Abstract
Aim: To evaluate the utility of a multigene real-time reverse transcriptase polymerase chain reaction (RT-PCR) assay to detect circulating tumor cells in peripheral blood specimens of breast cancer patients during or after treatment.
Method: Using this assay, peripheral blood samples were analyzed for expression levels of mammaglobin and three complementary transcribed breast cancer-specific genes: B305D, γ-aminobutyrate type A receptor π subunit (GABA π; GABRP), and B726P. We examined 172 blood specimens from 82 breast cancer patients during or after therapy for the presence of circulating tumor cells using the multigene real-time RT-PCR assay.
Results: In 63.4% of the blood samples, a positive signal for mammaglobin and/or three breast cancer-associated gene transcripts was detected. Of breast cancer patients, 75.6% had at least one positive blood sample. Blood specimens from 51 of 53 healthy female volunteers tested negative in the assay whereas two samples had a low expression signal. In addition, three patients were monitored for more than a year during their adjuvant therapy treatment.
Conclusion: This assay could be a valuable tool for monitoring breast cancer patients during and after therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Disseminated tumor cells are considered the main cause for disease progression and metastatic relapse after treatment in breast cancer. Histological and immunological protocols are routinely used to detect metastatic cancer cells in lymph node and more recently in bone marrow specimens.[1] In addition to conventional pathology procedures, polymerase chain reaction (PCR) has been proposed as a sensitive tool to detect micrometastatic cells.[2] A continuously growing number of studies have demonstrated the use of reverse transcriptase (RT)-PCR to detect neoplastic mammary cells in sentinel and axillary lymph nodes, in bone marrow, and in peripheral blood. Several RNA markers have been described, including tumor-associated transcripts (e.g. carcinoem-bryonic antigen [CEA][3]), transcripts of epithelial tissue-specific genes (e.g. cytokeratin 19 and 20 [KRT19; KRT20][4]), mucin family members (e.g. MUC1[5]), and the breast tissue-specific gene mammaglobin (hMAM, SCGB2A2)[6] To date, mammaglobin is the most promising molecular marker for breast cancer because of its high specificity and absence of background expression in normal hematopoietic tissues.[6,7] Mammaglobin was first identified by Watson and colleagues in 1996[8] as a mammary tissue-specific member of the uteroglobin gene family. The uteroglobin/Clara cell protein family consists of small epithelial secretory proteins and has recently been named secretoglobins, with currently 23 known family members.[9] All six human member genes are localized on chromosome 11 and form a dense cluster.[10] Mammaglobin[11,12] is known to be overexpressed in 70–80% of primary and metastatic breast tumor specimens.[13–16] Additional marker genes could be utilized to increase detection sensitivity for breast tumor cells. Our group identified three genes, B305D, γ-ami-nobutyrate type A receptor π subunit (GABA π; GABRP), and B726P, which complement the expression profile of mammaglobin in breast cancers.[16,17] The novel gene designated as B305D is predicted to encode a type II membrane protein. GABA π is a member of the GABAA receptor family.[18] B726P is a novel gene located on chromosome 10 with several different putative open reading frames yielded by mRNA splicing. One of these splice forms, referred to as NY-BR-1, has been recently identified using reactivity with autologous breast cancer patient sera.[19]
Recently, we described the development of a multigene realtime RT-PCR assay detecting the expression profiles of mammaglobin and these three genes simultaneously in order to increase detection sensitivity for breast cancer cells.[20,21] We showed the application of this assay in breast cancer lymph node analysis with high specificity and sensitivity in all tissue samples tested.[20] In addition, we demonstrated the ability of this assay to detect circulating tumor cells in peripheral blood in 77% of untreated Senegalese breast cancer patients.[21]
The aim of the current study was to test the application of this assay in a clinical oncology setting for its ability to detect and monitor tumor cell levels in patient specimens during or after adjuvant therapy. We received 172 peripheral blood specimens from 82 breast cancer patients, and these were enriched for circulating tumor cells by CD45 depletion, and analyzed using the multigene real-time RT-PCR assay.
