Abstract
Background
Macrophage migration inhibitory factor (MIF) was one of the first lymphokine activities to be discovered and was described almost 30 years ago to be a soluble factor(s) produced by activated T lymphocytes. In more recent studies, MIF has been “rediscovered” to be an abundant, pre-formed constituent of the anterior pituitary gland and the macrophage, and to be a critical component in the host response to septic shock. Pituitary-derived MIF enters the circulation after infectious or stressful stimuli and appears to act to counterregulate glucocorticoid suppression of cytokine production.
Materials and Methods
Immunoelectron microscopy utilizing a combination of anti-MIF and anti-pituitary hormone-specific antibodies was used to study the ultrastructural localization of MIF within the anterior pituitary gland. Pituitaries were obtained from resting, unstimulated mice and from mice 16 hr after endotoxin administration. The release of MIF also was investigated in vitro by examining the effect of corticotropin-releasing hormone (CRH) on the AtT-20, corticotrophic cell line.
Results
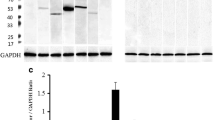
MIF localizes to granules present exclusively in ACTH and TSH secreting cells. Within each cell type, a subset of granules was found to contain both MIF and ACTH, or MIF and TSH. The pituitary content of MIF-containing granules decreased significantly after experimentally induced endotoxemia. In seven pituitaries examined 16 hr after LPS injection, the number of MIF-positive granules diminished by 38% in corticotrophic cells and by 48% in thyrotrophic cells when compared with controls (p < 0.05). CRH was observed to be a potent MIF secretagogue in vitro, inducing the release of MIF from corticotrophic cells at concentrations lower than that required for ACTH release.
Conclusions
These data provide ultrastructural information that identify MIF to be a novel anterior pituitary hormone, support earlier studies showing a time-dependent release of pituitary MIF during endotoxemia, and suggest an important, systemic role for MIF in the stress response to infection and other stimuli.







Similar content being viewed by others
References
Bloom BR, Bennett B. (1966) Mechanism of a reaction in vitro associated with delayedtype hypersensitivity. Science 153: 80–82.
David J. (1966) Delayed hypersensitivity in vitro: Its mediation by cell-free substances formed by lymphoid cell-antigen interaction. Proc. Natl. Acad. Sci. U.S.A. 56: 72–77.
Bernhagen J, Calandra T, Mitchell RA, et al. (1993) MIF is a pituitary-derived cytokine that potentiates lethal endotoxaemia. Nature 365: 756–759.
Calandra T, Bernhagen J, Mitchell RA, Bucala R. (1994) The macrophage is an important and previously unrecognized source of macrophage migration inhibitory factor. J. Exp. Med. 179: 1895–1902.
Bernhagen J, Mitchell RA, Calandra T, Voelter W, Cerami A, Bucala R. (1994) Purification, bioactivity, and secondary structure analysis of mouse and human macrophage migration inhibitory factor (MIF). Biochemistry 33: 14144–14155.
Calandra T, Bernhagen J, Metz CN, et al. (in press). Identification of MIF as a glucocorticoid-induced mediator and counter-regulator of glucocorticoid inhibition of cytokine production. Nature.
Bendayan M. (1982) Double immunocyto-chemical labeling applying the protein Agold technique. J. Histochem. Cytochem. 30: 81–85.
Zar JH. (1984) Biostatistical Analysis. Prentice-Hall, Englewood Cliffs, NJ.
Kurosumi K. (1986) Cell classification of the rat anterior pituitary by means of immunoelectron microscopy. J. Clin. Electron Microscopy 19: 299–319.
Hook VYH, Heisler S, Sabol SL, Axelrod J. (1982) Corticotropin releasing factor stimulates Contributed by A. Cerami on August 29, 1995. adrenocorticotropin and β-endorphin release from AtT-20 mouse pituitary cells. Biochem. Biophys. Res. Commun. 106: 1364–1371.
Axelrod J, Reisine TD. (1984) Stress hormones: Their interaction and regulation. Science 224: 452–459.
Koenig JI, Snow K, Clarke BD, et al. (1990) Intrinsic pituitary interleukin-1β is induced by bacterial lipopolysaccharide. Endocrinology 126: 3053–3058.
Pernicone PJ, Scheithauer BW, Horvath E, Kovács K. (1992) Pituitary and sellar region. In: Sternberg SS (ed). Histology for Pathologists. Raven Press, New York, pp. 279–299.
Sapolsky R, Rivier C, Yamamoto G, Plotsky P, Vale W. (1986) Interleukin-1 stimulates the secretion of hypothalamic corticotropin-releasing factor. Science 238: 522–524.
Uehara A, Gottschall PE, Dahl RR, Arimura A. (1987) Interleukin-1 stimulates ACTH release by an indirect action which requires endogenous corticotropin releasing factor. Endocrinology 121: 1580–1582.
Sharp BM, Matta SG, Peterson PK, Newton R, Chao C, McAllen K. (1989) Tumor necrosis factor-α is a potent ACTH secretagogue: comparison to Interleukin-1β. Endocrinology 124: 3131–3133.
Spangelo BL, Judd AM, Isakson PC, MacLeod RM. (1989) Interleukin-6 stimulates anterior pituitary hormone release in vitro. Endocrinology 125: 575–577.
Elenkov IJ, Kovács K, Kiss J, Bertók L, Vizi ES. (1992) Lipopolysaccharide is able to bypass corticotrophin-releasing factor in affecting plasma ACTH and corticosterone levels: Evidence from rats with lesions of the paraventricular nucleus. Endocrinology 133: 231–236.
Acknowledgments
We are grateful to Drs. C. Metz and M. Wuttke for performing the MIF ELISA and AtT-20 experiments and to Dr. A. Cerami for helpful discussions. This work was supported by National Institutes of Health Grant AI35931.
Author information
Authors and Affiliations
Additional information
Contributed by A. Cerami on August 29, 1995.
Rights and permissions
About this article
Cite this article
Nishino, T., Bernhagen, J., Shiiki, H. et al. Localization of Macrophage Migration Inhibitory Factor (MIF) to Secretory Granules within the Corticotrophic and Thyrotrophic Cells of the Pituitary Gland. Mol Med 1, 781–788 (1995). https://doi.org/10.1007/BF03401892
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03401892