Abstract
Background
Csx/Nkx2.5, a murine nonclustered homeobox gene expressed primarily in the heart, has significant sequence similarity to the Drosophila tinman gene. Tinman is essential for heart and gut formation in Drosophila. Targeted mutation in the mouse gene, Csx/Nkx25, arrests cardiac development during early embryonic stages, suggesting an evolutionary conservation in cardiogenesis.
Materials and Methods
We have isolated and characterized a human homolog, hCsx, from an adult cardiac cDNA library. Northern blotting and ribonuclease protection was used to define the pattern of expression during normal development and in disease states. Chromosomal localization of the gene was determined by somatic cell hybrid analysis and fluorescent in situ hybridization.
Results
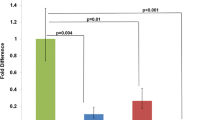
The predicted amino acid sequence of hCsx has 87% overall homology to the murine gene with 100% identity in the homeodomain. The homeodomain sequence of hCsx is 95% identical to its Xenopus homolog, and 65% to tinman. hCsx mRNA was detected exclusively in the heart. hCsx transcript was detected at 12 weeks in human embryonic heart, the earliest time point examined, and was up-regulated 5-fold between 12 and 19 weeks. There was no significant alteration of hCsx message level in the myocardium of 14 patients with end stage heart failure compared to a normal control. The human gene mapped to the distal portion of chromosome 5, the 5q34–q35 region. This defines a new synteny region between human chromosome 5q and the t-locus of mouse chromosome 17, where the mouse Csx gene is located.
Conclusions
hCsx, the human homolog of Drosophila tinman, is expressed in heart in a tissue restricted manner. Distal 5q trisomies produce several phenotypic abnormalities, including a high incidence of congenital heart disease. Isolation of the hCsx gene will allow further studies of mutations in this gene and their potential associations with some forms of congenital heart disease in humans.







Similar content being viewed by others
References
Weintraub H, Davis R, Tapscott S, et al. (1991) The myoD gene family: Nodal point during specification of the muscle cell lineage. Science 251: 761–766.
Olson E, Klein WH. (1994) bHLH factors in muscle development: Deadlines and commitment, what to leave in and what to leave out. Gene Dev. 8: 1–8.
Rudnicki MA, Schnegelsberg PNJ, Stead RH, Braun T, Arnold HH, Jaenisch R. (1993) MyoD or Myf-5 is required for the formation of skeletal muscle. Cell 75: 1351–1359.
Gehring WJ. (1994) A history of the homeobox. In: Duboule D (ed). Guidebook to the Homeobox Genes. Oxford University Press, Oxford, pp. 3–10.
Gehring WJ, Qian YQ, Billeter M, et al. (1994) Homeodomain-DNA recognition. Cell 78: 211–223.
Burglin TR. (1994) A Comprehensive classification of the homeobox genes. In: Duboule D (ed). Guidebook to the homeobox genes. Oxford University Press, Oxford, pp. 27–71.
Beeman RW. (1987) A homeotic gene cluster in the red flour beetle. Science 327: 247–249.
Gehring WJ, Affolter M, Burglin T. (1994) Homeodomain proteins. Annu. Rev. Biochem. 63: 487–526.
Kim Y, Nirenberg M. (1989) Drosophila NK-homeobox genes. Proc. Natl. Acad. Sci. U.S.A. 86: 7716–7720.
Dohrmann C, Azpiazu N, Frasch M. (1990) A new Drosophila homeobox gene is expressed in mesodermal precursor cells of distinct muscles during embryogenesis. Gene Dev. 4: 2098–2111.
Duboule D. (1994) Description of the homeobox genes. In: Duboule D (ed). Guidebook to the Homeobox Genes. Oxford University Press, Oxford, pp. 75–246.
Price M, Lazzaro D, Pohl T, et al. (1992) Regional expression of the homeobox gene Nkx2.2 in the developing mammalian forebrain. Neuron 8: 241–255.