Materials and Methods
Patients
Breast cancer patients at the Swedish Medical Center (Seattle, WA, USA) and at the Good Samaritan Cancer Center (Puyallup, WA, USA) participated in this study. Written informed consent was obtained in compliance with the Human Subjects Institutional Review Boards of the Swedish Medical Center and the Western Institutional Review Board. Fifty-three blood samples from healthy women with an average age of 38.5 (range 26–59) years were collected through an in-house blood donor program with Western Institutional Review Board approval.
Blood Processing and Multigene Real-Time RT-PCR Analysis
Patients’ blood (10mL) was drawn into EDTA-containing vaccutainers and processed within 3 hours. Tumor cell enrichment, RNA extraction, cDNA synthesis, and multigene real-time RT-PCR were performed as previously described.[21] Results were reported as positive if any expression signal was detected by multigene real-time RT-PCR analysis, and quantitative multigene copy numbers were determined using DNA calibrator dilutions containing plasmid standards for all four genes. PCR products of specimens that tested positive were analyzed by agarose gel electrophoresis and the identity of the expressed tumor marker gene was determined by amplicon size (as described previously).[21]
Results
From October 2001 until July 2003 we received 172 peripheral blood samples from 82 breast cancer patients attending two local clinics. For 55 patients, samples were collected during therapy, and for 17 patients, samples were collected after therapy (currently no treatment). For ten patients, the treatment status was unknown. Patient clinical information, and the number of consecutive specimens collected from breast cancer patients, are listed in table I.
A multigene signal was detected by real-time analysis in 63.4% of the 172 total blood specimens. Blood samples from 62 of 82 individual breast cancer patients (75.6%) tested positive by the real-time multigene RT-PCR. An average expression signal of 49.4 multigene copies (range 0–2201.8 multigene copies) was detected in the 82 patients (table II, figure 1). An average expression signal of 4.2 copies was detected in 13 of 20 patients (65%) with stage I breast cancer and no current metastasis, whereas 26 of 36 patients (72%) with stage II-IV disease and no current evidence of metastasis tested positive with an average copy number of 16.5. In 23 of 26 patients (88.5%) with current metastatic disease, an average expression signal of 129.6 copies was detected. In addition, we tested peripheral blood specimens of 53 healthy female donors as controls. Only two samples tested positive for mammaglobin expression by gel electrophoresis with real-time multigene copy numbers of 2.6 and 1.1, respectively. One of the positive donors was pregnant, in her first trimester.
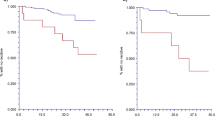
Two patients (Cr009 and Cr072) were monitored over the treatment time course of 17 months and one patient (Cr118) for 25 months. Peripheral blood specimens were received from patient Cr118 with invasive ductal carcinoma and bone recurrence during the treatment course from 24 April 2001 until 28 May 2003 (figure 2). The first three blood draws from April 2001 until February 2002 tested negative for circulating tumor cells using the multigene real-time RT-PCR assay. Until January 2003, the bone recurrence was stable and treated with hormone therapy (table III). Metastatic spread to the liver was diagnosed in March 2003 and treatment was changed to chemotherapy. Metastatic disease progression continued and the chemotherapy regimen was changed in May 2003. A signal was detected by the multigene real-time RT-PCR assay and a mammaglobin amplicon was identified by agarose gel electrophoresis in the blood specimen from June 2002, 8 months before diagnosis of liver metastasis. The expression level detected by the multigene real-time RT-PCR assay increased in the two subsequent blood draws in July and October 2002. Interestingly, CA-27.29 antigen levels were also elevated from 17.2 to 54.8 units/mL in this timeframe. From January until May 2003, positive multigene real-time RT-PCR assay results were indicative for the presence of circulating tumor cells, whereas the multigene expression signal increased significantly during the two draws in May. The breast cancer-specific gene transcript detected changed from mammaglobin alone to GABRP on 20 May 2003 and GABRP + mammaglobin on 28 May 2003. The change in gene expression occurred after chemotherapy was changed on 13 May 2003.
