Abstract
The results of one-dimensional calculations of the height profiles of nucleated sulfate aerosol particles for the northern mid-latitudes and tropics in winter are presented. Numerical calculations were performed using a three-dimensional model of the transport and transformation of multicomponent gas and aerosol substances in the atmosphere, incorporating photochemistry, nucleation involving neutral molecules and ions, as well as condensation/evaporation and coagulation. It is found that the resulting dynamics of the formation of aerosol particle nuclei is not a simple sum of ion and binary (water vapor/sulfuric acid) nucleation rates. This dynamics is determined by the ratio of critical radii of nucleated particles due to binary and ion nucleation of these substances (rcr_bin and rcr_ion) depending on temperature, relative humidity, and ionization rate. This should be taken into account in modeling the gas and aerosol composition of the atmosphere and comparing calculated and observed data.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
The investigation of aerosol particle formation remains a relevant area in atmospheric physics and chemistry [15]. The presence of aerosol in the atmosphere affects climate [11, 16] and the concentration of trace gases in air [4, 10]. Along with the emission of particles to the atmosphere, the binary nucleation (sulfuric acid and water vapor [6, 14]) is usually considered as their source. In view of this, the involvement of atmospheric ions in the nucleation also attracts attention [13]. Their role is indicated by the field data on the bursts of concentration of nanoscale particles in the surface atmosphere at not too low temperatures [9, 17]. The authors of the present paper reported earlier [1, 3, 5] the creation of a three-dimensional model of the transport of multicomponent gas and aerosol species in both hemispheres, taking into account chemical and kinetic processes of their transformation, as well as the binary nucleation of sulfuric acid and water vapor. This model complemented with a block for the description of dynamics of nucleated sulfate aerosol particles involving ions (ion-induced nucleation) allowed us to analyze the impact of ions on the nucleation dynamics in the mid-latitudes and tropics [2]. Preliminarily, the analysis of vertical profiles of particle concentrations revealed a negative effect of ions on the dynamics of the binary nucleation of sulfuric acid and water vapor. The objective of the present paper is to find the reasons for the negative impact of ions on the dynamics of binary nucleation in the process of sulfate aerosol formation in the atmosphere based on these data and the results of additional numerical calculations.
SULFATE AEROSOL FORMATION MODEL
The calculations of the formation and transport of multicomponent gas and aerosol substances in the lower stratosphere using the three-dimensional model were performed in the coordinate system (λ, ψ, z), where λ is longitude, ψ is colatitude, z is altitude. The equations for the rate of variations in the concentration of trace gases and aerosol in the atmosphere presented in [1, 3, 5] have the following form:
(1)
(2)
The equations were integrated in the domain Dt= G × [0, T], where G = S × [0, H], S = {(λ, ψ): 0 ≤ λ ≤ 2π, 0 ≤ ψ ≤ π}, H is the top of the computational domain. Here, Ci(i = 1,…, Ng), φk (k = 1,…, Na) are the concentrations of gases and aerosol, respectively; Ng and Na are the number of gas components and aerosol fraction, respectively; (u, v, w) are the wind speed components in the directions λ, ψ, z, respectively; wgr is the gravitational settling velocity; a is the average radius of the Earth; μ and ν are the coefficients of turbulent exchange in the horizontal and vertical directions, respectively; \(F_{i}^{\text{gas}}\) and \(F_{i}^{\text{aer}}\) are the sources of trace gases and aerosols, respectively; \(P_{i}^{\text{nucl}}\), \(P_{i}^{\text{cond}}\), \(P_{i}^{\text{coag}}\), and \(P_{i}^{\text{phot}}\) are the operators of nucleation, condensation, coagulation, and photochemical transformation, respectively. Along with the binary nucleation of sulfuric acid and water vapor (Jbin, cm–3 s–1), the process of nucleation of these vapors due to atmospheric ions was considered (Jion, cm–3 s–1). The nucleation rate for these two channels (\(P_{i}^{\text{nucl}}\) = Jbin + Jion) was calculated as the flow of particles passing through the critical size of nuclei (rcr_bin and rcr_ion). The nucleation rate is a principally important element for the new particle formation from gas-precursors; it is taken into account in the corresponding equations for the condensational growth of particles (denoted in the right-hand side of equations (1), (2) as \(P_{i}^{\text{cond}}\)). The resulting nucleation operators \(P_{i}^{\text{nucl}}\) were used to calculate the variability of gas and sulfate aerosol concentrations.
