Abstract
A new mathematical model of the global transport of multicomponent gas impurities and aerosols has been constructed. The model includes the formation of sulfate aerosols in the atmosphere in both hemispheres. Calculations of the nucleation rates and particle number concentrations are performed for the winter period in the troposphere using a unified model that takes into account chemical and kinetic transformation processes (photochemistry, nucleation, condensation/evaporation, and coagulation). Along with the binary nucleation of sulfuric acid and water vapors, the formation of particle nuclei with the participation of atmospheric ions is also considered. The results indicate a significant role of ion nucleation in aerosol formation in the Northern and Southern hemispheres in winter. The factors controlling the dynamics of ionic processes and their influence on the spatiotemporal distribution of aerosol particles in the atmosphere include not only the level of air ionization, but also temperature, relative humidity, and the content of sulfuric acid and water vapors in the air.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Studies of aerosol formation and monitoring of aerosol particles in the atmosphere are still high on the agenda. This is caused by the participation of aerosol particles in scattering and absorption of solar radiation, as well as in heterogeneous chemical reactions (HCRs), which has an effect both on the climate and on the content of small admixtures in the air. Despite the importance of the climate-forming potential of aerosol particles, their influence on the heat balance of the atmosphere remains uncertain (Lowe and MacKenzie, 2008; Akimoto, 2016). The causes are the large variety of components of aerosol particles, differences in their morphology (Zuend and Seinfeld, 2012), etc. Among atmospheric particles, an essential role in the atmosphere is played by sulfate aerosol (SA) appearing from the joint condensation of water and sulfuric acid vapors and playing the part of condensation nuclei. HCRs takes place on the surface and in the volume of SA in the atmosphere.
When developing models of the atmosphere, it is fundamentally important to correctly take into account the dynamics of SA formation in the troposphere and lower stratosphere. Their formation is a complex sum of nucleation, condensation/evaporation, and coagulation processes with the participation of water and sulfuric acid vapors, as well as photochemical transformation of gas impurities emitted in the atmosphere. The variability in the dynamics of each of them during fluctuations of weather conditions leads to changes in the chemical and phase SA composition in the atmosphere. The equilibrium in the disperse system is established rather slowly, and calculations of the dynamics in the formation of SA particles and prediction of their properties must involve a nonequilibrium particle size distribution function.
Previously, in (Aloyan, 2000; Aloyan et al., 2012, 2018), we reported about construction of a three-dimensional model of the transfer of multicomponent gas and aerosol microimpurities in the Northern Hemisphere with allowance for chemical and kinetic processes of their transformation, as well as processes of binary nucleation of water and sulfuric acid vapors and processes of condensation growth and particle coagulation. However, field experiments demonstrate that the considered mechanism of generation of secondary aerosol particle nuclei does not explain the nucleation enhancements of their formation (Kirkby et al., 2011). Attention is also attracted by processes of aerosol particle formation in the atmosphere with the participation of atmospheric ions. This work is aimed at complementing our three-dimensional model with the module of ion nucleation processes (Aloyan et al., 2021) to estimate the effect of ions on the spatiotemporal distribution of number concentrations of sulfate aerosol in the atmosphere.
MODEL OF AEROSOL FORMATION
The model of the global transfer of multicomponent small gas impurities and aerosols and formation of sulfate aerosols in the troposphere and lower stratosphere is considered as applied to the spherical Earth in the coordinate system (λ, ψ, z), where λ is the longitude, ψ is the colatitude, and z is the height. The fundamental equations for the variation rate of concentrations of gas impurities and aerosol have the following form (Aloyan, 2000; Aloyan et al., 2012, 2018):
were uj (\(j = \overline {1,3} \)) (u1 = u, u2 = v, u3 = w) (x1 = x, x2 = y, x3 = z) are components of the wind velocity vector in the directions x, y, and z, respectively; \({{C}_{i}}{\text{ }}(i = 1,...,{{N}_{{\text{g}}}})\) is the concentration of gaseous substances; \({{\varphi }_{k}}{\text{ }}(k = 1,...,{{N}_{{\text{a}}}})\) is the concentration of aerosol particles of the corresponding fraction; Ng and Na are the numbers of the gaseous substances and aerosol fractions, respectively; wg is the gravity sedimentation rate; Fgas and Faer are the sources of the gaseous substances and aerosols; and Pnucl, Pion-nucl, Pcond, Pcoag, and Pchem are the operators of homogeneous and ion nucleation, condensation, coagulation, and photochemical transformation, respectively.
