Abstract
Proactive interference (PI) occurs when memories of past events or stimuli intrude in the present moment, causing working memory (WM) errors. These errors are often measured through WM tests such as matching-to-sample (MTS). When the repetition of individual stimuli increases, there is a greater chance of these intrusions, and thus there can be a decrease in accuracy in such tasks. In two experiments, we explored the nature of PI on dog working memory. First, we manipulated the size of the set of odors (2, 6, trial-unique) used to construct each session to maximize (2-odor set) and minimize (trial-unique) within-session proactive interference during an olfactory MTS task. Matching-to-sample accuracy decreased with greater PI. Second, we adapted procedures originally designed for pigeons and rhesus macaques to determine the locus of PI in dogs. To test for proactive interference, probe trials were inserted into MTS sessions where sample odors from earlier trials reappeared as incorrect comparisons. Incorrect responses on these probe trials indicated proactive interference. These probe tests were conducted with a 0-s or 20-s retention interval in separate sessions. We found that dogs performed worse on the matching task when the source of interference (odor stimulus) was from the immediately preceding trial compared with when they were from trials further back in the session but only for the 0-s retention interval. These results are compared with previous work examining the effects of proactive interference on working memory in other species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In natural settings, animals are constantly encountering new objects and information. At some point, memory for these stimuli can be taxed to the point of failure (e.g., forgetting). Cognitive psychology has long been interested in understanding these failures of memory (Wixted, 2004), and interference from previously encountered stimuli is one possible explanation (Keppel & Underwood, 1962). Like humans, nonhuman animals, are susceptible to intrusions of stimuli into memory. These intrusions can produce interference that can cause confusion during remembering and can be either proactive (earlier memories cause confusion at a later moment) or retroactive (later memories cause confusion of past moments). Together, these sources of interference can help account for the nature of memory (Wright, 2012).
Proactive interference (PI) is a common explanation for forgetting and its effects are primarily assessed in tasks that use working memory (WM), or the ability to hold and use memories of a stimulus for the length of a session (Dudchenko, 2004). Proactive interference is a critical process of interference theory (Keppel & Underwood, 1962). That is, the influence of PI is one way to explain how information can enter the brain and be processed and stored, but cannot be accurately retrieved (Ceraso, 1967). Proactive interference can increase both within a session and across sessions, and is due to the repetition of stimuli, either during a single session (within-session PI) and/or from reusing the same stimulus set each session (across-session PI). Both types of PI can be mitigated, or bolstered, by decreasing or increasing the number of repeated stimuli used throughout the experiment, respectively (Wright, 2018).
A favored procedure for comparative studies of PI on memory is the delayed matching-to-sample task (dMTS). In a typical dMTS experiment, each trial consists of a series of phases. First is the sampling phase, where a sample stimulus (e.g., red circle) is presented to the subject before being removed or made unavailable. Second is the retention phase, where subjects must retain information from the sample item for a certain amount of time (i.e., retention interval) during which the sample stimulus is absent. The retention interval can vary greatly in duration from seconds to hours (e.g., Overman & Doty, 1980). Third is the comparison phase, where a copy of the sample (e.g., red circle) and a nonmatching comparison stimulus (e.g., red triangle) are presented at the same time. The correct response is to choose the comparison stimulus (i.e., red circle) that matches the sample presented in the first phase, prior to the retention interval.
A similar task, the same/different (S/D) procedure, requires subjects to compare pairs of stimuli. A key difference between S/D tasks and dMTS is that in S/D, subjects must make a differential response depending on whether the stimuli in the pair are the same or different. For example, Katz, Wright, and Bachevalier (2002) trained monkeys on a S/D task where monkeys were first presented with a sample stimulus (e.g., a photograph of buildings) and then presented with either a copy of the same stimulus or a different one. For trials in which the two stimuli were the same, the monkeys were rewarded for touching the second (matching) image, and for trials in which they were different, they were rewarded for touching a small rectangle, denoting a “different” response. Importantly, animals in these experiments benefit greatly from an observing response to the sample stimulus (Katz et al., 2007). An observing response can vary between sensory modality and task, but typically requires the subject to physically contact the stimulus (e.g., touch a sample stimulus 10 times). Results from studies using each task have shown that PI can be mitigated, or bolstered, by decreasing or increasing the number of repeated stimuli used (as either sample or incorrect comparison) throughout the experiment (Wright, 2018).
In MTS as well as S/D tasks, PI occurs when the same stimuli switch roles as sample or incorrect comparisons continuously within an experimental session. Thus, repeating stimuli causes an increase in PI (Wright et al., 1986) within a session. One way to manipulate this repetition in MTS is to change the size of the pool from which stimuli are drawn to construct each session, referred to as the set size (Wright, 2007). Proactive interference builds up most quickly, and to the highest degree, with a small set size. For example, consider an MTS session with a set size of 2 (e.g., orange circle, blue circle). In this case the stimuli are repeated every trial, sometimes as the sample/correct comparison and sometimes as the incorrect comparison, and within-session PI continues to increase as the session progresses. As set size increases, PI decreases to the point where there can be no within-session interference (e.g., trial-unique). In trial-unique sessions, stimuli are not repeated during a session, and hence there is no within-session PI. The effect of set size on memory has been a point of interest for decades (for a review see Wright, 2007).
An additional factor in this procedure is how interference transfers from one trial to the next (Wright et al., 1986), a measure of intertrial PI. On any given pair of consecutive trials, there are several ways for intertrial PI to transition from one trial to another, depending on specific pairings. When the sample is the same from one trial to the next, it is considered a positive transfer trial, where the effects of PI will be mitigated due to the lack of change. Negative transfer trials occur when the previous trial sample changes between trials and is now the incorrect comparison. For such negative transfer trials, performance drops relative to positive transfer trials. Another factor is the outcome of the previous trial. Animals tend to perform worse on rewarded negative transfer trials, whereas rewarded positive transfer trials typically lead to better accuracy (Moise, 1976). That is, if on a negative transfer trial, the previous trial was rewarded, there is often worse accuracy on the trial following the rewarded trial (i.e., the second trial in a negative transfer pair of trials). The inverse of these is also true, where non-rewarded negative transfer trials tend to lead to higher accuracy than non-rewarded positive transfer trials.
