Abstract
The total concentrate of heavy lanthanides, zirconium, scandium, as well as emanating uranium and thorium can be recovered from the eudialyte ore concentrate breakdown solutions with help of 5-(diphenylphosphoryl)hexan-3-one within one technological extraction stage. The method makes it possible to separate the components of rare metal raw materials of various origins and generate concentrates suitable for further processing. The proposed new reagent of a series of phosphoryl ketones can be successfully used in the extraction processing of mineral and technogenic raw materials, enabling extraction and concentration of rare earth metals, as well as to separate them from related impurities, in particular, from radioactive uranium, thorium and their decay products.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
During the processing of mineral raw materials containing rare earth and rare metals (REM and RM), both natural and technogenic origin, low-level radioactive waste (LLW) is formed, mainly due to the presence of emanating uranium and thorium. The requirements of state regulatory agencies provide for the transfer of these LLW for storage with subsequent disposal, which significantly reduces the economic efficiency of the production of concentrates and other products based on RM and REM, as well as the attractiveness of projects to create new manufacturings [1–3]. At the same time, the resulting waste contains valuable components, uranium and thorium, which can be separated and used in the nuclear fuel cycle of nuclear power plants (NPPs). The use of a promising thorium–uranium fuel cycle will significantly expand the fuel resources of the domestic nuclear power industry [4–8]. Thus, the separation of RM and REM, both in the form of concentrates and in individual form, is an urgent radiochemical problem.
Due to the acute scarcity and high cost of RM and REM, it is necessary to consider not only traditional polymetallic raw materials, but also new natural and technogenic sources of these metals as the feedstock for their production. In this regard the mineral eudialyte is of interest, which is a complex silicate of sodium, calcium, and zirconium [9–13]. Eudialyte contains up to 2.5% lanthanides, and it has a high content of metals of the heavy yttrium subgroup, the most valuable, scarce, and demanded by the nuclear power manufacturing, since gadolinium and erbium are widely used as burnable absorbers in NPP fuel elements [14–16]. Eudialyte is widespread in mineral deposits of the Kola Peninsula [12], contains, in addition to REM, valuable uranium, thorium, zirconium, and scandium; furthermore, it is easily broken down by acids even without preliminary activation, which simplifies its processing [13]. The processing of eudialyte for the recovering REM is reported, in particular, in [17–20].
Of the technogenic raw materials containing rare metals, phosphogypsum, a large-tonnage waste from apatite processing, which accounts for most of the REM contained in the rock, is a very important potential source of raw materials [21, 22]. In addition to gypsum itself (calcium sulfate), phosphogypsum also contains significant amounts of residual phosphoric acid, fluorine, iron, aluminum, and strontium. The REM content in phosphogypsum is low (0.3–1%), but its processing is also of interest for the production of high-quality building materials and for solving an important environmental problem caused by the accumulation of huge amounts of phosphogypsum in the dumps of mineral fertilizer and phosphoric acid production. Previously, we studied the extraction of rare earth elements, uranium(VI) and thorium(IV) from phosphogypsum breakdown solutions with a number of known organophosphorus compounds: tributyl phosphate, trioctylphosphine oxide, bis(2-ethylhexyl)phosphoric acid, and also phosphoryl ketone—5-(diphenylphosphoryl)hexan-3-one [23]. It was established that the indicated phosphoryl ketone is the most effective reagent for obtaining the collective concentrate of lanthanides [23].
During processing raw materials containing rare metals in fairly low concentrations, it is especially important to choose the right technology that would allow both the concentration of valuable components and their purification from simultaneously recovered metals. In addition, it is important to provide the possibility of selective separation of individual elements with the required level of purity from concentrates. Liquid extraction utilized in the technology of rare metals does not always meet the entire set of requirements, including the economic feasibility and environmental acceptability of the process [24, 25]. One of the most important ways to control the technological process of hydrometallurgical processing of rare metal raw materials is the choice of an extractant. Traditionally used neutral organophosphorus reagents, in particular, tributyl phosphate, have rather low coefficients of distribution and separation of lanthanides; therefore, there is a need to search for new, more effective, but at the same time simple and inexpensive chemical reagents to solve these problems.
Earlier, the possibility of application of phosphoryl ketones [26–28] for the recovery of REMs of the following structural type was mentioned:

We found that phosphoryl-containing ketones are very effective and selective extractants for the extraction and group separation of f-elements during extraction from model nitric acid solutions into chloroform. Therewith, the corresponding P,P-diphenylphosphoryl alkanones generated from commercial raw materials are the most accessible in terms of synthesis.