ION-INDUCED NUCLEATION MODEL
In the kinetic model of ion-induced nucleation [18, 19] of aerosol formation, the primary ions (N2+, О2+, Н+, О+) and electrons are transformed into protonated (H3O+(Н2О)n, NH4+(Н2О)n, etc.) and conjugated (for example, NО3–(НNO3)х(Н2О)y) cluster ions [3]. The authors of [7, 8] applied quantum chemistry methods to compute the dynamics of elementary ion-molecule processes of the formation of these complex ions and the growth of ion clusters, that finally lead to the formation of the condensed particle nuclei. To integrate these processes in the three-dimensional model, we used a look-up table of the calculated values of Jion [18], which correspond to the stationary conditions at the concentrations of nucleating sulfuric acid molecules, temperature (T, K), relative humidity, ionization rate (J, cm–3 s–1), and the total surface of aerosol particles (S, μm2/cm3), that are typical of the atmosphere. The interpolation scheme for the values of these parameters at their intermediate values was also taken from that study. More details on the ion nucleation model and the three-dimensional model updated by the authors and used in the calculations are given in [2].
RESULTS AND DISCUSSION
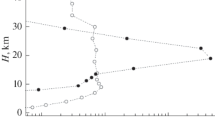
The calculations of the dynamics of sulfuric acid and water vapor nucleation in the atmosphere were performed with respect to winter (January 2002) for the mid-latitudes (the interfluve of the Lena and Olenka rivers: 70° N, 120° E), the Czech Republic (west of Karlovy Vary: 50° N, 12.5° E), and a geographic point located close to the tropics (southern Nigeria, near the ocean coast: 5° N, 5° E). These geographic points differ in vertical variations in temperature and relative humidity. Their comparison revealed general variability patterns for the dynamics of formation of sulfate aerosol and its concentration in the atmosphere, depending on air temperature and relative air humidity. Figure 1 demonstrates the profiles of the total rate of particle nucleation (JΣ = Jbin + Jion) calculated at the synchronous processes of binary and ion-induced nucleation of sulfuric acid and water vapor. Here, Jbin and Jion are the components of the resulting rate of binary and ion-induced nucleation of their vapors, respectively, computed in the framework of the single model in the atmosphere. It is clear that an increase in JΣ is observed to the height of 10 km in the mid-latitudes. Then, despite variations, the quasiconstant value of JΣ is maintained as the height above the ground increases: JΣ ≈ 20 ± 10 cm–3 s–1 (Fig. 1а). Close to the tropics, the profile of JΣ is characterized by the pronounced maximum at the altitude of about 18 km (see Fig. 1b). The levels of the “break” for the profile of JΣ in the mid-latitudes (~10 km) and close to the tropics (~18 km) are close to the location of the maxima of the profiles for the rates of binary (\(\ J_{\text{bin}}^{*}\)) and ion-induced (\({\ J}_{\text{ion}}^{*}\)) nucleation calculated in the absence of ion and binary nucleation, respectively. Their position is also close to the location of the maximum for the sum (\({\ J}_{\text{bin}}^{*}\) + \(J_{\text{ion}}^{*}\)). Despite this, the absolute values of JΣ in the mid-latitudes and tropics are much smaller than the values of \(J_{\text{bin}}^{*}\) + \(J_{\text{ion}}^{*}\) (see Fig. 1). At the same time, the numerical values of JΣ are rather close to the rate of ion-induced nucleation \(J_{\text{ion}}^{*}\), which indicates a negative effect of ions on the dynamics of binary nucleation of sulfuric acid and water vapor at their simultaneous realization.
The vertical profiles of the nucleation rate (a) in the mid-latitudes (70° N, 120° E) and (b) tropics (5° N, 5° E) in winter (January 2002): (1) the calculated nucleation rate under conditions of the simultaneous realization of binary and ion-induced nucleation; (2, 3) the nucleation rates \(J_{\text{bin}}^{*}\) and \(J_{\text{ion}}^{*}\) calculated in the absence of ion-induced and binary nucleation, respectively; (4) the sum of the rates \(J_{\text{bin}}^{*}\) and \(J_{\text{ion}}^{*}\).