Another way to manipulate repetition is to add interfering probes during trials in which there is otherwise no interference (i.e., trial-unique; no-PI) sessions herein referred to as probe trials. Two previous studies used this method for manipulating the locus of PI in pigeons (Wright et al., 2012) and monkeys (Devkar & Wright, 2016). In these studies, interfering probe trials (PI probes) were inserted into trial-unique S/D sessions. These probes were trials in which the incorrect comparison stimulus had previously appeared as a correct sample. The presentation of probe trials varied in the number of trials since the comparison stimulus had last appeared in the session. For example, in some probe trials the comparison stimulus might have appeared on the immediately previous trial, or it could be 2, 4, 6, 8, or 16 trials since it first appeared in the session. Additionally, retention intervals occurred between the sample and comparison stimuli. One delay was short (1 s), and the other was a longer delay of 10 s for pigeons (Wright et al., 2012) and 10 and 20 s for monkeys (Devkar & Wright, 2016). This manipulation allowed the researchers to examine PI both as a function of time (e.g., delay) and number of intervening trials (e.g., probe displacement), and as an interaction between these effects. Importantly, pigeons (see Fig. 1) showed worse interference with an increase in time (1 s vs. 10 s) and number of intervening trials, as well as an interaction between these two factors, while monkeys (see Fig. 2) showed an effect of number of intervening trials only. This indicates a potential qualitative difference between pigeon and monkey working memory. For monkeys, interference is only due to the increase of relevant events (i.e., the number of intervening trials) but is not time-based. That is, monkeys experienced interference when there was stimulus repetition, regardless of the duration of the retention interval. This explains the differences in PI found by Devkar and Wright (2016) and other studies where monkeys show much stronger PI effects. For example, when composing MTS or dMTS experiments, smaller set sizes will necessarily increase the repetition of relevant events (trials), in turn increasing the overall amount of PI and decreasing accuracy. Indeed, early evidence of dMTS with monkeys suggested they had almost no working memory duration, as their accuracy over delays of just a few seconds was near chance when PI increased to a maximum degree, while reducing PI improved accuracy (Overman & Doty, 1980; Wright et al., 2018). Conversely, pigeons show significant effects of intervening trials, delay, and an interaction between these factors. The interaction is the critical component of these experiments, and fully shows an effect of time-based interference. These data cannot be interpreted as loss due to decay because there is no effect of time at baseline trials, where there is no within-session interference and almost no across-session interference (see Wright et al., 2012 for modeling of the data).
Mean percent correct for pigeons at each trial number of interfering stimulus (1,2,4,8,16) from Wright et al., (2012). Pigeons show main effects of delay, trial number of interfering stimulus, and an interaction between these factors
Mean monkey performance by delay and trial number of interfering stimulus, from Devkar and Wright (2016). Note that the lines overlap, indicating no effect of delay. Monkeys are only impacted by proactive interference when the repeating events occur immediately after one another
Expanding the number of species beyond pigeons and rhesus monkeys tested in this procedure can provide information on how time-based and event-based interference have evolved. One species to consider is the dog, for which there is great increasing interest across scientific disciplines in understanding their cognitive processing (Bruce et al., 2021). This is especially important as dogs play a variety of roles in human society, from security and protection, explosives and disease detection, emotional and physical support, and companionship, to potential models of human aging (Ruple et al., 2022). Memory and proactive interference can influence performance in each of these roles and has implications for training dogs to perform these roles. Therefore, developing procedures for exploring memory and cognition can lead to improvements in dog welfare and the efficacy of dogs in specialized roles.
Recently, we developed a procedure for training canines to perform olfactory MTS and dMTS to test for abstract-concept learning and working memory (Krichbaum et al., 2021; Lazarowski et al., 2021). Krichbaum et al., (2021) tested the effects of PI at different retention intervals to disassociate the effects of time and PI in dMTS. Dogs experienced dMTS sessions that had been constructed using three different set sizes, each corresponding to the degree of increasing PI (e.g., high, moderate, and no-PI) in a 24-trial session, with varying delays. First, there was the no-PI session, which was trial-unique (i.e., each odor appears only once). Second was the moderate-PI condition, where six odors were used equally often, leading to some PI in each session. Lastly, there was the high-PI condition, where two odors were used in each session, creating a high amount of PI due to constant repetition (i.e., each odor reappears on every trial). Dogs did worse in the high-PI condition than in both moderate and trial-unique sessions. In the high-PI sessions, dogs’ accuracy was above chance (50% correct) only when there was a 0-s retention interval. The results demonstrated that dogs can be trained to a high level of accuracy on dMTS (greater than 85% correct) yet are still susceptible to the effects of PI.
In the current study, two experiments further tested the effects of PI in dogs. In Experiment 1, set size was manipulated to explore the effects of repetition within a session on dog performance in dMTS only at the 0-s delay instead of using multiple delays within a session (cf. Krichbaum et al., 2021). As in Krichbaum et al., the set size for each session varied in the amount of repetition and, therefore, the amount of within-session PI. Each session comprised 2, 6, or 48 odors. In Krichbaum et al., (2021) there was only an effect of PI at the 2-odor set during 0-s delays. Thus, for this experiment, we expected that performance would be worse in the 2-odor set in relation to the 6-odor and 48-odor sets. We also analyzed the 2-odor set for the effects of intertrial interference (cf., Wright et al., 1986). Negative transfer trials where the previous trial was rewarded were expected to have worse overall accuracy than other combinations, especially positive transfer trials where the previous trial was rewarded. Alternatively, dogs may have a tendency to perseverate on their previous choice, meaning that regardless of reward, dogs will suffer from intertrial interference effects when the previous choice and current sample differ. On trials where the current sample is the same as the dog’s previous choice, there will be higher accuracy and no deleterious effect of intertrial PI. In this view, interference would occur because memories of the current trial choice conflict with memories of the previous trial choice. When the stimuli were the same, there were no deleterious effects of PI, but when the stimuli were different, there was a deleterious effect of PI. Worse accuracy in the dMTS task would indicate conflicting memories of the current sample and previous samples. In Experiment 2, to characterize dogs’ representation of PI as either time- or event-based, we adapted the PI probe experiments of Wright et al., (2012) for olfactory dMTS. The effects of delay (0 s vs. 20 s) and number of intervening trials (n−1, n−6, n−12, and no-PI) were tested. If PI in dogs is functionally due to the effects of time, then the effects of delay, the number of intervening trials, and the interaction between these two would be significant. This result would suggest that dogs are more like pigeons in their representation (i.e., the reference memories of the sample used at test) of PI. Otherwise, if neither delay nor the interaction is significant, this would suggest that dogs are more like monkeys and that event-based PI might be shared among mammals broadly.