Blood samples from patient Cr072 with stage IV invasive ductal carcinoma were analyzed for breast cancer-specific gene expression by the multigene real-time RT-PCR assay from October 2001 to March 2003 (figure 3). The patient received several courses of chemotherapy for the treatment of liver and bone metastasis and was diagnosed with stable disease in October 2001 and with disease progression in November 2001 (table IV). No signal was detected by the multigene real-time RT-PCR assay in the first peripheral blood specimen from October 2001; however, the following specimen in October tested positive by the multigene assay, and mammaglobin expression was identified by gel electrophoresis. Serum CA-27.29 level was also elevated from 9.3 to 27.7 units/mL in the two October blood specimens. Chemotherapy was changed and the disease status was stable from February 2002 until July 2002. No multigene expression signal was detected by real-time PCR in the next two blood samples in February and March of 2002; however, the specimen analyzed on 12 March 2002 tested positive, 6 months prior to disease progression in September 2002. Significantly, no change was detected in CA-27.29 and CEA sera levels (data not shown), whereas the multigene expression signal was elevated in September 2002 and January 2003 as disease progressed.
Positive multigene expression signals were indicative for circulating tumor cells in all blood specimens from patient Cr009 who had invasive ductal carcinoma and disease recurrence in liver and bone (figure 4). Both B726P and mammaglobin transcripts were detected by gel electrophoresis after multigene real-time RT-PCR analysis in specimens prior to chemotherapy change in March 2002 (table V). Analysis of subsequent samples demonstrated mammaglobin expression only. The expression signal rose from 0.2 copies on 9 July 2002 to 318.3 copies on 21 January 2003. Similarly, CA-27.29 serum levels were increased from 63 in July 2002 to 261 in January 2003. In March 2003 the patient died.
Discussion
In this study, we examined the application of our previously described multigene real-time RT-PCR assay to detect circulating breast tumor cells in the blood of breast cancer patients during and after therapy. Whereas our previous studies demonstrated the complementary expression of mammaglobin and three novel breast cancer tumor marker genes in primary cancers[16] and in metastatic lymph node specimens,[20] and the ability of a multigene real-time RT-PCR assay to detect circulating tumor cells in untreated Senegalese breast cancer patients at the time of diagnosis,[21] the data presented in this article outline the application of this assay to detect and monitor circulating tumor cell loads in peripheral blood of US breast cancer patients during or after treatment.
We tested 172 blood specimens originating from 82 breast cancer patients. Of all blood specimens, 63.4% tested positive for circulating tumor cells by real-time RT-PCR, whereas 75.6% of the breast cancer patients had a positive blood sample. Analyzing multiple blood draws from single patients increased the sample reactivity by 12%. One reason could be that multiple blood samples increase the likelihood of detecting micrometastasis in the blood. In addition, response to treatment could cause fluctuating levels of disseminated tumor cells in the bloodstream.
Different levels of multigene expression were detected in patients who had stage I or stage II-IV disease with no evidence of metastasis compared with patients with current metastatic disease. The average real-time multigene signals detected in patients with metastatic disease were 8 times higher than those occurring in patients with stage II-IV disease and no evidence of metastasis, and 30 times higher than in stage I patients with no evidence of metastasis. In addition, the multigene assay detection rate increased with stage and presence of metastasis. A positive expression signal indicating circulating tumor cells was detected in 65% of stage I patients with no current disease, in 72% of stage II-IV patients with disease but no metastasis, and in 88.5% of patients with current metastatic spread. Peripheral blood samples of 51 healthy female volunteers tested negative in the assay; however, a low real-time multigene expression signal was detected in two control donors. One donor who tested positive was pregnant, in the first trimester. It is possible that hormonal changes influencing cell proliferation in the breast tissue during pregnancy may lead to upregulation of mammaglobin expression and, in addition, to cell dissemination in the bloodstream. Further specimens from pregnant and breastfeeding women have to be examined to investigate this relationship.
