Abstract
The features of the extraction technology for the separation of rare-earth elements (REEs) of the yttrium group are considered with regard to the sharp reduction in the price of individual oxides. The price reduction has the same nature as the low prices of lanthanum and cerium oxides and is associated with a predominant increase in the consumption of praseodymium and neodymium and a slow increase in the consumption of other REEs, with the exception of terbium and dysprosium. Since all REEs are extracted from rare-earth concentrates, less in demand ones are stored or sold at very low prices. Elements such as samarium, europium, gadolinium, and dysprosium are used in high-tech instruments and devices. In this case, it is possible to allow the operation of low-profit production, but technological solutions must certainly be built taking into account the minimum costs and be the most economically effective. The authors propose a technology for separating elements of the yttrium group including the stages of isolation of yttrium in a single-stage mode by extraction with a mixture of three extractants (25 vol % trialkylmethylammonium nitrate–20 vol % tributyl phosphate–20 vol % higher isomeric carboxylic acid), followed by separation of the triad of elements samarium–europium–gadolinium by extraction with organophosphoric acids (30 vol % solution of di-2-ethylhexylphosphoric acid or 30 vol % solution of bis(2,4,4-trimethylpentyl)-phosphinic acid). In the last operation, concentrates of the yttrium group REEs are isolated simultaneously. The process is carried out in the mode of complete internal irrigation using a 30 vol % solution of bis(2,4,4-trimethylpentyl)-phosphinic acid as an extractant. First, all cells of the cascade are filled with the initial solution. Separation zones are formed in the cells of the cascade with the accumulation of terbium–dysprosium, holmium–erbium, and thulium–ytterbium–lutetium concentrates. After the accumulation of products, the solution of concentrates is drained from the cells and the process starts again. If there is a need for any element of the yttrium group, the corresponding binary or ternary concentrate is separated to isolate the required element.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Owing to the fact that many rare-earth elements (REEs) of the yttrium group have unique properties and are in demand in industry, researchers focus increased attention on their separation, especially the isolation of yttrium [1] as a metal widely used in a variety of industries. Organic compounds are used as extractants, in particular, carboxylic acids of various structures [2]. The latter is due to the fact that the distribution coefficient of yttrium during extraction with carboxylic acids shifts to the region of neodymium–samarium [3–5], and yttrium can be separated from most rare-earth elements. Work continues on the isolation of yttrium by extraction with tributyl phosphate (TBP) from rhodanide media to obtain yttrium containing 99.9% of the basic substance [6]. Extraction systems based on carboxylic acids and a complexing agent in the aqueous phase have a disadvantage associated with a low concentration of REEs in the aqueous phase [7]. Extraction with organophosphorus acids is free of this drawback [8–12]. In [13, 14], the results of synthesis and the parameters of extraction with a new branched alkylphosphinic acid are presented as applied to the separation of REEs of the yttrium group. Liquid extraction with di-2-ethylhexylphosphoric acid (D2EHPA) was used to isolate a group of elements, namely, samarium, europium, and gadolinium, from monazite, followed by obtaining oxides of individual elements [15]. Significant efforts of researchers are aimed at finding more selective systems, in particular, based on the use of mixtures of extractants with a synergistic effect [16–18] and ionic liquids [19, 20].
Rare-earth elements of the yttrium group are much less common in rare-earth concentrates and are mined in smaller quantities, but their properties are so unique that it would be impossible to develop modern high-performance materials and equipment without them. At the same time, there is an excess of available capacity and slow growth of demand for rare-earth elements of the yttrium group. The latter conclusion is perfectly confirmed by the long-term decrease in the price of oxides of all elements of the yttrium group [21]. It is known that the profitability of the REE separation technology provides a high recovery (99%) of praseodymium and neodymium with a basic substance content of 99.95–99.99 wt % [22]. Preliminary calculations show that the complete separation of the elements of the yttrium group is generally unprofitable.
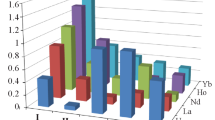
Figure 1 shows the share of realization of each individual REE isolated from 100 kg of Sm–Lu + Y concentrate (isolated from apatite). It can be seen that, despite the significant content of yttrium in the concentrate (more than 44%), the sales volume from the extraction of yttrium will be only 2–3%. At the same time, while the content of terbium and dysprosium is 10%, their sales volume increases to 76% of the total amount. In addition, it should be noted that terbium and dysprosium have the highest liquidity.