Equations (1) and (2) are considered in the domain Dt = G × (0, T), where G = S × (0, H), S = {(λ, ψ): 0 ≤ λ ≤ 2π, 0 ≤ ψ ≤ π}, and H is the upper boundary of the computational domain. At the lateral boundaries, the periodicity conditions are specified for all functions in longitude; at the poles, it is assumed that the functions are bounded in ψ. The initial conditions (at t = 0) are specified in the following form:
At the lateral boundaries of the domain, the following boundary conditions are specified for the functions:
Here, Ω is the lateral surface, n is the external normal to Ω, un is the normal component of the velocity vector, and Cb and φb are the background concentrations of gaseous substances and aerosols, respectively.
The equations of transfer and transformation of multicomponent gas impurities and aerosols in the atmosphere were solved within the boundaries of the specified computational domain. On the earth’s surface (z = h), the absorption of gas impurities was calculated with allowance for the sum of aerodynamic drag, quasi-laminar resistance of the surface layer, molecular diffusion, and resistance to surface deposition.
To specify the boundary condition at the first computational level (at the upper boundary of the surface layer of the atmosphere) z = h, the resistance to the impurity entry on the earth’s surface was specified by a sum of three summands: aerodynamic drag ra, quasi-laminar resistance of the surface layer rb appearing due to molecular diffusion near the Earth, and surface resistance rc caused by surface deposition. The flows of impurities in the surface layer of the atmosphere were considered in the form
where \({{{r}_{{\text{b}}}} = \frac{{\text{2}}}{{{{\kappa }}u}}{{{\left( {\frac{{{{S}_{{\text{c}}}}}}{{{\text{Pr}}}}} \right)}}^{{{2 \mathord{\left/ {\vphantom {2 3}} \right. \kern-0em} 3}}}}},\) Sc = 1.5 × 10–5 m2 s–1, Pr = 0.71 is the Prandtl number, and u* is the dynamic velocity.
Aerodynamic drag was calculated by the formula presented in (Aloyan, 2008). The surface resistance coefficient rc over dry land is determined from the model describing the impurity transfer in the soil and active layer of the ocean with the simultaneous use of flows at the atmosphere–soil and atmosphere–ocean interfaces. The coefficients of horizontal and vertical turbulent exchange coefficient are determined using the method proposed in (Kurihara and Tuleya, 1974).
Ion Nucleation
Experiments demonstrate (Ferguson, 1979; Froyd and Lovejoy, 2003) that the process of ion nucleation involves mostly negative ions, which is related to the well-known sign preference (Kusaka et al., 1995). In the presence of H2SO4 vapors in the air, \({\text{NO}}_{3}^{ - }\)(HNO3)х(H2O)y ions are transformed into cluster \({\text{HSO}}_{4}^{ - }\) the growth of the size of which in processes of clusterization and recombination with counterions leads, in the end, to nuclei of aerosol particles. The nucleation rate (Jion) is calculated here as a flow of particles passing through the critical size of the nucleus (rcr). Neutral clusters, simultaneously appearing in the air and including molecules of sulfuric acid and water, coagulate both with neutral and negatively charged clusters. At the same time, their capture by neutral aerosol particles leads to a decrease in Jion (Yu, 2010).
However, using this algorithm for finding Jion in three-dimensional numerical models meets great difficulties in view of the necessity to involve large computational resources. For this reason, the nucleation rate depending on the sulfuric acid concentration, temperature, relative humidity, ionization rate, and surface area of previous particles is determined in this model using the parameterization of Jion on these parameters (Yu, 2010). A detailed description of the ion nucleation model and algorithm for finding its rate was presented in (Yu, 2010).
Model of Photochemical Transformation
As precursors of secondary aerosol particles, processes of photooxidation of sulfur-containing anthropogenic and biogenic components emitted from the surface (including dimethyl sulfide (CH3SCH3) emitted by the oceans) were considered. In total, 72 individual gas components were taken into account (Aloyan et al., 2018).