General procedures
Methods
Subjects
Six Labrador retrievers (four female) served as subjects for this experiment. Age ranged from two to six years, (mean age: 4.17 years). Dogs had previous training history in scent detection. After initial training, tests of MTS abstract-concept learning (Lazarowski et al., 2021) and subsequent dMTS training with set sizes of 2, 6, and 48 odors with delays of 0, 30, 60, and 90 s (Krichbaum et al., 2021). The data for Experiment 1 were collected alongside the data reported by Krichbaum et al., (2021). After the abstract-concept learning testing (Lazarowski et al., 2021), dogs began dMTS training with variable delays using the trial-unique 48-odor set. After this initial dMTS training, the dogs were tested on each of the smaller set sizes, starting with the 0-s delay sessions reported here. Thus, each dog completed the 0-s delay 2-odor and 6-odor sessions before the corresponding session of 2- and 6-odor dMTS as in Krichbaum et al., (2021). The data from Experiment 2 were all collected immediately following the last dMTS session. Dogs were housed in kennels with indoor/outdoor runs at Auburn University’s College of Veterinary Medicine. Auburn University’s Institutional Animal and Care Use Committee approved the animal use. Approval was granted by the Auburn University Institutional Animal Care and Use Committee (protocol: #2018-3334). Dogs were tested in the following sequence: initial MTS training, abstract-concept learning as described by Lazarowski et al., (2021), Experiment 1 of this study and experiments reported by Krichbaum et al., (2021) and ending with Experiment 2 of this study. Dogs were tested on one condition at a time, and only one session per day.
Stimuli and apparatus
Stimuli were 48 household spices (e.g., ground cinnamon; from The Great American Spice Company, Rockford, MI, USA) and essential oils (e.g., almond extract; Anjou Naturals, Fremont, CA) used primarily for cooking (see Table 1 for list of odors). To present odors, cotton pads (Swisspers® 100% cotton rounds pads) were first saturated with the odor by storing them in glass mason jars with approximately 28 g of powder or 3–4 drops of each essential oil. Cotton pads were placed into a perforated tin at the beginning of each session. These perforated tins were kept in a container unique to each odor.
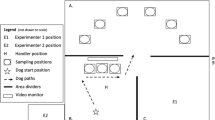
Testing took place in an enclosed arena (6.5 × 6 m; Fig. 3) in a temperature-controlled building at Auburn’s Canine Performance Sciences. In the arena, there were six cinderblocks (19 × 19 × 19 cm) placed on wooden platforms (28 × 28 × 18 cm) that served as possible locations for odors. Within each cinderblock were paint canisters that could hold an odor tin. Each location, regardless of the presence of an odor tin, looked the same from the entrance of the arena. Cinderblocks were arranged in a semicircle formation, approximately .5 m apart from each other, and 2.7 m from the entrance to the arena. Thus, dogs had to search each location to find odors. A sample odor, which provided the dogs with the correct choice in the arena, was presented to the dogs before they could enter the test arena. The sample odor could be in one of the three cinderblock/paint canister locations outside the arena. Each session was recorded by an HD camera (GoPro Hero 5), which was also used as a closed-circuit television (i.e., CCTV) to observe and live-score each session.
Schematic representation of the testing arena. Previously published in Krichbaum et al., (2021). The dotted line represents the path each dog takes on each trial. Prior to each trial, Experimenter 1 places each odor in the predetermined location and then returns to the location marked by E1. At the beginning of each trial, the handler (H) directs the dog from its starting location at the star along the path indicated by the dotted arrowhead lines. Along this path, the dog is commanded to search the three potential sample odor locations. At the end of the line, the dog is released into the enclosure to search the six potential locations in whatever order it chooses. Handler 1 remained out of the enclosure, out of view of the dog, and observed the dog via a monitor that displayed a live feed of the enclosure and signaled when the dog made a choice. Experimenter 2 stayed in location E2, separated by a low wall. Experimenter 2 confirmed whether the choices were correct or incorrect and scored each trial
Task: Olfactory dMTS
There were 24 trials in each session. A trial began when experimenter 1 signaled to the handler that the trial was ready. At the beginning of each trial, dogs were directed to search the sample location, which varied randomly across three possible locations. When they made an observing response, defined as fully putting their snout in a paint canister and freezing for 1 s (often referred to as a “change of behavior,” Minhinnick et al., 2016), the handler marked the response with a clicker and the dog entered the arena to search the array off-leash with all experimenters and handlers out of view. A dog could search in any direction, and typically searched from right to left (all odors were balanced so that either search direction led to the correct choice first an equal number of times). A dog indicated a choice by sitting next to the cinderblock with the odor in it. Handlers, who were kept blind to the location of each odor, signaled when a response had been made and experimenter 2 relayed whether the dog was correct or incorrect. For correct responses, handlers again clicked at which point the dog ran back to the handler for play with a Chuckit!® ball. For incorrect responses, handlers recalled the dog and did not reward it with the ball or engagement. The handler held the dog until the next trial was ready. During delay trials, the sample canister was removed from the sample area and the dog was held at the entrance to the arena before sending the dog into the arena in order to remove access to the odor during the delay. There was an intertrial interval (ITI) of about 30 s, varying only slightly depending on individual dogs, handlers, and experimenters. Between sessions, the experimenters swept and cleaned the arena. Odors were always handled with gloves, and odorized cotton pads were replaced prior to the first session of the day.
Experiment I: Set size testing
Dogs began set size testing after testing on three sessions of 48 odors (trial-unique). Dogs were then tested with two different odor set sizes: 2 odors and 6 odors. Each set size was tested twice, counterbalanced for order across subjects. A different set of odors were used each session to avoid across-session PI. In the 2-odor set, carob and amaretto were used in one session, and coriander and apple in the other session. For the 6-odor set, allspice, apricot, cotton candy, pecan, parsley, and chamomile were used in one session, and butterscotch, cinnamon, garlic, mustard, root beer, and thyme were used in the other session. Six-odor sessions were balanced such that each odor appeared both as the sample and incorrect distractor four times each. The same odors were used for each dog to rule out potential differences between dogs due to odor, which may have been more likely with only six dogs as subjects.
The 2-odor sessions were arranged to test for four intertrial progressions (Wright et al., 1986). Specifically, the four types of intertrial progression are as follows: odor A repeats as sample, odor A switches from sample to incorrect comparison, odor B repeats as sample, and odor B switches from sample to incorrect comparison. These four intertrial progressions have been called positive (PT) and negative transfer (NT). When the sample repeats, there is a positive transfer between trials (i.e., the previous trial memories will not cause a disruptive intrusion). When the sample changes, there is a negative transfer (i.e., previous trial memories will cause a disruptive intrusion). Each transfer type occurred 12 times in each session, allowing us to analyze the intertrial PI effects.