We were able to monitor the presence of circulating tumor cells using the multigene real-time RT-PCR assay during the course of treatment of three patients for 17 (two patients) and 25 months. In two patients (Cr118 and Cr072), the occurrence of circulating tumor cells preceded disease progression and observation of additional metastasis. In one patient the upregulation of multigene copy numbers correlated with the elevation of CA-27.29 levels (Cr118). Interestingly, CA-27.29 levels were not indicative of disease progression in one patient (Cr072), whereas multigene copy numbers were significantly elevated. This finding demonstrates the potential application of circulating tumor cell detection to evaluate treatment response, in cases where conventional serum markers do not correlate with disease progression. In a recent study, Weigelt et al.[22] showed that the presence of circulating tumor cell mRNA in peripheral blood predicts significantly shortened survival. Ninety-four breast cancer patients with metastatic disease were studied using real-time RT-PCR assays for four genes (KRT19, p1B, PS2, TACSTD1); however, circulating tumor cells could be detected in only 31% of patients.
Moreover, it is interesting to note that in two patients a change of gene expression in the circulating tumor cells was detected after the chemotherapy regimen was altered. Therefore, the detection and molecular analysis of micrometastatic cells could provide an important tool to assess the status of residual disease for future individualized antibody and vaccine therapy applications. In patient Cr072, mammaglobin expression was detected by gel electrophoresis in six of nine specimens that tested positive by the multigene real-time RT-PCR. In two specimens, B726P expression was detected instead of mammaglobin. One potential explanation for the difference in detected gene expression could be that the circulating breast tumor cells expressed both B726P and mammaglobin mRNA; however, only the dominant amplification product was detected by gel electrophoresis. In addition, potential clonal selection due to therapy could also cause changes or specific loss in the tumor marker expression profile.
Conclusion
In summary, this current study demonstrates the utility of the mammaglobin multigene real-time RT-PCR assay to detect circulating tumor cells in breast cancer patients after or during treatment and its potential application for monitoring therapy. We recently reported data generated with peripheral blood specimens from Senegalese breast cancer patients using the multigene mammaglobin PCR assay in combination with a mammaglobin sandwich ELISA.[21] The sensitivity of the multigene real-time PCR assay was slightly higher in our previous study (77%), which can be explained by the fact that the Senegalese patient population was not treated by chemotherapy at the time of testing. We conclude that the described mammaglobin multigene real-time RT-PCR assay might be a valuable tool to detect and monitor circulating breast cancer tumor cells. As shown recently by Cristofanilli and colleagues[23] in a prospective, multicenter study, circulating tumor cells are the most significant predictors of progression-free and overall survival in metastatic breast cancer.