For example, at the height of 2.2 km over the interfluve of the Lena and Olenka rivers, Jion ≈ 1 cm–3 s–1, while the rate of binary nucleation in such conditions Jbin ≈ 0. At the same time, \(J_{\text{bin}}^{*}\) + \(J_{i\text{on}}^{*}\) ≈ \(J_{\text{ion}}^{*}\) ≈ JΣ. The negative impact of ion-induced nucleation on binary nucleation in these conditions cannot be considered due to too high temperature (~254 К). At the altitude of 5.6 km, \(J_{\text{bin}}^{*}\) ≈ \(J_{\text{ion}}^{*}\) (≈8 and ≈6 cm–3 s–1). At the same time, JΣ is about three times smaller than the sum (\({\ J}_{\text{bin}}^{*}\) + \(J_{\text{ion}}^{*}\)), despite the favorable combination of temperature and relative humidity at this height providing \(J_{\text{bin}}^{*}\) ≈8 cm–3 s–1. The negative impact of ions on the dynamics of sulfate aerosol particle nucleation is obvious. This negative effect of ions intensifies still more as the height increases by 1.6 km (H = 7.2 km), which leads to the more than 10-fold decline in JΣ as compared to (\({\ J}_{\text{bin}}^{*}\) + \(J_{\text{ion}}^{*}\)). This occurs despite the ~130-fold growth of \(J_{\text{bin}}^{*}\) (~1090 cm–3 s–1) at the lifting by 1.6 km over the ground.
The negative impact of ions on the dynamics of sulfate aerosol particle formation in the atmosphere can be naturally related to the difference in the critical size of sulfate aerosol particle nuclei that are formed in the process of ion-induced (rcr_ion) and binary (rcr_bin) nucleation of sulfuric acid and water vapor. At the height of 2.2 km over the interfluve of the Lena and Olenka rivers, for example, rcr_ion ≈ 0.59 nm. Here, the subscript “ion” indicates only the origin but not the charge of the critical nuclei. The size of the critical cluster during the binary nucleation under the considered conditions is roughly 1.5 times smaller (rcr_bin ≈ 0.4 nm). The calculations show that JΣ ≈ Jion ≈ \(J_{\text{ion}}^{*}\) ≈ 1 cm–3 s–1 and Jbin ≈ 0. This means that the sulfate aerosol particles in these conditions are formed only as a result of ion-induced nucleation. The critical size of sulfate aerosol particle nuclei at the analyzed values of temperature and relative humidity cannot be smaller than rcr_ion ≈ 0.59 nm. Therefore, the nuclei of the smaller particles formed as a result of binary nucleation are thermally unstable and mainly evaporate. This is also confirmed by the data of calculations of \(J_{\text{bin}}^{*}\) (see above). Their evaporation in the analyzed conditions means that the rate of the growth of such clusters is lower than the rate of their evaporation, which leads to Jbin ≈ 0. It may be stated that the use of the sum of \(J_{\text{bin}}^{*}\) and \(J_{\text{ion}}^{*}\) for estimating the resulting effect of binary and ion nucleation in the process of sulfate aerosol particle nucleation in the atmosphere is correct only if rcr_ion = rcr_bin. This conclusion is made when considering data on the particle nucleation at large heights. At the height of 5.6 km, rcr_bin/rcr_ion ≈ 0.93 and JΣ ≈ 0.3(\({\ J}_{\text{bin}}^{*}\) + \(J_{\text{ion}}^{*}\)) ≈ \(J_{\text{ion}}^{*}\). Despite the similarity of the calculated values of \(J_{\text{bin}}^{*}\) and \(J_{\text{ion}}^{*}\) at this height, only a small part of the clusters arising in the binary nucleation over the time of their evaporation has time to grow from rcr_bin to rcr_ion and to take part in the formation of sulfate aerosol particles. Let us estimate their portion in the mid-latitudes at the height of 5.6 km using the ratio JΣ/\(J_{\text{bin}}^{*}\). The maximum value of JΣ/\(J_{\text{bin}}^{*}\) ≈ 0 in the mid-latitudes and ≈0.1 in the tropics (see Fig. 1). This growth of JΣ/\(J_{\text{bin}}^{*}\) is basically associated with an increase in \(J_{\text{bin}}^{*}\) (Jbin) caused by the temperature drop.