Despite the rather high content of yttrium in the concentrates under consideration (up to 40–70%), the volume of possible sales of yttrium is insignificant. To reduce costs, it seems appropriate to remove yttrium at the first stage by single-cascade extraction with the production of varietal yttrium oxide (99.9% of the basic substance), simultaneously reducing the volume of processed REEs in half. Small batches of concentrates of the yttrium group elements should be combined and organized for the separation of these elements with a capacity of 400–500 t/year (more than 100 t/year). With separation of ≥500–600 t/year of yttrium REEs, it is feasible to base the separation process on the standard countercurrent extraction with successive isolation of all elements, which ensures a sufficiently high productivity. If there is a need to process 50–100 t/year of concentrate to obtain elements that are in demand by the consumer but in a small amount, the proposed technological solutions should be optimized to maximally reduce the costs. In this case, it is reasonable to first extract binary rare-earth concentrates, which are then subjected to separation to obtain the required individual oxides. The solution of the problem is best approached by technologies with a nonstationary mode and accumulation of elements or concentrates in the cascade cells.
In this paper, we consider a technology for the separation of small amounts of yttrium REEs (50–100 t/year) with the isolation of binary or ternary concentrates. The initial concentrate was obtained by the separation of REEs isolated from apatite along the neodymium–samarium interface. The concentrate contains all elements from samarium to lutetium, including yttrium. The technological scheme is based on the sequential isolation of yttrium first to reduce the amount of rare-earth elements subjected to further separation. At the next stage, a concentrate containing samarium, europium, and gadolinium is isolated. This decision is justified by the fact that the separation coefficient between gadolinium and terbium is one of the highest in this group of elements and reaches a value of 3–4 for some systems. At the next stage, a terbium–dysprosium concentrate is isolated with simultaneous isolation of holmium–erbium and thulium–ytterbium–lutetium concentrates. Pure individual elements are recovered from the obtained concentrates on the basis of the prevailing market conditions and prices for certain elements.
EXPERIMENTAL
Experimental Technique
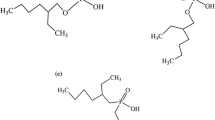
The following reagents were used: nitric acid, ammonia, oxalic acid, calcium nitrate of chemically pure grade, and extractant tributyl phosphate (corresponding to the technical standard TU 2435-305-05763458–2001). Commercial D2EHPA was purified from impurities according to the procedure described in [23]. Trialkylammonium nitrate (TAMAN) and the fraction of the higher isomeric carboxylic acid (HICC-2) and bis(2,4,4-trimethylpentyl)-phosphinic acid (Cyanex-272) contained more than 99% of the basic substance. Technical hydrogen peroxide (A) of chemically pure grade (GOST 177–88) was used for the reduction of cerium.
Extraction was carried out according to the usual method by shaking the organic and aqueous phases in a separating funnel at a temperature of 20 ± 2°C. The total REE content was determined by precipitation of oxalates followed by their roasting to oxides at t = 800–850°C. The content of individual elements in re-extracts and aqueous solutions was determined on a Profile Plus spectrometer (Teledyne Leeman Labs, USA) with inductively coupled plasma. After separating the elements of the cerium group, the yttrium REE concentrate contained (wt %) 19.1Sm, 6.3Eu, 15.4Gd, 0.9 Tb, 9.1Dy, 0.9Ho, 2.7Er, 0.1Tm, 0.9Yb, 0.2Lu, and 44.4Y.
During the separation of rare-earth concentrates, these elements are concentrated together with yttrium in an isolated fraction and are subjected to separation in extraction cascades using mainly organophosphorus acids. D2EHPA has become the most common owing to its availability. To reduce production costs, it is reasonable to use phosphonic or phosphine derivatives, for example, di-2-ethylhexyl ether of 2-ethylhexylphosphonic acid (P-507, PC88A) or bis(2,4,4-trimethylpentyl)-phosphinic acid (Cyanex-272). A 30–35% solution of alkyl phosphoric acid was applied as an extractant. It appears preferable to use alkylphosphonic acid because of the need to use less concentrated solutions of mineral acids for the re-extraction of rare-earth elements from the organic phase. Phosphinic acids complicate the process because of the need to control the pH of the aqueous phase.