References
Zehentner BK. Detection of disseminated tumor cells: strategies and diagnostic implications. Expert Rev Mol Diagn 2002; 2(1): 41–8
Ghossein RA, Rosai J. Polymerase chain reaction in the detection of micrometastases and circulating tumor cells. Cancer 1996; 78(1): 10–6
Gerhard M, Juhl H, Kalthoff H, et al. Specific detection of carcinoembryonic antigen-expressing tumor cells in bone marrow aspirates by polymerase chain reaction. J Clin Oncol 1994; 12(4): 725–9
Slade MJ, Smith BM, Sinnett HD, et al. Quantitative polymerase chain reaction for the detection of micrometastases in patients with breast cancer. J Clin Oncol 1999; 17(3): 870–9
de Cremoux P, Extra JM, Deni MG, et al. Detection of MUC1-expressing mammary carcinoma cells in the peripheral blood of breast cancer patients by real-time polymerase chain reaction. Clin Cancer Res 2000; 6(8): 3117–22
Zach O, Kasparu H, Krieger O, et al. Detection of circulating mammary carcinoma cells in the peripheral blood of breast cancer patients via a nested reverse transcriptase polymerase chain reaction for mammaglobin mRNA. J Clin Oncol 1999; 17(7): 2015–9
Corradini P, Voena C, Astolfi M, et al. Maspin and mammaglobin genes are specific markers for RT-PCR detection of minimal residual disease in patients with breast cancer. Ann Oncol 2001; 12(12): 1693–8
Watson MA, Fleming TP. Mammaglobin, a mammary-specific member of the uteroglobin gene family, is overexpressed in human breast cancer. Cancer Res 1996; 56(4): 860–5
Klug J, Beier HM, Bernard A, et al. Uteroglobin/Clara cell 10-kDa family of proteins: nomenclature committee report. Ann N Y Acad Sci 2000; 923: 348–54
Ni J, Kalff-Suske M, Gentz R, et al. All human genes of the uteroglobin family are localized on chromosome 1 1q12.2 and form a dense cluster. Ann N Y Acad Sci 2000; 923: 25–42
Min CJ, Tafra L, Verbanac KM. Identification of superior markers for polymerase chain reaction detection of breast cancer metastases in sentinel lymph nodes. Cancer Res 1998; 58(20): 4581–4
Colpitts TL, Billing-Medel P, Friedman P, et al. Mammaglobin is found in breast tissue as a complex with BU101. Biochemistry 2001; 40(37): 11048–59
Watson MA, Dintzis S, Darrow CM, et al. Mammaglobin expression in primary, metastatic, and occult breast cancer. Cancer Res 1999; 59: 3028–31
Fleming TP, Watson MA. Mammaglobin, a breast-specific gene, and its utility as a marker for breast cancer. Ann N Y Acad Sci 2000; 923: 78–89
Leygue E, Snell L, Dotzlaw H, et al. Mammaglobin, a potential marker of breast cancer nodal metastasis. J Pathol 1999; 189: 28–33
Houghton RL, Dillon DC, Molesh DA, et al. Transcriptional complementarity in breast cancer: application to detection of circulating tumor cells. Mol Diagn 2001; 6(2): 79–91
Jiang Y, Harlocker SL, Molesh DA, et al. Discovery of differentially expressed genes in human breast cancer using subtracted cDNA libraries and cDNA microarrays. Oncogene 2002; 21(14): 2270–82
Hedblom E, Kirkness EF. A novel class of GABAA receptor subunit in tissues of the reproductive system. J Biol Chem 1997; 272: 15346–50
Jager D, Stocken E, Gure AO, et al. Identification of a tissue-specific putative transcription factor in breast tissue by serological screening of a breast cancer library. Cancer Res 2001; 61: 2055–61
Zehentner BK, Dillon DC, Jiang Y, et al. Application of a multigene reverse transcription-PCR assay for detection of mammaglobin and complementary transcribed genes in breast cancer lymph nodes. Clin Chem 2002; 48(8): 1225–31
Zehentner BK, Persing DH, Deme A, et al. Mammaglobin as a novel breast cancer biomarker: multigene reverse transcription-PCR assay and sandwich ELISA. Clin Chem 2004; 50(11): 2069–76
Weigelt B, Bosma AJ, Hart AA, et al. Marker genes for circulating tumour cells predict survival in metastasized breast cancer patients. Br J Cancer 2003; 88(7): 1091–4
Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 2004; 351(8): 781–91
Acknowledgements
This study was supported in part by NIH grants CA-75794 and CA-86673.
The authors have no conflicts of interest that are directly relevant to the content of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zehentner, B.K., Secrist, H., Hayes, D.C. et al. Detection of Circulating Tumor Cells in Peripheral Blood of Breast Cancer Patients During or After Therapy Using a Multigene Real-Time RT-PCR Assay. Mol Diag Ther 10, 41–47 (2006). https://doi.org/10.1007/BF03256441
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03256441