Data analysis
Data were analyzed in R (version 4.0.3) using a generalized linear mixed-effects model (GLMM), binomial family distribution, with individual dog ID as a random factor (lme4 package; Bates et al., 2015). To fit a binomial distribution, accuracy was coded as 1 for correct and 0 for incorrect (as opposed to using a percent correct score as in linear regression). Accuracy was determined as a function of set size (2, 6, 48), coded categorically as small, medium, and trial-unique. Trial (1 to 24) was also included as a variable. The initial formula for the logistic regression was as follows: Accuracy ~ set size + trial + trial * set size + (1|Dog), where the term (1|Dog) specifies random effects of individual dog.
A second analysis explored the effects of positive and negative transfer in the 2-odor condition. Each trial was coded as either positive transfer (PT) or negative transfer (NT). Reward was also included as a factor and coded as whether or not (yes or no) the previous trial was rewarded. The formula for this logistic regression was as follows: Accuracy ~ trial type + reward + reward * trial type, (1|Dog).
Results and discussion
Figure 4 shows the effect of set size on mean accuracy. Proactive interference had an effect in the 2- and 6-odor conditions relative to the 48-odor condition. There was a significant main effect of set size, such that dogs had lower accuracy on the 2-odor (M = 74.53, SE = 4.40) than the 48-odor (M = 87.15, SE = 1.08; z = −3.34, p < .001) and 6-odor (M = 76.01, SE = 3.77; z = −3.60, p <.001) set sizes. There was no significant difference between the 2-odor and 6-odor set sizes (M = 76.01, SE = 3.77; z = −0.31, p = .756). Five of six dogs showed an effect of interference (i.e., lower percent correct) in the 2- and 6-odor sessions. The main effect of trial and the interaction between set size and trial (z = −1.19, p = .235) were not significant; therefore, the final model was accuracy ~ set size + trial + (1|Dog).
Figure 5 illustrates the combined effects of intertrial PI and the impact of reward (i.e., that the previous trial had been rewarded) on mean accuracy (i.e., percent correct) for the 2-odor set size. There were significant effects of reward (reward: M = 74.77, SE = 5.19; non-reward: M = 73.43, SE = 6.13; z = −2.89, p = .004) and trial type (PT: M = 76.92, SE = 6.06; NT: M =71.94, SE = 4.10; z = −2.6, p = .009) and the trial type × reward interaction was also significant (z = 3.45, p < .001). Therefore, two separate GLMMs examining the effect of transfer type (PT or NT) were analyzed for previous rewarded or non-rewarded trials separately. When the previous trial was rewarded, accuracy on PT (M = 81.31, SE = 5.22) was higher than on NT (M = 68.47, SE = 1.47) trials (z = 2.37, p = .018), while when the previous trial was not rewarded, dogs performed better on NT (M = 85.71, SE = 2.93) than PT (M = 63.89, SE = 6.71) trials (z = −2.47, p = .013).
In Experiment 1, the effects of within-session and intertrial PI on accuracy in dogs were shown. Repetition from the 2- and 6-odor sets caused increased PI and worse overall accuracy compared to trial-unique sessions in this task. These results are in line with the data from 0-s delay condition of Krichbaum et al., (2021), where dogs perform worse with a 2-odor set (72.22%) than 6-odor (85.56%) and 48-odor (87.78%) sets. However, we found a significant difference in accuracy between 6- and 48-odor sets, indicating that by removing variable delays from each session, the effects of PI were more robust for 6 odors at the 0-s delay. Perhaps in the previous study, the more difficult longer delays created a contrast effect which allowed the dogs to combat PI at the 0-s delay. In addition, the longer delays (30, 60, 90 s) created greater time differences over trials which may have helped to combat PI in the previous experiment and diminished PI in the 6-odor condition. The lack of a trial effect indicates that the observed effects of PI occurred early in a session and did not increase throughout the session. Perhaps sessions longer than 24 trials would show an increasing effect of PI due to increasing stimulus repetition. The lack of effect of trial and the interaction suggests that the effects of PI occurred toward the start of the session, and did not increase further over trials.
In the trial type analysis (Fig. 5), there was a significant interaction between reward and trial type where dogs tended to perseverate on behaviors that were recently rewarded, causing severe interference on negative transfer trials when they were previously rewarded, but not when the previous trial was unrewarded (17.24% difference). On positive transfer trials, dogs performed best when the previous trial was rewarded, and worse when the previous trial was not rewarded (17.42% difference). Dogs did better on both rewarded positive transfer trials and non-rewarded negative transfer trials, and worse on rewarded negative transfer trials and non-rewarded positive transfer trials. These results replicated similar findings in pigeons (Roberts, 1980) and pig-tailed macaque monkeys (Moise, 1976). Table 2 (adapted from Wright et al., 1986) shows the effects of PI as a function of transfer and either reward or previous choice in our study, as well as Roberts (1980) and Moise (1976). On the left column is transfer type, either positive transfer (PT) or negative transfer (NT), as described previously. The next column describes the outcome of the previous trial (N−1) in terms of reward or non-reward. Under % correct on trial N is the accuracy for each species as a function of trial transfer type and reward. The last column reinterprets the accuracy under % correct on Trial N as a function of trial type and the animals' choice on N−1, where “same” means the current sample is the same as the previous trial choice, and “different” means the current sample is not the same as the previous trial choice. When the previous choice and the current sample are the same, dogs show no effects of PI. However, when the previous choice and the current sample are different, dogs show a significant effect of PI. Monkeys and pigeons show similar effects. What these data show is that intertrial interference is less influenced by reward outcomes than by what stimulus the animal just selected during the choice phase on the previous trial (cf. Roberts, 1980; Wright et al., 1986). Of note, while these findings show a qualitative similarity across species, there is a quantitative difference. The effect is on the order of 5–8% in pigeons and monkeys but a 17% difference in dogs. That is, dogs seem to be more influenced by what stimulus was selected on the previous trial than the consequences of that choice. It is important to note that the sources of intertrial interference are not all or none. For example, if dogs were only controlled by their previous choice, then the difference between the positive transfer rewarded vs. non-rewarded trials would be 50%.