Azpiazu N, Frasch M. (1993) Tinman and bagpipe: Two homeobox genes that determine cell fates in the dorsal mesoderm of Drosophila. Gene Dev. 7: 1325–1340.
Bodmer R, Jan LY, Jan YN. (1990) A new homeobox-containing gene, msh-2, is transiently expressed early during mesoderm formation of Drosophila. Development 110: 661–669.
Bodmer R. (1993) The gene tinman is required for specification of the heart and visceral muscles in Drosophila. Development 118: 719–729.
Bodmer R. (1995) Heart development in Drosophila and its relation to vertebrates. Trends Cardiovasc. Med. 5: 21–28.
Komuro I, Izumo S. (1993) Csx: A murine homeobox-containing gene specifically expressed in the developing heart. Proc. Natl. Acad. Sci. U.S.A. 90: 8145–8149.
Lints TJ, Parsons LM, Hartley L, Lyons I, Harvey RP. (1993) Nkx-2.5: A novel murine homeobox gene expressed in early heart progenitor cells and their myogenic descendants. Development 119: 419–431.
Lions I, Parsons LM, Hartley L, Harvey RP. (1995) Myogenic and morphogenetic defects in the heart tubes of murine embryos lacking the homeobox gene Nkx2.5. Gene Dev. 9: 1654–1666.
Heikinheimo M, Scandrett JM, Wilson DB. (1994) Localization of transcription factor GATA-4 to regions of the mouse embryo involved in cardiac development. Devel. Biol. 164: 361–373.
Edmondson DG, Lyons GE, Martin JF, Olson EN. (1994) Mef2 gene expression marks the cardiac and skeletal muscle lineages during mouse embryogemesis. Development 120: 1251–1263.
Tamkun MM, Knoth KM, Walbridge JA, Kroemer H, Roden DM, Glover DM. (1991) Molecular cloning and characterization of two voltage-gated K channel cDNA from human ventricle. FASEB J 5: 331–337.
Takahashi T, Allen PD, Izumo S. (1992) Expression of A-, B-, and C-type natriuretic peptide genes in failing and developing human ventricles. Circ. Res. 71: 9–17.
Sambrook J, Fritsch EF, Maniatis T. (1989) Molecular Cloning: A Laboratory Manual. 2nd Ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
Church GM, Gilbert W. (1984) Genomic sequencing. Proc. Natl. Acad. Sci. U.S.A. 81: 1991–1995.
Shepherd NS, Pfrogner BD, Coulby JN, et al. (1994) Preparation and screening of an arrayed human genomic library generated with the P1 cloning system. Proc. Natl. Acad. Sci. U.S.A. 91: 2629–2633.
Pierce JC, Sauer B, Stenberg N. (1992) A positive selection vector for cloning high molecular weight DNA by the bacteriophage P1 system: Improved cloning efficacy. Proc. Natl. Acad. Sci. U.S.A. 89: 2056–2060.
Wechsler DS, Hawkins AL, Li X, Wang Jabs E, Griffin CA, Dang CV. (1994) Localization of the human Mxi1 transcription factor gene (MXI1) to chromosome 10q24–q25. Genomics 21: 669–672.
Kozak M. (1987) An analysis of 5′-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acid. Res. 15: 8125–8132.
Tonissen KF, Drysdale TA, Lints TJ, Harvey RP, Krieg PA. (1994) XNkx-2.5, a Xenopus gene related to Nkx-2.5 and tinman: Evidence for a conserved role in cardiac development. Devel. Biol. 162: 325–328.
Himmelbauer H, Harvey RP, Copeland NG, Jenkins NA, Silver LM. (1994) High-resolution genetic analysis of a deletion on mouse chromosome 17 extending over the fused, tufted, and homeobox Nkx2.5 loci. Mamm. Genome 5: 814–816.
Forejt J, Artzt K, Barlow D, et al. (1994) Mouse chromosome 17. Mamm. Genome 5(Suppl): S238–S258.