Condensation Nuclei
To describe the spatiotemporal variability in concentrations of condensation nuclei in the atmosphere and the size spectrum, 20 discrete intervals from ≈3.5 nm to 0.58 µm were used in the calculations. Note that, according to the data of field experiments, the number concentration of condensation nuclei varies with height within the range from ∼10 to several hundred particles per 1 cm3 (Hofmann, 1990). It was assumed that the condensation nuclei were inert particles turning into sulfate aerosol only after the molecules of sulfuric acid and water settled on them or after the capture of small sulfate aerosol particles arising in the nucleation processes. The distribution of concentration of condensation nuclei was set in the range from 0.18 to 9.98 × 108 m–3 (Aloyan, 2008). The total specific surface area of condensation nuclei at the initial time was ≈0.26 µm2/m3. According to (Yu, 2010), this minimizes their influence on the dynamics of ion nucleation.
The numerical experiments were performed as applied to both hemispheres at different geographical points in winter in the Northern Hemisphere at the following input parameters. The number of levels along the vertical is 20 (from 100 to 46 000 m). The rate of binary nucleation of H2O and H2SO4 vapors, the threshold H2SO4 concentration necessary for nucleation, the critical cluster size, and the temporal variability of the particle size distribution function were calculated in numerical experiments. Meteorological information was borrowed from the database of the European Centre for Medium-Range Weather Forecasts (ECMWF) for January 2002.
RESULTS OF NUMERICAL CALCULATIONS
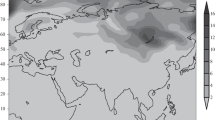
Figures 1–3 present the calculated spatial variability of ion nucleation rates of water and sulfuric acid vapors in the atmosphere. These data for the heights of 4.2, 7.2, and 10.4 km and winter correspond to the picture of 18 h after the model initialization. Close patterns of the spatial distribution of Jion are also observed 6 and 12 h after the model initialization, which indicates the establishment of an almost stationary spatiotemporal distribution of Jion in the atmosphere.
It follows from these calculations that Jion is characterized by a considerable spatial inhomogeneity. For example, at a height of 4.2 km, the formation of particle nuclei with the participation of ions is implemented only over regions located over Lake Baikal, regions of Kamchatka, over regions occupying the northeast and northwest of the American continent. Within the localization areas of this process in the atmosphere, the nucleus generation rate in them changes by more than an order of magnitude. The appearance of these contrasts indicates a high sensitivity of the ion nucleation process to changes in weather conditions, ionization and insolation levels, content of impurities in the atmosphere, etc. Despite the high level of air ionization and low temperatures, regions of particle nucleus formation at this height are not revealed in the atmosphere over the Arctic and Antarctic, which indicates a shortage of anthropogenic and natural sulfur-containing impurities in the air of these regions.
The character of the inhomogeneity in the distribution of Jion noticeably changes with height over the underlying surface. In contrast to what was described above, regions of occurrence of the ion nucleation process at a height of 7.2 km occupy almost the whole atmosphere over both the Arctic and Antarctic. At the same time, a considerable difference in Jion within these regions is also observed. With a further rise to 10.4 km, the generation of particle nuclei in the ion nucleation process covers almost the whole atmosphere with the simultaneous decrease in differences in Jion in particle nucleation sites, which, therefore, indicates the nearly uniform participation of ions in the nucleation process at this height.
The inhomogeneity in the spatial distribution of Jion seems to be caused by the variability in temperature, relative humidity, content of sulfuric acid and water vapors in the air, and differences in the ionization level. In particular, the ionization influence on Jion clearly manifests itself at the height of 7.2 km in the localization of regions of particle nucleus generation over the Arctic and Antarctic (see Fig. 2), i.e., in regions with the maximum air ionization level (Yu, 2010). The influence of atmosphere contamination by sulfuric acid vapors arising due to the oxidation of sulfur dioxide, dimethyl sulfate, and other sulfur-containing components is observed to a greatest extent at the height of 4.2 km over the regions adjacent to Lake Baikal and located in Kamchatka, as well as in northeast and northwest of the American continent. This takes place despite the decrease in the ionization level when moving from the pole to the equator (Yu, 2010) and relatively high temperatures by winter standards, which indicates the predominantly anthropogenic origin of atmospheric contamination by emissions of sulfur dioxide and other components in the regions listed above. The influence of temperature on the ion nucleation dynamics clearly manifests itself at a height of 10.4 km, i.e., under the lowest temperatures. It follows from the computed data (see Fig. 3) that the generation of particle nuclei with the participation of ions at this height occupies almost the entire atmosphere. At the same time, their formation in the sources characterizes feebly marked differences in Jion.