Influence from the previous choice has also been shown in a variation of the dMTS task, the delayed matching-to-position task (DMTP; Dunnett & Martel, 1990). Rats were trained to press a lever that was inserted into one of two locations in an operant chamber. Pressing the lever started the delay period, where the lever was removed for the duration of a retention interval (such intervals ranged from 0 to 24 s in this study). After the period, levers were inserted into both locations, and rats had to press the lever at the same location as before in order to obtain a reward. Pressing the nonmatching lever led to a time-out period. Rats were susceptible to the effects of interference from the preceding trial only, especially at longer retention intervals. Directly comparing the effects of previous sample vs. previous choice found that choice produced stronger intertrial interference effects. This study (Dunnett & Martel, 1990) also found that longer intertrial intervals reduced the interference effects. Roberts (1980) and Moise (1976) used intertrial intervals of 1 s or 20 s, and 15 s, respectively. The current study on average had a 30-s intertrial interval. It would be worth exploring the effects of different intertrial intervals on interference in dogs in follow-up experiments. An important takeaway from these experiments is that this effect is not bound to olfactory stimuli as in our experiment, but is also found in spatial (Dunnett & Martel, 1990) and visual (Moise, 1976; Roberts, 1980) matching tasks. This suggests that the effect of previous choice is a true effect of PI (Wright et al., 1986), one that reflects common memory processes in each sensory modality.
Experiment II: Proactive interference probe
Experiment 1 found that proactive interference is not only influenced by the memory of repeated stimuli (2- and 6-odor sets had worse accuracy than the trial-unique set) but also the memory of the previously chosen stimulus. In Experiment 2, we addressed whether such memories are event- or time-based.
Procedure
Each session was 24 trials long and had three of each interference trial separation (n−1, n−6, n−12) embedded within a session (Table 3 is a representation of a session). For example, in Table 3, trial 6 has an n−1 trial separation because the incorrect choice (maple) was the sample on trial 5 (n−1), trial 8 has an n−6 trial separation because the incorrect choice (parsley) was the sample on trial 2 (n−6), trial 15 has an n−12 trial separation because the incorrect choice (oregano) was the sample on trial 3 (n−12). The remaining 15 trials are all trial-unique for that session (referred to as no-PI).
Sessions were conducted with a 0-s delay or a 20-s delay condition. In the 0-s delay condition, dogs would immediately enter the arena after they made an observing response to the sample, indicating they had smelled the sample odor. In the 20-s delay condition, handlers held the dog just outside and out of view of the arena for 20 s before sending the dogs into the arena. There was one session conducted for each condition, which was counterbalanced between dogs. All other procedural details were the same as in Experiment 1.
Data analyses
Data were analyzed in R (version 4.0.3) using a generalized linear mixed-effects model (GLMM), binomial family distribution, with individual dog ID as a random factor (lme4 package; Bates et al., 2015). To fit a binomial distribution, accuracy was again coded as 1 for correct and 0 for incorrect (as opposed to using a percent correct score as in linear regression), just like in the previous analysis. Accuracy was determined as a function of trial separation (i.e., number of trials since the interfering stimulus last appeared: 1, 6, 12, no-PI), delay (0-s, 20-s), and a trial separation x delay interaction. The logistic regression had the following formula: Accuracy ~ trial separation + delay + trial separation * delay, (1|dog), where the (1|dog) specifies random effects.
Results and discussion
Dogs showed an effect of trial separation, that interacted with delay such that as the trials were further separated in the sequence (i.e., there is greater trial separation) accuracy improves with the 0-s delay but not the 20-s delay, as shown in Fig. 6. The analyses revealed the significant main effect of trial separation (n−1: M = 77.78%; n−6: M = 69.44%; n−12: M = 91.67%; no-PI: M = 85.56%; z = 2.62, p = .009). The main effect of delay was not significant (z = −0.70, p = .486), but the interaction between trial separation * delay was significant (z = −1.97, p = .049). Two separate GLMMs found that the interaction was driven by the significant effect of trial separation in the 0-s delay condition (z = 2.79. p = .005), and a nonsignificant effect of trial separation in the 20-s delay condition (z = 0.09, p = .925).
In Experiment 2, we sought to better characterize the proactive interference of odors in dogs. To do this, we adapted procedures from Wright et al., (2012) and Devkar and Wright (2016). Wright et al., (2012) found evidence in pigeons of a type of PI characterized by intrusive memory cues due to time, whereas Devkar and Wright (2016) found that monkeys suffer from intrusive proactive memories based on the number of similar events. Only when interfering events occurred in immediate succession did monkeys suffer significant decreases in accuracy. The key difference between monkeys and pigeons in the locus of PI is the influence of time. Interference in monkeys is functionally related to the number of events between the probe and the first time the stimulus is experienced regardless of the amount of time between each event (Wright, 2018). Pigeons, by contrast, showed strong effects of time. When pigeons are comparing the stimuli during the test phase of the S/D task, they are comparing them to memories of the sample. Exactly which memories of the sample depends on the elapsed time since the sample was viewed. Whereas monkeys remember the sample, interference comes from other recent events (i.e., the previous trial). Our results are tentative but suggest that dogs might be using event-based memory in the task. To elaborate, they did not show a significant effect of delay; however, the interaction between delay and trial separation was significant. Further analysis indicated that the interaction was due only to an effect of interference during the 0-s condition, and there was no effect of trial separation in the 20-s session. Perhaps the 20-s delay periods created an effect where dogs were no longer confusing the previous trial samples as they could better represent each sample as a separate event. While not exactly replicating the results from Devkar and Wright (2016), the fact that the effect was limited to the 0-s delay condition suggests that dogs were more similar to monkeys suggesting event-based memory in dMTS. If memory was time-based the effect of PI over trial separation would have been stronger in the 20-s than the 0-s delay condition.
However, there are some caveats to these conclusions. First, procedurally the experiments are different. The pigeons and monkeys in the previous studies were trained on a same/different (S/D) discrimination which, while similar to MTS, might require different memory systems (Shettleworth, 2010, p. 201–202). Second, pigeons and monkeys were tested for more sessions than in the present study, therefore diminishing power in the present study. While the results were statistically significant, they were variable, and the result could change with a larger sample size or more sessions. In summary, we view these results as tentatively suggesting that dogs may have an event-based experience of PI yet replicating these results in the future is important.