Moorman AFM, Lamers WH. (1994) Molecular anatomy of the developing heart. Trends Cardiovasc. Med. 4: 257–264.
Litvin J, Montgomery M, Gonzalez-Sanchez A, Bisaha JG, Bader D. (1992) Commitment and differentiation of cardiac myocytes. Trends Cardiovasc. Med. 2: 27–32.
John Sutton MG, Raichlen JS, Reichek N, Huff DS. (1984) Quantitative assesment of right and left ventricular growth in the human fetal heart: A pathoanatomic study. Circulation 70: 935–941.
John Sutton MG, Gewitz MH, Shah B, et al. (1984) Quantitative assessment of growth and function of the cardiac chambers in the normal fetus: A prospective longitudinal echocardiographic study. Circulation 69: 645–654.
Kenny JF, Plappert T, Doubilet P, et al. (1986) Changes in intracardiac blood flow velocities and right and left ventricular stroke volumes with gestational age in the normal human fetus: A prospective Doppler echocardiographic study. Circulation 74: 1208–1216.
Dietz HCI, Pyeritz RE. (1994) Molecular genetic approaches to the study of human cardiovascular disease. Annu. Rev. Physiol. 56: 763–796.
Nora JJ. (1993) Causes of congenital heart diseases: Old and new modes, mechanisms, and models. Am. Heart J. 125: 1409–1419.
Bouvagnet P, Sauer U, Debrus S, et al. (1994) Deciphering the molecular genetics of congenital heart disease. Herz 19: 119–125.
Zavala C, Jimenez D, Rubio R, Castillo-Sosa ML, Diaz-Arauzo A, Salamanca F. (1992) Isolated congenital heart defects in first degree relatives of 185 affected children. Prospective study in mexico city. Arch. Med. Res. 23: 177–182.
Perry LW, Neill CA, Ferencz C, Rubin JD, Loffredo CA. (1994) Infants with congenital heart disease: the cases. In: Ferencz C, Rubin JD, Loffredo CA, Magee CA (eds). Perspectives in Pediatric Cardiology, Epidemiology of Congenital Heart disease. Futura Publishing Company, Mount Kisco, NY. Vol. 4, pp. 33–62.
Borgaonkar DS. (1991) Chromosomal Variation in Man: A Catalog of Chromosomal Variants and Anomalies. 6th Ed. Wiley-Liss, New York.
McDonald M, Maynard S, Sheldon S, Innis J. (1994) Unbalanced 5;16 translocation in a boy with papillary Thyroid carcinoma. Am. J. Med. Genet. 49: 288–293.
Fryns JP, Kleczkowska A, Kubien E, Petit P, Van den Berghe H. (1984) Cytogenetic survey in couples with recurrent fetal wastage. Hum. Genet. 65: 336–354.
Byrne J, Warburton D, Kline J, Blanc W, Stein Z. (1985) Morphology of early fetal deaths and their chromosomal characteristics. Teratology 32: 297–315.
Lyon MF, Bechtol K. (1977) Derivation of mutant t-haplotypes of the mouse by presumed duplication or deletion. Genet. Res. 30: 63–76.
Acknowledgments
We thank Ann Burgess, Madhu Prasad, and Mary Richmond for excellent technical assistance; T. Takahashi and P. D. Allen for RNA samples; and N. G. Copeland, T. W. Glover, S. A. Camper, and D. Law for helpful comments on the manuscript. This work was supported in part by grants from the National Institutes of Health and the American Heart Association to SI.
Author information
Authors and Affiliations
Additional information
Contributed by T. Yamada on October 11, 1995.
Rights and permissions
About this article
Cite this article
Turbay, D., Wechsler, S.B., Blanchard, K.M. et al. Molecular Cloning, Chromosomal Mapping, and Characterization of the Human Cardiac-Specific Homeobox Gene hCsx. Mol Med 2, 86–96 (1996). https://doi.org/10.1007/BF03402205
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03402205