Isolation of Yttrium
These extractants recover REEs with an increasing distribution coefficient from samarium to lutetium. According to its distribution coefficients, yttrium is located in the region of holmium–erbium, which is typical of ionic bonds. To separate yttrium from other REEs within a single countercurrent cascade, a 25% TAMAN–20% TBP–20% HICC-2 system in kerosene is proposed. Ammonia is added to the aqueous solution to neutralize hydrogen ions. Table 1 shows the results of the interfacial distribution of REEs during extraction with mixtures of extractants from solutions containing 154 g/dm3 REEs (to oxides).
It can be seen from Table 1 that, during extraction with TAMAN and HICC-2 mixtures for samarium, europium, gadolinium, and terbium, there is a decrease in separation coefficients with respect to yttrium. During extraction with a ternary mixture of extractants, this disadvantage disappears. The separation coefficient βREE/Y varies within 1.8–2.2 depending on the experimental conditions, which is quite acceptable for organizing an effective countercurrent process with the production of yttrium with a basic substance content of 99.9–99.99%. The separation of the countercurrent cascade and the flow balance were calculated in accordance with the recommendations given in [24].
Table 2 shows the results regarding the effect of the initial parameters on the number of steps and the phase ratio in the extraction part of the cascade. It can be seen that, to obtain yttrium with a basic substance content of 99.95–99.99% and a separation coefficient βREE/Y = 2, the sufficient number of steps in the extraction part of the cascade is 17–26.
Table 3 shows the results of the influence of various parameters on the efficiency of the separation process in the washing part of the cascade. The main task to be solved in the washing part of the cascade is to maximally separate yttrium from other REEs but without deteriorating the quality of yttrium in the raffinate. To extract more than 99% of yttrium (the content of yttrium in the Sm–Lu concentrate is 2%), 10–12 steps in the washing part of the cascade are sufficient.
Isolation of Sm–Eu–Gd Concentrate
The technological scheme is built on the sequential isolation of yttrium first to reduce the volume of rare-earth elements subjected to further separation. Owing to the fact that the price of individual elements is very low, the isolation of these elements and their subsequent sale do not ensure the profitability of production as a whole. The separation of the samarium–europium–gadolinium concentrate is simplified to a large extent because of the high separation coefficient βTb/Gd = 2.3–3.0 as a result of the manifestation of the tetrad effect (filling of the 4f 7 orbital in gadolinium). Table 4 shows the distribution coefficients of the elements of the yttrium group during extraction with organophosphorus acids D2EHPA and Cyanex-272.
During the extraction with D2EHPA, the concentration of REEs in the aqueous phase was maintained at 92 g/dm3 and the concentration of nitric acid was maintained at 1.2 mol/dm3. When using Cyanex-72, the initial aqueous solution containing nitrates of rare-earth elements (100 g/dm3) with pH 4–5 was in contact with the Cyanex-272 solution for 3 min. Simultaneously, the aqueous phase was mixed with an amount of ammonium hydroxide equivalent to the amount of REEs that passed into the organic phase. Owing to the high separation coefficient, it is possible to add 10–20 vol % TBP to the organic phase.
The use of a mixture of di-2-ethylhexylphosphoric acid and TBP leads to a slight decrease in βTb/Gd to 2.0–2.3, but the probability of precipitation in the organic phase (solvation of extractable compounds by TBP molecules) decreases, the working capacity of the organic phase slightly increases (by 10–15%), and the hydrodynamic parameters of the system improve (phase separation rate, viscosity). Since the system also contains heavy REEs (thulium, ytterbium, and lutetium), it is advisable to use phosphonic acid as an extractant.
It has been found that the distribution coefficient increases in the series Sm–Eu–Gd–Tb (Table 4); the REE concentration in the organic phase reaches 30 g/dm3; the separation coefficient βTb/Gd ranges within 2.1–2.9. It should be noted that the phases separate rather quickly, and at the chosen concentration of nitric acid, the formation of neutral salts with precipitation is not observed. The main parameters of the separation process along the Gd–Tb interface in the extraction part of the cascade are given in Table 5.