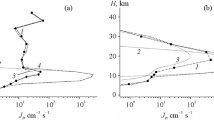
Summarizing all of the above, one can state that the deciding factor in the dynamics in the formation of particle nuclei in nucleation in the troposphere and especially in its lower part, i.e., under conditions of relatively high temperatures in winter, is apparently the influence of the complex ion charge on heat stability of the clusters. At the same time, in the upper troposphere, i.e., under conditions of low temperatures, the influence of the ion charge on stability of clusters turns out to be deciding due to deficiency of air humidity. In particular, despite intense air ionization in high latitudes in the Northern Hemisphere, a noticeable decrease in Jion is observed with an increase in height over the underlying surface, which is related to the sharp decrease in the concentration of water vapors in the atmosphere to ≈107 cm–3. In the Northern Hemisphere, e.g., this results in the value Jion ≈ 2 cm–3 s–1 at the height of 10.4 km. At the same time, the water vapor content in the Southern Hemisphere in winter is one and a half orders of magnitude higher, which leads to much higher values of Jion.
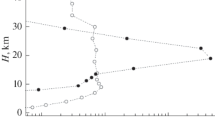
Figures 4–6 show the form of the calculated distributions of number concentrations of aerosol particles with different sizes (from 3.5 to ≈60 nm) 18 h after the model initialization at the height of 4.2 km. Here, along with the formation of aerosol particles over the Baikal region, vast regions of their formation are observed over the north of Russia (the Barents and Kara seas), over the Norwegian Sea, and over Scandinavia, as well as over regions covering the northeast and northwest of the American continent. The formation of aerosol particles in these regions, which begins at heights of ≈2 km (see also (Yu et al., 2010)), is also clearly observed at the height of 4.2 km. The formation of particles in the Baikal region is caused by high anthropogenic load and, as a result, high concentrations of sulfuric acid vapors (≥5 × 107 cm–3). Ion nucleation is also favored by sufficiently low temperatures and a sufficiently high level of relative air humidity (0.6–0.7), which, in the aggregate, leads to a level of such threshold concentrations of sulfuric acid vapors that are lower than the vapor content over this region by an order of magnitude. We meet a similar situation when considering the results of calculations over the northeast and northwest of the American continent. At the same time, the appearance of particles traced at this height over Yakutia, Kamchatka, and Sakhalin, as well as over the basins of the Chukchee, Bering, and Okhotsk Seas, is observed under conditions of low insolation and low content of sulfur-containing impurities in the atmosphere. The formation of particles in these regions is mainly due to the transport of sulfur oxides and accompanying processes of oxidation followed by nucleation, which is caused in winter by low temperatures (220–245 K) and high relative humidity of the air (≥0.65).
The increase in the concentration present in the greatest concentration of first mode particles, as is observed in the calculations, is caused by the generation of the smallest new particles during the nucleation of water and sulfuric acid vapors and their capture by condensation nuclei. This capture of new particles is accompanied by an increase in the size of condensation nuclei. Molecules of sulfuric acid and water are captured from the air simultaneously with these condensation nuclei, which also leads to an increase in the size of first mode particles. As a result, both the processes, as well as the participation of first mode particles in coagulation processes, lead to the transition of particles from the first mode (3.5 nm) to the second mode (25.8 nm) and following (the third mode of 60.7 nm) particle modes (Kurihara and Tuleya, 1974). In Figs. 5 and 6, an increase in concentrations of particles from the first to the third mode over Arctic is also observed, which is related to the participation of ions in the process of particle formation.
CONCLUSIONS
The calculated distributions of the number concentration of secondary sulfate aerosol in winter have been presented. The calculations have been performed for a spherical atmosphere with the use of a physically complete model of the global transport of multicomponent gas impurities and aerosols on the regional scale and accompanying physicochemical processes. Along with binary nucleation of water and sulfuric acid vapors, their nucleation with the participation of atmospheric ions is also taken into account. It has been found that the key factors determining the influence of ions on the spatial distribution of aerosol particles in the atmosphere are the ionization level, as well as temperature, relative humidity, and the content of water and sulfuric acid vapors in the air. It has been shown that the influence of atmospheric ions on the distribution of aerosol particles is traced in the lower troposphere. For example, at a height of 4.2 km, the formation of particle nuclei with the participation of ions is implemented only over regions situated over Lake Baikal, regions of Kamchatka, and regions occupying the northeast and northwest of the American continent. Within the localization regions of this process in the atmosphere, the nucleus generation rate in them changes more than by an order of magnitude.
