol Lett 10:77–94. doi:10.1111/j.1461-0248.2006.00987.x
Miller AL, Diez JM, Sullivan JJ, Wangen SR, Wiser SK, Meffin R, Duncan RP (2014) Quantifying invasion resistance: the use of recruitment functions to control for propagule pressure. Ecology 95:920–929. doi:10.1890/13-0655.1
Minchinton TE, Bertness MD (2003) Disturbance-mediated competition and the spread of phragmites austraus in a coastal marsh. Ecol Appl 13:1400–1416
Mitchell CE, Power AG (2003) Release of invasive plants from fungal and viral pathogens. Nature 421:625
Mwangi PN, Schmitz M, Scherber C, Roscher C, Schumacher J, Scherer-Lorenzen M, Weisser WW, Schmid B (2007) Niche pre-emption increases with species richness in experimental plant communities. J Ecol 95:65–78. doi:10.1111/j.1365-2745.2006.01189.x
Naeem S, Knops JMH, Tilman D, Howe KM, Kennedy T, Gale S (2000) Plant diversity increases resistance to invasion in the absence of covarying extrinsic factors. Oikos 91:97–108. doi:10.1034/j.1600-0706.2000.910108.x
Nemec KT, Allen CR, Helzer CJ, Wedin DA (2013) Influence of richness and seeding density on invasion resistance in experimental tallgrass prairie restorations. Ecol Restor 31:168–185
Parepa M, Fischer M, Bossdorf O (2013) Environmental variability promotes plant invasion. Nat Commun 4:1604
Parker IM, Gilbert GS (2007) When there is no escape: the effects of natural enemies on native, invasive, and noninvasive plants. Ecology 88:1210–1224
Pauchard A, Shea K (2006) Integrating the study of non-native plant invasions across spatial scales. Biol Invasions 8:399–413. doi:10.1007/s10530-005-6419-8
Perelman SB, Chaneton EJ, Batista WB, Burkart SE, LeÓN RJC (2007) Habitat stress, species pool size and biotic resistance influence exotic plant richness in the Flooding Pampa grasslands. J Ecol 95:662–673. doi:10.1111/j.1365-2745.2007.01255.x
Perry L, Cronin S, Paschke M (2009) Native cover crops suppress exotic annuals and favor native perennials in a greenhouse competition experiment. Plant Ecol 204:247–259
Peter CR, Burdick DM (2010) Can plant competition and diversity reduce the growth and survival of exotic Phragmites australis invading a tidal marsh? Estuar Coasts 33:1225–1236. doi:10.1007/s12237-010-9328-8
Pokorny ML, Sheley RL, Zabinski CA, Engel RE, Svejcar TJ, Borkowski JJ (2005) Plant functional group diversity as a mechanism for invasion resistance. Restor Ecol 13:448–459. doi:10.1111/j.1526-100X.2005.00056.x
Price JN, Pärtel M (2013) Can limiting similarity increase invasion resistance? A meta-analysis of experimental studies. Oikos 122:649–656. doi:10.1111/j.1600-0706.2012.00121.x
Prieur-Richard AH, Lavorel S, Grigulis K, Dos Santos A (2000) Plant community diversity and invasibility by exotics: invasion of Mediterranean old fields by Conyza bonariensis and Conyza canadensis. Ecol Lett 3:412–422. doi:10.1046/j.1461-0248.2000.00157.x
Prober SM, Thiele KR, Lunt ID, Koen T (2005) Restoring ecological function in temperate grassy woodlands: manipulating soil nutrients, exotic annuals and native perennial grasses through carbon supplements and spring burns. J Appl Ecol 42:1073–1085
Procheş Ş, Wilson JRU, Richardson DM, Rejmánek M (2008) Searching for phylogenetic pattern in biological invasions. Glob Ecol Biogeogr 17:5–10. doi:10.1111/j.1466-8238.2007.00333.x
Pysek P, Chytry M (2014) Habitat invasion research: where vegetation science and invasion ecology meet. J Veg Sci 25:1181–1187. doi:10.1111/jvs.12146
Quinn LD, Holt JS (2009) Restoration for resistance to invasion by giant reed (Arundo donax). Invas Plant Sci Manage 2:279–291. doi:10.1614/ipsm-09-001.1
Reinhardt Adams C, Galatowitsch SM (2008) The transition from invasive species control to native species promotion and its dependence on seed density thresholds. Appl Veg Sci 11:131–138. doi:10.3170/2007-7-18335
Rinella MJ, Pokorny ML, Rekaya R (2007) Grassland invader responses to realistic changes in native species richness. Ecol Appl 17:1824–1831
Sax DF, Stachowicz JJ, Brown JH, Bruno JF, Dawson MN, Gaines SD, Grosberg RK, Hastings A, Holt RD, Mayfield MM, O Connor MI, Rice WR (2007) Ecological and evolutionary insights from species invasions. Trends Ecol Evolut (Personal edition) 22:465–471
Schamp B, Aarssen L (2010) The role of plant species size in invasibility: a field experiment. Oecologia 162:995–1004. doi:10.1007/s00442-009-1499-2
Shea K, Chesson P (2002) Community ecology theory as a framework for biological invasions. Trends Ecol Evol 17:170–176
Sheley RL, Half ML (2006) Enhancing native forb establishment and persistence using a rich seed mixture. Restor Ecol 14:627–635
Simberloff D (2005) Non-native species do threaten the natural environment! J Agr Environ Ethic 18:595–607. doi:10.1007/s10806-005-2851-0
Simberloff D (2009) The role of propagule pressure in biological invasions. Annu Rev Ecol Evol S 40:81–102. doi:10.1146/annurev.ecolsys.110308.120304
Simmons MT (2005) Bullying the bullies: the selective control of an exotic, invasive annual (Rapistrum rugosum) by oversowing with a competitive native species (Gaillardia pulchella). Restor Ecol 13:609–615