Thus, the value of the ratio rcr_bin/rcr_ion can be considered as an indicator characterizing the stability of the critical cluster in the binary nucleation at the simultaneous occurrence of ion-induced nucleation of sulfuric acid and water vapor. For example, Figure 2 presents the dependence of JΣ/(\({\ J}_{\text{bin}}^{*}\) + \(J_{\text{ion}}^{*}\)) on rcr_bin/rcr_ion for the mid-latitudes. Here, the ratio JΣ/(\({\ J}_{\text{bin}}^{*}\) + \(J_{\text{ion}}^{*}\)) shows a portion of critical nuclei taking part in the formation of sulfate aerosol particles at the synchronous realization of binary and ion nucleation (JΣ) relative to their maximum number \(J_{\text{bin}}^{*}\) + \(J_{\text{ion}}^{*}\). It is clear that some time after the model initialization (in 12–30 hours), the calculated dependence leads to the similar result. If rcr_bin/rcr_ion ≥ 0.85, which is typical of small heights (≤10 km), JΣ/(\({\ J}_{\text{bin}}^{*}\) + \(J_{\text{ion}}^{*}\)) → 1. In these conditions, Jbin << Jion (\({\ J}_{\text{ion}}^{*}\)). The nucleation of sulfate aerosol particles occurs both due to the ion-induced nucleation and due to a part of particles formed in the process of binary nucleation that escaped evaporation. A similar mechanism of sulfate aerosol formation is also valid if rcr_bin/rcr_ion < 0.75, i.e., at height above 10 km. In the interval 0.75 ≤ rcr_bin/rcr_ion ≤ 0.85, there is a “dip” in JΣ/(\({\ J}_{\text{bin}}^{*}\) + \(J_{\text{ion}}^{*}\)) (see Fig. 2). In these conditions, the formation of sulfate aerosol particles occurs almost completely due to the formation of their nuclei in the process of ion-induced nucleation. The similar dependence of JΣ/(\({\ J}_{\text{bin}}^{*}\) + \(J_{\text{ion}}^{*}\)) on rcr_bin/rcr_ion according to our calculations is also observed near the tropics, although the dip at this geographic point is slightly displaced towards the smaller values of rcr_bin/rcr_ion (~0.6). In this case, the zone with dominant ion-induced nucleation, considerably expands and is shifted toward greater altitudes.
The impact of the ratio of critical nuclei radii in the process of binary and ion-induced nucleation to the fraction of critical nuclei (JΣ/\(J_{\text{bin}}^{*}\) + \(J_{\text{ion}}^{*}\))) involved to the formation of sulfate aerosol particles at the synchronous occurrence of binary and ion-induced nucleation (JΣ) relative to their maximum number \(J_{\text{bin}}^{*}\) + \(J_{\text{ion}}^{*}.\) (1, 2, 3) The results of calculations 12, 24, and 30 hours after the model initialization.
CONCLUSIONS
The paper presents the results of one-dimensional calculations of the height profiles of nucleated sulfate aerosol particles for the mid-latitudes and tropics in winter. The calculations were performed using the three-dimensional model of the transport and transformation of multicomponent gas and aerosol substances in the atmosphere, incorporating photochemistry, nucleation involving neutral molecules and ions, as well as condensation/evaporation and coagulation. It is found that the resulting dynamics of the formation of aerosol particle nuclei is not a simple sum of ion and binary (water vapor/sulfuric acid) nucleation rates. This dynamics is determined by the ratio of the critical radii of nucleated particles due to binary and ion-induced nucleation of vapors of these substances (rcr_bin/rcr_ion) depending on temperature and relative humidity, as well as on the ionization rate and latitude. The nucleation rate nonlinearly depends on rcr_bin/rcr_ion, which should be taken into account in modeling the gas and aerosol composition of the atmosphere and comparing calculated and observed data. For example, at small and large heights in the mid-latitudes, the nucleation rate is caused by the participation in this process of both ions and a part of nuclei of particles formed in the binary nucleation that escaped evaporation. Between these levels, the formation of sulfate aerosol particles occurs almost solely due to the formation of their nuclei as a result of ion-induced nucleation. Such differentiation of the contribution of ion and binary nucleation to the formation of sulfate aerosol particles is also observed close to the tropics. At the same time, the zone with dominant ion-induced nucleation, considerably expands and is shifted toward greater altitudes.
REFERENCES
A. E. Aloyan, A. N. Yermakov, and V. O. Arutyunyan, “Aerosols in the Troposphere and Lower Stratosphere. Sulfate Particles in Northern Latitudes,” Optika Atmosfery i Okeana, No. 2, 31 (2018) [in Russian].
A. E. Aloyan, A. N. Yermakov, and V. O. Arutyunyan, “Modeling the Impact of Ions on the Dynamics of Atmospheric Aerosol Formation,” Izv. Akad. Nauk, Fiz. Atmos. Okeana, No. 1, 57 (2021) [in Russian].
A. E. Aloyan, A. N. Yermakov, and V. O. Arutyunyan, “Sulfate Aerosol Formation in the Troposphere and Lower Stratosphere,” in The Investigation of Possible Stabilization of Climate Using New Technologies (Roshydromet, Moscow, 2012) [in Russian].
H. Akimoto, Atmospheric Reaction Chemistry (Springer Japan, 2016).
A. E. Aloyan, “Mathematical Modeling of the Interaction of Gas Species and Aerosols in Atmospheric Dispersive Systems,” Russ. J. Num. Anal. Math. Model., No. 1–4, 15 (2000).