The samarium–europium–gadolinium concentrate remains in the aqueous phase, while the yttrium REEs from terbium to lutetium remain in the extract. In the raffinate, it is important to obtain a product containing less than 0.01% terbium and heavier REEs for the subsequent isolation of gadolinium in a single-cascade mode. With a separation coefficient βTb/Gd = 2.0–2.2, the sufficient number of steps is 22–28.
The conditions for the extraction part must be selected so as not to lose very valuable terbium and dysprosium; i.e., the content of terbium and dysprosium should not exceed 0.05 wt %. In the washing part of the cascade, it is important to remove europium and gadolinium as best as possible to a content of less than 0.05%. The main parameters of the separation process in the washing part of the cascade and the results of separation in the countercurrent mode are given in Tables 6 and 7.
In the extract, a dysprosium concentrate is formed, the isolation of which, along with terbium, determines the economic feasibility of separating the REEs of the yttrium group.
Separation of Terbium–Lutetium Concentrate. Extraction of Concentrates in the Accumulation Mode with the Obtainment of Three Products
The concentrate containing REEs from terbium to lutetium is subjected to separation in the mode of complete internal irrigation with the accumulation of three REE concentrates in the cascade cells: terbium–dysprosium, holmium–erbium, and thulium–ytterbium–lutetium in the extraction, middle, and washing parts of the cascade, respectively. The calculation of the process was carried out on the basis of the separation along two interfaces: dysprosium–holmium and erbium–thulium. The residual amount of yttrium from previous separations is distributed between holmium and erbium. It is advisable to use alkylphosphonic acid as an extractant.
The cascade contains 60–70 steps. Containers in which the released concentrates are accumulated are attached to the cascade cells: closer to the raffinate release, in the center of the cascade, and a few steps before the extract release. A container for the accumulation of terbium–dysprosium concentrate is connected to steps 8–10, a container for the accumulation of holmium–erbium concentrate is connected to steps 25–27, and a container for the accumulation of thulium–ytterbium–lutetium concentrate is connected to steps 45–46. At the beginning of the process, the containers and cells of the cascade are filled with the initial solution and the extractant. Ammonium hydroxide or ammonium carbonate is introduced into steps 2–3 before the raffinate is released to neutralize excess amounts of hydrogen ions freed owing to ion exchange. The process is carried out by extracting all the REEs from the aqueous solution and returning all the REEs to the washing solution cascade. Ammonium hydroxide is introduced into the first step of the extraction part of the cascade to neutralize excess mineral acid. The acid must be neutralized to such an extent that gadolinium and lighter elements are completely extracted into the organic phase. The re-extract is neutralized with ammonium hydroxide, evaporated, and, after adjustment, sent to the cascade as a washing solution. The washing solution is obtained from the extract with added ammonium hydroxide or ammonium carbonate to reduce the concentration of hydrogen ions to a minimum level, at which medium REE salts does not yet precipitate (LnR3). This is 1.0–1.5 mol/dm3 of the nitric acid solution for D2EHPA and 0.2–0.4 mol/dm3 for phosphonic acid.
At the start of the cascade, all chambers and all containers are filled with the initial solution containing 80–100 g/dm3 REEs and various amounts of mineral acids. The re-extracting solution contains 4.0–5.0 mol/dm3 nitric acid for D2EHPA and 2.0–3.0 mol/dm3 for alkylphosphonic acid. The organic and aqueous phases flow in countercurrent. The cascade cells accumulate rare-earth elements, the distribution of which over the steps of the cascade is shown in Fig. 2. The accumulation zone of dysprosium–terbium occupies 15 steps, and the accumulation zone of holmium–yttrium–erbium concentrate takes 16–17 steps. After the accumulation of the corresponding concentrates in the cascade cells, the supply of the initial solution is cut off, and the organic phase and the washing solution are introduced into the cascade to remove the REEs contained in the initial solution from the central part of the cascade. The concentrates are drained from the respective cells and the process can be repeated again.
The resulting concentrates are further separated with the segregation of the required individual elements, for example, terbium and dysprosium. Owing to the fact that market needs can vary significantly, individual concentrates can be stored, while other concentrates can be sent to production to isolate pure elements. This approach, in our opinion, provides the most adequate response to the changing conditions of a market economy.