General discussion
These experiments show the effects of proactive interference on dogs’ working memory in an olfactory dMTS task. In Experiment 1, we found that dogs were susceptible to proactive interference. Interference reduced accuracy in both the 2-odor and 6-odor conditions relative to the 48 trial-unique condition. We also found an interaction between reward and susceptibility to interference for PT and NT trials. When reward contingency was consistent with transfer (i.e., rewarded positive transfer trials and non-rewarded negative transfer trials) dogs showed very high accuracy relative to inconsistent trials (i.e., non-rewarded positive transfer and rewarded negative transfer trials). This interaction indicates that dogs were influenced more by the choice they made on the previous trial than the response outcome. In Experiment 2, we found that dogs were sensitive to PI probes only at the 0-s delay. There was also no overall effect of delay (0 s vs. 20 s), altogether suggesting, tentatively, that dogs may have an event-based memory for odors.
While there is past research on variations of the dMTS using dogs (Chan et al., 2002; Fiset et al., 2003; Kuœmierek & Kowalska, 2002), none of these studies other than Krichbaum et al., (2021) have specifically sought to examine the effects of set size on working memory. One study used trial-unique stimuli with dogs in an auditory variant of the dMTS and found that there was no within-session PI (Kuœmierek & Kowalska, 2002). Other studies have used a visual delay nonmatching-to-position task (vDNMP; Chan et al., 2002), where dogs were presented with a tray that had an object on either the left or right side of it. Dogs were rewarded for displacing the object, after which the tray was removed for a retention interval. After this interval the tray was brought back, this time with objects on both sides; the dog was rewarded for displacing the item on the opposite side from the first object. The limited number of locations would seem to generate a large amount of PI; however, younger dogs were still accurate up to delays of 110 s, while older dogs struggled with delays of 30 s.
Other animals have been evaluated for the effects of PI (for a review see Wright, 2006). For example, Overman and Doty (1980) found that monkeys’ memory during dMTS is greatly affected by set size. At a short 5-s delay, monkey accuracy drops to 70% for 2-stimuli sets, compared to over 90% for both 100-stimuli and novel stimuli sets. Pigeons similarly show these effects of PI when delay is zero or just a few seconds (Zentall & Hogan, 1974). As mentioned previously, Dunnett and Martel (1990) found effects of PI in a spatial DMTP task in rats. One point of interest is that both of the spatial delay tasks seemed to be set up for a large degree of interference through the small set sizes in each experiment. However, dogs and rats did not seem to have much of an effect of PI on accuracy. This could reflect a difference in the way spatial PI is experienced. Perhaps spatial information is represented differently from visual, auditory, and olfactory information, thus dampening the effects of PI.
Future directions
There are several relevant follow-up studies for both experiments. In the future, ITI is an important variable to manipulate. In Experiment 2, the dogs had an effect of interference for the 0-s delay, and on average an ITI of 30 s. Modulating ITI in an olfactory MTS task, both with dogs and with other species, would be an important next step in understanding this relationship. Devkar and Wright (2016) examined the effects of ITI in their PI study, using either 5-s or 15-s ITI durations. There was no effect of ITI, and the effects of interference were still strongest when the trial number of the interfering stimulus was n−1. While Devkar and Wright did not find an effect of shorter ITI durations, it is unknown how longer durations, such as 120 s, would affect interference. If there was an absence of an ITI effect with dogs this would provide additional evidence of event-based memory. Finding ways to replicate our dog studies with different sensory modalities could be another future direction. We used olfactory stimuli as opposed to visual stimuli in order to take advantage of olfaction, as it is dogs’ primary sensory modality. The effects of PI may vary with different sensory systems within a species (e.g., Wright, 1998). Additionally, expanding the species tested on these procedures, especially the PI probe experiment, would be of interest as this manipulation has only been tested in pigeons, rhesus monkeys, and now dogs. Human participants in particular would provide a valuable comparison point, and so would rats, as common laboratory animals. Previous studies with rats have shown similar effects of set size, such that rats using only 2 odors during MTS and nonmatching to sample (NMTS), failed to learn and generalize to new odors, but with larger sets the rats succeeded (Lazarowski et al., 2019; Peña et al., 2006). While these studies did not specifically seek to test the effects of set size and PI on rat (N)MTS performance, it is likely that PI played a role, similar to how it affected the dogs in the present studies. An additional reason to expand the PI probe experiment is that olfaction is likely rats’ primary sensory modality as well, providing a useful comparison with dogs. The use of a species’ preferred sensory modality has been shown to improve overall performance in dMTS tasks (Lind et al., 2015). The use of a favored sensory modality is likely shared among all three PI probe experiments (vision for monkeys and pigeons, odors for dogs). Testing these and other species on different sensory modalities could reveal functional relationships between modality and interference. The aforementioned future experiments will help to better understand familiarity and episodic memory as it has been argued that the difference between event-based and time-based memory are similar to the differences between episodic memory and familiarity, respectively (Devkar & Wright, 2016; Wright et al., 2018).
One consideration of both experiments is the breed used and that these dogs are purpose-bred detection dogs bred for certain characteristics. These traits and their rearing/training history may improve their cognitive abilities. Hence, there may be differences in motivation and trainability between working dogs and companion dogs (Lazarowski et al., 2018). Therefore, translating these findings to companion dogs and other breeds would be premature. Replicating these experiments with companion dogs of different breeds can elucidate this point of translation. Including other domesticated animals, such as cats and livestock, would be of interest as well. Such an expansion will allow for an improved understanding of the evolution of the functional relationships of interference processes and memory.
Conclusion
Dogs play an important role in society as companion, working, service, and model animals. It is important to understand the cognitive aspects of dog behavior as this can have implications for their welfare and service. Training working dogs is an expensive and time-consuming practice and understanding the cognitive processes of dogs can streamline training as well as reduce frustration from unrealistic expectations placed on dogs in training programs. Improving the process of training dogs improves both the services they provide and dog welfare (Cobb et al., 2015). Effective selection is one method for reducing financial cost and improving the welfare of training dogs for service (Bray et al., 2021) and requires careful observation of various characteristics. Sensitivity to PI, and willingness to continue working in confusing or frustrating conditions, could be relevant factors, especially as combatting PI may be related to working memory span (Conway et al., 2003; Jonides & Nee, 2006), which in turn is related to general fluid intelligence (Broadway & Engle, 2010). Understanding these complex cognitive processes in dogs may improve the selection and training of detection dogs (MacLean & Hare, 2018).