A. D. Clarke, D. Davis, V. N. Kapustin, F. Eisele, G. Chen, I. I. Paluch, D. Lenschow, A. R. Bandy, D. Thornton, K. Moore, L. Mauldin, D. Tanner, M. Litchy, M. A. Carroll, J. Collins, and G. Albercook, “Particle Nucleation in the Tropical Boundary Layer and Its Coupling to Marine Sulfur Sources,” Science, 282 (1998).
E. E. Ferguson, “Ion–Molecule Reactions in the Atmosphere,” in Kinetics of Ion–Molecule Reactions, Ed. by P. Ausloos (Springer, Boston, 1979).
K. D. Froyd and E. R. Lovejoy, “Experimental Thermodynamics of Cluster Ions Composed of H2SO4 and H2O. 1. Positive Ions,” J. Phys. Chem., A, No. 45, 107 (2003).
U. Horrak, J. Salm, and H. Tammet, “Bursts of Intermediate Ions in Atmospheric Air,” J. Geophys. Res., 103 (1998).
M. Kanakidou, J. H. Seinfeld, S. N. Pandis, I. Barnes, F. J. Dentener, M. C. Facchini, R. van Dingenen, E. Swietlicki, J. P. Putaud, Y. Balkanski, S. Fuzzi, J. Horth, G. K. Moortgat, R. Winterhalter, C. E. L. Myhre, K. Tsigaridis, E. Vignati, E. G. Stephanou, and J. Wilson, “Organic Aerosol and Global Climate Modeling: A Review,” Atmos. Chem. Phys., No. 4, 5 (2005).
M. Kulmala, H. Vehkamaki, T. Pe, M. Dal Maso, A. Lauri, V. M. Kerminen, W. Birmili, and P. H. McMurry, “Formation and Growth Rates of Ultrafine Atmospheric Particles: A Review of Observations,” J. Aerosol Sci., 35 (2004).
Y. Kurihara and R. E. Televa, “Structure of Tropical Cyclone Developed in Three-dimensional Numerical Simulation Model,” J. Atmos. Sci., No. 5, 31 (1974).
E. R. Lovejoy, J. Curtius, and K. D. Froyd, “Atmospheric Ion Induced Nucleation of Sulfuric Acid and Water,” J. Geophys. Res., 109 (2004).
M. Noppel, H. Vehkamaki, and M. Kulmala, “An Improved Model for Hydrate Formation in Sulfuric Acid–Water Nucleation,” J. Chem. Phys., 116 (2002).
J. H. Seinfeld and S. N. Pandis, Atmospheric Chemistry and Physics. From Air Pollution to Climate Change (Wiley-Interscience, New York, 1997).
M. Wang and P. E. Penner, “Aerosol Indirect Forcing in a Global Model with Particle Nucleation,” Atmos. Chem. Phys., 9 (2009).
R. J. Weber, J. J. Marti, P. H. McMurray, F. L. Eisele, D. J. Tanner, and A. Jefferson, “Measured Atmospheric New Particle Formation Rates: Implications for Nucleation Mechanisms,” Chem. Eng. Commun., 151 (1996).
F. Yu, “Ion-mediated Nucleation in the Atmosphere: Key Controlling Parameters, Implications, and Look-up Table,” J. Geophys. Res., 115 (2010).
F. Yu, G. Luo, T. S. Bates, B. Anderson, A. Clarke, V. Kapustin, R. M. Yantosca, Y. Wang, and Sh. Wu, “Spatial Distributions of Particle Number Concentrations in the Global Troposphere: Simulations, Observations, and Implications for Nucleation Mechanisms,” J. Geophys. Res., 115 (2010).
Funding
The research was supported by the Russian Foundation for Basic Research (project numbers 18-05-00289 and 19-05-50007 (Mikromir)), as well as by the governmental assignments of Marchuk Institute of Numerical Mathematics of Russian Academy of Sciences (RAS) and the Talrose Institute for Energy Problems of Chemical Physics of RAS (theme AAAA-0047-2018-0012).
Author information
Authors and Affiliations
Corresponding author
Additional information
Russian Text ©The Author(s), 2021, published in Meteorologiya i Gidrologiya, 2021, No. 1, pp. 53-60.
About this article
Cite this article
Aloyan, A.E., Yermakov, A.N. & Arutyunyan, V.O. The Role of Binary and Ion Nucleation of Sulfuric Acid and Water Vapor in the Dynamics of Sulfate Aerosol Formation in the Atmosphere. Russ. Meteorol. Hydrol. 46, 37–42 (2021). https://doi.org/10.3103/S1068373921010052
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1068373921010052