The proposed technical solutions can be used to separate yttrium elements isolated, for example, from the concentrates of the Yakutsk Tomtor deposit, apatite and loparite.
CONCLUSIONS
(1) A sequence of operations for separating elements of the yttrium group has been proposed that includes the stage of yttrium isolation by extraction with a ternary mixture of extractants and the subsequent separation of concentrates: samarium–europium–gadolinium, terbium–dysprosium, holmium–erbium, and thulium–ytterbium–lutetium.
(2) The technology of simultaneous extraction of binary and ternary concentrates of rare-earth elements terbium–dysprosium, holmium–erbium, and thulium–ytterbium–lutetium in the mode of complete internal irrigation has been considered.
REFERENCES
Bartonova, L., Serencisova, J., and Cech, B., Yttrium partitioning and associations in coal-combustion ashes prior to and after their leaching in HCl, Fuel Process. Technol., 2018, vol. 173, pp. 205–215. https://doi.org/10.1016/j.fuproc.2018.01.01
Singh, D.K., Singh, H., and Mathur, J.N., Extraction of rare earths and yttrium with high molecular weight carboxylic acids, Hydrometallurgy, 2006, vol. 81, nos. 3–4, pp. 174–181.
Kui Liu, Zengkai Wang, Xiaomeng Tang, and Shiquan Lu, Extraction of yttrium using naphthenic acid with different acid numbers, Sep. Sci. Technol., 2016, vol. 51, no. 17, pp. 1–11. https://doi.org/10.1080/01496395.2016.1222427
Yanliang Wang, Wuping Liao, and Deqian Li, A solvent extraction process with mixture of CA12 and Cyanex 272 for the preparation of high purity yttrium oxide from rare earth ores, Sep. Purif. Technol., 2011, vol. 82, pp. 197–201. https://doi.org/10.1016/j.seppur.2011.09.018
Sposato, C., Romanelli, A., Blasi, A., and Morgana, M., Behavior of sec-octylphenoxy acetic acid (CA-12) in yttrium recovery from high concentrated heavy rare earths mixture, in Rare Metal Technology 2017, The Minerals, Metals and Materials Series, Kim, H., Alam, S., Neelameggham, N., Oosterhof, H., Ouchi, T., and Guan, X., Eds., Cham: Springer, 2017, pp. 225–233. https://doi.org/10.1007/978-3-319-51085-9_24
Deshpande, S.M., Mishra, S.L., Gajankush, R.B., Thakur, N.V., and Koppiker, K.S., Recovery of high purity Y2O3 by solvent extraction route using organo-phosphorus extractants, Miner. Process. Extr. Metall. Rev., 1992, vol. 10, no. 1, pp. 267–273. https://doi.org/10.1080/08827509208914089
Wang, Y.G., Xiong, Y., Meng, S.L., and Li, D.Q., Separation of yttrium from heavy lanthanide by CA-100 using the complexing agent, Talanta, 2004, vol. 63, no. 2, pp. 239–243. https://doi.org/10.1016/j.talanta.2003.09.034
Agarwal, V., Safarzadeh, M.S., and Galvin, J., Solvent extraction and separation of Y(III) from sulfate, nitrate and chloride solutions using PC88A diluted in kerosene, Miner. Process. Extr. Metall. Rev., 2018, vol. 39, no. 4, pp. 258–265. https://doi.org/10.1080/08827508.2017.1415210
Desouky, O.A., Daher, A.M., Abdel-Monem, Y.K., and Galhoum, A.A., Liquid–liquid extraction of yttrium using primene-JMT from acidic sulfate solutions, Hydrometallurgy, 2009, vol. 96, no. 4, pp. 313–317. https://doi.org/10.1016/j.hydromet.2008.11.009
Xiaobo Sun, Junmei Zhao, Shulan Meng, and Deqian Li, Synergistic extraction and separation of yttrium from heavy rare earths using mixture of sec-octylphenoxy acetic acid and bis(2,4,4-trimethylpentyl)phosphinic acid, Anal. Chim. Acta, 2005, vol. 533, no. 1, pp. 83–88. https://doi.org/10.1016/j.aca.2004.11.005
Fontana, D. and Pietrelli, L., Separation of middle rare earths by solvent extraction using 2-ethylhexylphosphonic acid mono-2-ethylhexyl ester as an extractant, J. Rare Earths, 2009, vol. 27, no. 5, p. 830. https://doi.org/10.1016/S1002-0721(08)60344-0
Shengting Kuang, Zhifeng Zhang, Yanling Li, Haiqin Wei, and Wuping Liao, Extraction and separation of heavy rare earths from chloride medium by aminophosphonic acid HEHAPP, J. Rare Earths, 2018, vol. 36, no. 3, pp. 304–310. https://doi.org/10.1080/07366299.2018.1431079
Junlian Wang, Guang Chen, Shengming Xu, and Linyan Li, Synthesis of novel nonsymmetric dialkylphosphinic acid extractants and studies on their extraction-separation performance for heavy rare earths, Hydrometallurgy, 2015, vol. 154, pp. 129–136.