Dogs are potential models of aging and dementia, both of which are associated with declines in olfaction and increased susceptibility to proactive interference. Dogs are phenotypically diverse and share many of their habitats with humans (Ruple et al., 2022). Dogs, like humans, show white matter demyelination as a sign of aging (Chambers et al., 2012; Gunning-Dixon et al., 2009; Guttmann et al., 1998). In humans, white matter degradation has been linked to increased susceptibility to PI (Andersson et al., 2022). Additionally, declines in olfactory functioning are an early sign of forms of cognitive decline, including mild cognitive impairment (MCI) and Alzheimer’s disease (AD) (Jung et al., 2019; Windon et al., 2020). Dogs may be of use in modeling these olfactory-related declines, and examining whether these age-related PI effects are also found in dogs performing olfactory tasks could provide important behavioral correlates of MCI and AD in dogs and humans.
In this study, dogs showed susceptibility to within-session and intertrial PI solely through the manipulation of the repetition of events, regardless of factors of time. This clearly indicates a role of PI in the forgetting processes of dogs. Previous studies have not explicitly shown intertrial PI effects or the effects of interference completely separate from any within-session effects of delay. This is important for improving success among working dogs, but replicating these results in applied settings would be useful to see how well these findings translate. In a live detection scenario, for example, at a sporting event, there are far more distractions and therefore possible intrusions into the dog’s memory, potentially affecting the observed effects of PI. Scent lineup dogs would be an important application as well, as many countries (Ferry et al., 2019) use dogs to link suspects to possible crimes. PI as outlined in the present paper could have disastrous consequences in such situations. Overall, these two experiments represent a further step in understanding dog cognition and memory and have relevant applications to real-world situations.
References
Andersson, P., Xin, L., & Persson, J. (2022). The association between control of interference and white-matter integrity: A cross-sectional and longitudinal investigation. Neurobiology of Aging, 114, 49–60. https://doi.org/10.1016/j.neurobiolaging.2022.03.002
Bates, D., Mächler, M., Bolker, B., & Walker, S. (2015). Fitting linear mixed effects models using lme4. Journal of Statistical Software, 67(1), 1–48. https://doi.org/10.18637/jss.v067.i01
Bray, E. E., Otto, C. M., Udell, M. A., Hall, N. J., Johnston, A. M., & MacLean, E. L. (2021). Enhancing the selection and performance of working dogs. Frontiers in Veterinary Science, 8, 430.
Broadway, J. M., & Engle, R. W. (2010). Validating running memory span: Measurements of working memory capacity and links with fluid intelligence. Behavior Research Methods, 42(2), 563–570. https://doi.org/10.3758/BRM/42.2.563
Bruce, K., Leavens, D. A., & Boysen, S. T. (2021). Current perspectives in cognitive processing by domesticated animals. Frontiers in Psychology, 12, 1–3. https://doi.org/10.3389/fpsyg/2021.736717
Ceraso, J. (1967). The interference theory of forgetting. Scientific American, 217(4), 117–127.
Chambers, J. K., Kazuyuki, U., & Nakayama, H. (2012). White matter myelin loss in the brains of aged dogs. Experimental Gerontology, 47(3), 263–269.
Chan, A. D. F., Nippak, P. M. D., Murphey, H., Ikeda-Douglas, C. J., Muggenburg, B., Head, E., Cotman, C. W., & Milgram, N. W. (2002). Visuospatial impairments in aged canines (Canis familiaris): The role of cognitive-behavioral flexibility. Behavioral Neuroscience, 116(3), 443–454. https://doi.org/10.1037/0735-7044.116.3.443
Cobb, M., Branson, N., Lill, A., & Bennett, P. (2015). The advent of canine performance science: Offering a sustainable future for working dogs. Behavioural Processes, 110, 96–104.
Conway, A. R. A., Kane, M. J., & Engle, R. W. (2003). Working memory capacity and its relation to general intelligence. Trends in Cognitive Sciences, 7(12), 547–552. https://doi.org/10.1016/j.tics.2003.10.005
Devkar, D. T., & Wright, A. A. (2016). Event based proactive interference by rhesus monkeys. Psychonomic Bulletin and Review, 23, 1474–1482. https://doi.org/10.3758/s13423-016-1005-x
Dudchenko, P. (2004). An overview of the tasks used to test working memory in rodents. Neuroscience and Biobehavioral Reviews, 28, 699–709. https://doi.org/10.1016/j.neubiorev.2004.09.002
Dunnett, B. S., & Martel, L. F. (1990). Proactive interference effects on short-term memory in rats: I. Basic parameters and drug effects. Behavioral Neuroscience, 104(5), 655–665.
Ferry, B., Ensminger, J. J., Schoon, A., Bobrovskij, Z., Cant, D., Gawkowski, M., ... & Jezierski, T. (2019). Scent lineups compared across eleven countries: Looking for the future of a controversial forensic technique. Forensic Science International, 302, 1–18. https://doi.org/10.1016/j.forsciint.2019.109895
Fiset, S., Beaulieu, C., & Landry, F. (2003). Duration of dogs’ (Canis familiaris) working memory in search for disappearing objects. Animal Cognition, 6(1), 1–10. https://doi.org/10.1007/s10071-002-0157-4
Gunning-Dixon, F. M., Brickman, A. M., Cheng, J. C., & Alexopoulos, G. S. (2009). Aging of cerebral white matter: A review of MRI findings. International Journal of Geriatric Psychiatry, 24, 109–117. https://doi.org/10.1002/gps.2087
Guttmann, C. R., Jolesz, F. A., Kikinis, R., Killiany, R. J., Moss, M. B., Sandor, T., & Albert, M. S. (1998). White matter changes with normal aging. Neurology, 50(4), 972–978. https://doi.org/10.1212/wnl.50.4.972
Jonides, J., & Nee, D. E. (2006). Brain mechanisms of proactive interference in working memory. Neuroscience, 139, 181–193.
Jung, H. J., Shin, I. S., & Lee, J. E. (2019). Olfactory function in mild cognitive impairment and Alzheimer's disease: A meta-analysis. The Laryngoscope, 129(2), 362–369. https://doi.org/10.1002/lary.27399
Katz, J. S., Wright, A. A., & Bachevalier, J. (2002). Mechanisms of same/different abstract-concept learning by rhesus monkeys (Macaca mulatta). Journal of Experimental Psychology: Animal Behavior Processes, 28, 358–368.
Katz, J. S., Wright, A. A., & Bodily, K. D. (2007). Issues in the comparative cognition of abstract-concept learning. Comparative Cognition Behavior Review, 2, 79–92.