Stachowicz JJ, Tilman D (2005) Species invasions and the relationships between species diversity, community saturation, and ecosystem functioning. In: Sax DF, Stachowicz JJ, Gaines SD (eds) Species invasions: insights into ecology, evolution, and biogeography. Sinauer, Sunderland, pp 41–64
Stuble KL, Souza L (2016a) Priority effects: natives, but not exotics, pay to arrive late. J Ecol 104:987–993
Stuble KL, Souza L (2016b) Priority effects: natives, but not exotics, pay to arrive late. J Ecol 104(4):987–993
Symstad AJ (2000) A test of the effects of functional group richness and composition on grassland invasibility. Ecology 81:99–109
Thomas CD, Palmer G (2015) Non-native plants add to the British flora without negative consequences for native diversity. Proc Natl Acad Sci 112:4387–4392
Thomsen MA, Corbin JD, D’Antonio CM (2006a) The effect of soil nitrogen on competition between native and exotic perennial grasses from northern coastal California. Plant Ecol 186:23–35
Thomsen MA, D’Antonio CM, Suttle KB, Sousa WP (2006b) Ecological resistance, seed density and their interactions determine patterns of invasion in a California coastal grassland. Ecol Lett 9:160–170. doi:10.1111/j.1461-0248.2005.00857.x
Tilman D, Knops J, Wedin D, Reich P, Ritchie M, Siemann E (1997) The influence of functional diversity and composition on ecosystem processes. Science 277:1300–1302. doi:10.1126/science.277.5330.1300
Tilman D, Reich PB, Knops JMH (2006) Biodiversity and ecosystem stability in a decade-long grassland experiment. Nature 441:629–632
Van Driesche R, Carruthers R, Center T, Hoddle M, Hough-Goldstein J, Morin L, Smith L, Wagner D, Blossey B, Brancatini V (2010) Classical biological control for the protection of natural ecosystems. Biol Control 54:S2–S33
Van Kleunen M, Fischer M (2009) Release from foliar and floral fungal pathogen species does not explain the geographic spread of naturalized North American plants in Europe. J Ecol 97:385–392. doi:10.1111/j.1365-2745.2009.01483.x
Van Kleunen M, Johnson SD (2007) South African Iridaceae with rapid and profuse seedling emergence are more likely to become naturalized in other regions. J Ecol 95:674–681. doi:10.1111/j.1365-2745.2007.01250.x
van Ruijven J, de Deyn GB, Berendse F (2003) Diversity reduces invasibility in experimental plant communities: the role of plant species. Ecol Lett 6:910–918. doi:10.1046/j.1461-0248.2003.00516.x
Verdú M, Traveset A (2005) Early emergence enhances plant fitness: a phylogenetically controlled meta-analysis. Ecology 86:1385–1394
Von Holle B (2005) Biotic resistance to invader establishment of a southern Appalachian plant community is determined by environmental conditions. J Ecol 93:16–26. doi:10.1111/j.0022-0477.2004.00946.x
Von Holle B, Simberloff D (2004) Testing Fox’s assembly rule: does plant invasion depend on recipient community structure? Oikos 105:551–563. doi:10.1111/j.0030-1299.2004.12597.x
Wang J, Seliskar D, Gallagher J, League M (2006a) Blocking Phragmites australis reinvasion of restored marshes using plants selected from wild populations and tissue culture. Wetl Ecol Manag 14:539–547. doi:10.1007/s11273-006-9006-6
Wang Q, Wang C, Zhao B, Ma Z, Luo Y, Chen J, Li B (2006b) Effects of growing conditions on the growth of and interactions between salt marsh plants: implications for invasibility of habitats. Biol Invasions 8:1547–1560
Warren RJ, Bahn V, Bradford MA (2012) The interaction between propagule pressure, habitat suitability and density-dependent reproduction in species invasion. Oikos 121:874–881
Weiher E, Keddy PA (1995) Assembly rules, null models, and trait dispersion: new questions from old patterns. Oikos 74:159–164
Weltzin JF, Muth NZ, Von Holle B, Cole PG (2003) Genetic diversity and invasibility: a test using a model system with a novel experimental design. Oikos 103:505–518. doi:10.1034/j.1600-0706.2003.12389.x
Yeates AG, Schooler SS, Garono RJ, Buckley YM (2012) Biological control as an invasion process: disturbance and propagule pressure affect the invasion success of Lythrum salicaria biological control agents. Biol Invasions 14:255–271
Young TP (2001) Community succession and assembly Ecological restoration, North America 19:5
Zedler JB, Kercher S (2004) Causes and consequences of invasive plants in wetlands: opportunities, opportunists, and outcomes. Crit Rev Plant Sci 23:431–452. doi:10.1080/07352680490514673
Acknowledgements
This work was funded by grants from the Natural Sciences and Engineering Research Council of Canada to SdB and JB and from the Fonds de recherche Nature et Technologies to SdB. This research was also supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2015R1A6A3A01058185).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Byun, C., de Blois, S. & Brisson, J. Management of invasive plants through ecological resistance. Biol Invasions 20, 13–27 (2018). https://doi.org/10.1007/s10530-017-1529-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-017-1529-7