Junlian Wang, Guang Chen, Shengming Xu, Zhili Yin, and Qin Zhang, Solvent extraction of rare earth ions from nitrate media with new extractant di-(2,3-dimethylbutyl)-phosphinic acid, J. Rare Earths, 2016, vol. 34, no. 7, pp. 724–730. https://doi.org/10.1016/S1002-0721(16)60088-1
Rabie, K.A., A group separation and purification of Sm, Eu and Gd from Egyptian beach monazite mineral using solvent extraction, Hydrometallurgy, 2007, vol. 85, nos. 2–4, pp. 81–86. https://doi.org/10.1016/j.hydromet.2005.12.012
Gaikwad, A.G. and Damodaran, A.D., Synergistic extraction studies of thiocyanate complexes of gadolinium, dysprosium and erbium with mixture of tributyl phosphate and tricaprylmonomethylammonium chloride, Anal. Sci., 1990, vol. 6, no. 6, pp. 871–875. https://doi.org/10.2116/analsci.6.871
Abreu, R.D. and Morais, C.A., Study on separation of heavy rare earth elements by solvent extraction with organophosphorus acids and amine reagents, Miner. Eng., 2014, vol. 61, pp. 82–87. https://doi.org/10.1016/j.mineng.2014.03.015
Belova, V.V., Development trends of extraction processes for the extraction and separation of rare earth metals, Khim. Tekhnol., 2016, vol. 17, no. 5, pp. 228–240.
Yanliang Wang, Chao Huang, Fujian Li, Yamin Dong, and Xiaoqi Sun, The development of sustainable yttrium separation process from rare earth enrichments using bifunctional ionic liquid, Sep. Purif. Technol., 2016, vol. 162, pp. 106–113. https://doi.org/10.1016/j.seppur.2016.01.042
Yurasova, O.V., Samieva, D.A., Ivanova, S.N., Ermochenkov, I.M., and Vasilenko, S.A., Extraction of yttrium-subgroup rare earth elements with Aliquat 336, Russ. J. Appl. Chem., 2021, vol. 94, no. 7, pp. 903–911. https://doi.org/10.1134/S1070427221070065
Institute of Rare Earths and Strategic Metals. Prices for Rare Earth Elements in December 2020. https://ru.institut-selteneerden.de/unser-service-2/metall-preise/seltene-erden-preise/.
Val'kov, A.V., Rational technology for the separation of rare earth concentrates, Tsvetn. Met. (Moscow, Russ. Fed.), 2020, no. 2, pp. 43–51. https://doi.org/10.17580/tsm.2020.02.0
Acharya, S. and Nayak, A., Separation of D2EHPA and M2EHPA, Hydrometallurgy, 1988, vol. 19, no. 3, pp. 309–320. https://doi.org/10.1016/0304-386X(88)90037-0
Mikhlin, E.B. and Korpusov, G.V., Extraction of rare earth elements of the cerium subgroup with di-isoamyl ether of methyl-phosphonic acid, Zh. Neorg. Khim., 1965, vol. 10, no. 12, pp. 2787–2795.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by M. Chubarova
About this article
Cite this article
Valkov, A.V., Petrov, V.I. Rational Technology for Separation of Rare-Earth Elements of the Yttrium Group. Russ. J. Non-ferrous Metals 63, 385–391 (2022). https://doi.org/10.3103/S1067821222040125
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1067821222040125