REFERENCES
Akimoto, H., Atmospheric Reaction Chemistry, Springer, 2016.
Aloyan, A.E., Mathematical modeling of the interaction of gas species and aerosols in atmospheric dispersive systems, Russ. J. Numer. Anal. Math. Modell., 2000, vol. 15, nos. 1–4, pp. 211–224.
Aloyan, A.E., Modelirovanie dinamiki i kinetiki gazovykh primesei i aerozolei v atmosfere (Modeling the Dynamics and Kinetics of Gaseous Admixtures and Aerosols in the Atmosphere), Moscow: Nauka, 2008.
Aloyan, A.E., Yermakov, A.N., and Arutyunyan, V.O., Sulfate aerosol formation in the troposphere and lower stratosphere, in Issledovanie vozmozhnoi stabilizatsii klimata s pomoshch’yu novykh tekhnologii (Investigation of the Possible Climate Stabilization Using New Technologies), Moscow: Rosgidromet, 2012, pp. 75–98.
Aloyan, A.E., Yermakov, A.N., and Arutyunyan, V.O., Aerosol in the upper troposphere and lower stratosphere. Sulfate particles in northern latitudes, Opt. Atmos. Okeana, 2018, vol. 31, no. 2, pp. 136–142.
Aloyan, A.E., Yermakov, A.N., and Arutyunyan, V.O., Modeling the influence of ions on the dynamics of formation of atmospheric aerosol, Izv., Atmos. Ocean. Phys., 2021, vol. 57, no. 1, pp. 104–109.
Ferguson, E.E., Ion–molecule reactions in the atmosphere, in Kinetics of Ion–Molecule Reactions, Ausloos, P., Ed., Boston: Springer, 1979, pp. 377–403.
Froyd, K.D. and Lovejoy, E.R., Experimental thermodynamics of cluster ions composed of H2SO4 and H2O. 1. Positive ions, J. Phys. Chem. A, 2003, vol. 107, no. 45, pp. 9800–9811.
Hofmann, D.J., Measurement of the concentration nuclei profile to 31 km in the arctic in January and comparison with Antarctic measurements, Geophys. Res. Lett., 1990, vol. 17, no. 4, pp. 357–360.
Kirkby, J., Curtius, J., Almeida, J., et al., Role of sulphuric acid ammonia and galactic cosmic rays in atmospheric aerosol nucleation, Nature, 2011, vol. 476, pp. 429–433.
Kurihara, Y. and Televa, R.E., Structure of tropical cyclone developed in three-dimensional numerical simulation model, J. Atmos. Sci., 1974, vol. 31, no. 5, pp. 893–919.
Kusaka, I., Wang, Z.-G., and Seinfeld, J.H., Ion-induced nucleation: A density functional approach, J. Chem. Phys., 1995, vol. 102, no. 2, pp. 913–924.
Lowe, D. and Mackenzie, R., Review of polar stratospheric cloud microphysics and chemistry, J. Atmos. Sol.-Terr. Phys., 2008, vol. 70, no. 1, pp. 13–40.
Yu, F., Ion-mediated nucleation in the atmosphere: Key controlling parameters, implications, and look-up table, J. Geophys. Res., 2010, vol. 115, p. D03206.
Yu, F., Luo, G., Bates, T.S., et al., Spatial distributions of particle number concentrations in the global troposphere: Simulations, observations, and implications for nucleation mechanisms, J. Geophys. Res., 2010, vol. 115, p. D17205.
Zuend, A. and Seinfeld, J.H., Modeling the gas–particle partitioning of secondary organic aerosol: The importance of liquid–liquid phase separation, Atmos. Chem. Phys., 2012, vol. 12, no. 9, pp. 3857–3882.
Funding
This work was supported by the Russian Foundation for Basic Research, project no. 19-05-50007 (Mikromir), as well as within the framework of state contracts for the Institute of Numerical Mathematics, Russian Academy of Sciences, and the Talrose Institute for Energy Problems of Chemical Physics, Russian Academy of Sciences, topic no. AAAA-0047-2018-0012.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Aloyan, A.E., Yermakov, A.N. & Arutyunyan, V.O. On the Influence of Atmospheric Ions on Aerosol Formation in the Troposphere: Numerical Simulation. Izv. Atmos. Ocean. Phys. 59, 405–410 (2023). https://doi.org/10.1134/S0001433823040035
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0001433823040035