Keppel, G., & Underwood, B. J. (1962). Proactive inhibition in short-term retention of single items. Journal of Verbal Learning and Verbal Behavior, 1, 153–161. https://doi.org/10.1016/S0022-5371(62)80023-1
Krichbaum, S., Lazarowski, L., Davila, A., Cox, E., Smith, J. G., & Katz, J. S. (2021). Dissociating the effects of delay and interference on dog (Canis familiaris) working memory. Animal Cognition, 24(6), 1259–1265.
Kuœmierek, P., & Kowalska, D. M. (2002). Effect of sound source position on learning and performance of auditory delayed matching-to-sample task in dogs. Acta Neurobiologiae Experimentalis, 62(4), 251–262.
Lazarowski, L., Haney, P., Brock, J., Fischer, T., Rogers, B., Angle, C., Katz, J. S., & Waggoner, L. P. (2018). Investigation of the behavioral characteristics of dogs purpose-bred and prepared to perform vapor wake® detection of person-borne explosives. Frontiers in Veterinary Science, 5, 50. https://doi.org/10.3389/fvets.2018.00050
Lazarowski, L., Goodman, A., Galizio, M., & Bruce, K. (2019). Effects of set size on identity and oddity abstract-concept learning in rats. Animal Cognition, 22, 7331–74210.
Lazarowski, L., Davila, A., Krichbaum, S., Cox, E., Smith, J. G., Waggoner, L. P., & Katz, J. S. (2021). Matching-to-sample abstract-concept learning by dogs (Canis familiaris). Journal of Experimental Psychology: Animal Learning and Cognition, 47(3), 393–400. https://doi.org/10.1037/xan0000281
Lind, J., Enquist, M., & Ghirlanda, S. (2015). Animal memory: A review of delayed matching-to-sample data. Behavioral Processes, 117, 52–58. https://doi.org/10.1016/j.beproc.2014.11.019
MacLean, E. L., & Hare, B. (2018). Enhanced selection of assistance and explosive detection dogs using cognitive measures. Frontiers in Veterinary Science, 5, 1–14.
Minhinnick, S., Papet, L. E., Stephenson, C. M., & Stephenson, M. R. (2016). Training fundamentals and the selection of dogs and personnel for detection work. In T. Jezierski, J. Ensminger, & L. E. Papet (Eds.), Canine olfaction science and law: Advances in forensic science, medicine, conservation, and environmental remediation (pp. 155–172). CRC Press.
Moise, L. S. (1976). Proactive effects of stimuli, delays, and response position during delayed matching from sample. Animal Learning and Behavior, 4(1), 37–40.
Overman, W. H., & Doty, R. W. (1980). Prolonged visual memory in macaques and man. Neuroscience, 5(11), 1825–1831. https://doi.org/10.1016/0306-4522(80)90032-9
Peña, T., Pitts, R. C., & Galizio, M. (2006). Identity matching-to-sample with olfactory stimuli in rats. Journal of the Experimental Analysis of Behavior, 85(2), 203–221.
Roberts, W. A. (1980). Distribution of trials and intertrial retention in delayed matching to sample with pigeons. Journal of Experimental Psychology: Animal Behavior Processes, 6(3), 217–237. https://doi.org/10.1037/0097-7403.6.3.217
Ruple, A., MacLean, E., Snyder-Mackler, N., Creevy, K. E., & Promislow, D. (2022). Dog models of aging. Annual Review of Animal Biosciences, 10, 419–439. https://doi.org/10.1146/annurev-animal-051021-080937
Shettleworth, S. J. (2010). Cognition, evolution, and behavior. Oxford University Press.
Windon, M. J., Kim, S. J., Oh, E. S., & Lin, S. Y. (2020). Predictive value of olfactory impairment for cognitive decline among cognitively normal adults. The Laryngoscope, 130(4), 840–847. https://doi.org/10.1002/lary.28166
Wixted, T. J. (2004). The psychology and neuroscience of forgetting. Annual Review of Psychology, 55, 235–269. https://doi.org/10.1146/annurev.psych.55.090902.141555
Wright, A. A. (1998). Auditory and visual serial position functions obey different laws. Psychonomic Bulletin & Review, 5(4), 564–584.
Wright, A. A. (2006). Memory processing. In E. A. Wasserman & T. R. Zentall (Eds.), Comparative Cognition: Experimental Explorations of Animal Intelligence (pp. 164–185). Oxford University Press.
Wright, A. A. (2007). An experimental analysis of memory processing. Journal of the Experimental Analysis of Behavior, 88, 405–433.
Wright, A. A. (2012). Memory processing. In T. R. Zentall & E. A. Wasserman (Eds.), The Oxford handbook of comparative cognition (pp. 239–260). Oxford University Press.
Wright, A. A. (2018). Testing complex animal cognition: Concept learning, proactive interference, and list memory. Journal of the Experimental Analysis of Behavior, 109, 87–100.
Wright, A. A., Urcuioli, P. J., & Sands, S. F. (1986). Proactive interference in animal memory research. In D. F. Kendrick, M. Rilling, & R. Denny (Eds.), Theories of animal memory (pp. 101–125). Erlbaum.
Wright, A. A., Katz, J. S., & Ma, W. (2012). How to be proactive about interference: Lessons from animal memory. Psychological Science, 23, 453–458. https://doi.org/10.1177/0956797611430096
Wright, A. A., Kelly, D. M., & Katz, J. S. (2018). Comparing cognition by integrating concept learning, proactive interference, and list memory. Learning & Behavior, 46(2), 107–123. https://doi.org/10.3758/s13420-018-0316-3
Zentall, T., & Hogan, D. (1974). Abstract concept learning in the pigeon. Journal of Experimental Psychology, 102(3), 393–398. https://doi.org/10.1037/h0035970
Acknowledgements
We also thank Brook Caudill, Jordan Farrell, Anna Beth Freeman, Kierra Kuhlman, and Lindsey Rogers for assistance with data collection and Lane Montgomery for assistance with editing.
Funding
Funding was provided by grants from the Association of Professional Dog Training Foundation. We thank Auburn University’s Canine Performance Sciences and the Auburn University College of Veterinary Medicine Department of Lab Animal Health for logistical support and care of the dogs in this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Open practices statement
Data and materials for the experiments reported here are available upon reasonable request. The experiments were not preregistered.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Davila, A., Krichbaum, S., Lazarowski, L. et al. Effects of proactive interference on olfactory memory in dogs. Learn Behav 51, 108–119 (2023). https://doi.org/10.3758/s13420-022-00555-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13420-022-00555-z