The production of an enlarged batch of ligand I was conducted on the basis of a two-stage one-pot process using industrially available diphenylchlorophosphine Ph2PCl as an organophosphorus starting compound (Scheme 1).
At the first stage of the process, this chlorophosphine and the corresponding enone at room temperature in the absence of a solvent form a solid 1 : 1 adduct, which after treatment with absolute ethanol at 0°C turns into a phosphorylated alkanone, 5-(diphenylphosphoryl)-hexan-3-one I. It was isolated from the reaction mixture in a yield close to quantitative by filtration through Al2O3. The composition of I was confirmed by elemental analysis, and its structure was proved by 1Н and 31Р{1H} NMR spectra [23].
The model ligand II was synthesized according to the known method [27].
On the example of phosphoryl ketone II and its isomeric phosphoryl ketone I, it was shown for the first time that a change in the structure of the alkyl part of isomeric phosphoryl ketones can lead to a significant increase in their efficiency and selectivity as extractants.
Such a modification—the transfer of the Me group from the α- to the δ-carbon atom of the P-alkyl substituent containing the C=O fragment—should simultaneously lead to a decrease in steric hindrance in the coordination of the phosphoryl oxygen of the extractant molecule to the metal cation and to an increase in lipophilicity of both the extractant itself and the corresponding extractable complexes. It was shown that during the extraction of f-elements from model nitric acid solutions into chloroform, the extraction ability and selectivity of phosphoryl ketone I is significantly higher than that of its isomeric phosphoryl ketone II, as well as known neutral organophosphorus compounds (NPOCs): tributyl phosphate (TBP), trioctylphosphine oxide (TOPO), and (dibutylcarbamoylmethyl)diphenylphosphine oxide (CMPO). The possibility of using phosphoryl ketone I for the efficient extraction of valuable components (zirconium, scandium, and total heavy REE concentrate) from eudialyte stripping solutions within one technological extraction stage was demonstrated [29].
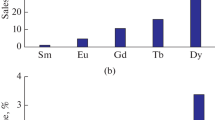
Initially, the positive effect of the above transformation of phosphoryl ketones was revealed in model experiments by the extraction of a number of f-elements from nitric acid solutions into chloroform. It was shown that the efficiency and selectivity of phosphoryl ketone I in the extraction of lanthanides is significantly higher than that of its prototype II and known NPOC : TBP, TOPO, and CMPO (Fig. 1).
At the same time, both phosphoryl ketones do not extract thorium and extract uranium relatively poorly, which is very useful when these compounds are employed to separate f-elements from natural raw materials, since the collective extract of lanthanides will practically not contain impurities of radioactive actinides. The radioactive decay products of thorium and uranium, in particular, radium isotopes, will also not pass into the organic phase during extraction, which at REM isolation will make it possible to remove all radioactive impurities and significantly reduce the cost and simplify the scheme for processing mineral and technogenic raw materials. The possibility of phosphoryl ketone I application for the extraction separation of valuable components from ore material breakdown solutions was studied in experiments simulating a liquid extraction countercurrent cascade.
The object under study was the eudialyte concentrate of the Lovozero Mining and Processing Plant. Before breakdown, we determined the granulometric and elemental composition of the concentrate. The granulometric composition of the concentrate was studied by low-angle laser light scattering (LALLS, laser diffraction) using an Analyzette 22 Compact diffractometer (Fritsch), particle size distribution was calculated with the Fraunhofer theory. The average particle size obtained by the described technique was 120 μm, the sizes of ~90% of the particles are in the range from 30 to 200 μm.
Elemental analysis of an eudialyte concentrate sample from the Lovozero Mining and Processing Plant was performed by non-standard X-ray fluorescence spectrometry with an ARLAdvant’X wave-dispersive XRF spectrometer and UniQuant software. The UniQuant software was utilized without corrections, the results were derived as weight percent without taking into account the oxygen content (Table 1) and in the form of weight percent of individual components relative to the REE amount (Table 2).
The breakdown of the eudialyte concentrate (6 g) was carried out with 6 M H2SO4 under stirring at a temperature of 70°С for 1.5 h. From the obtained sulfuric acid solution, the metals were recovered in the form of hydroxides with NH4OH. Due to the high content of zirconium in the breakdown solutions, precipitation in the form of hydroxides makes it possible to significantly reduce the concentration of ZrO2+ and Zr2O32+ oxo cations in the solution sent for extraction, which adversely affect the process by forming third phases [18]. The resulting precipitate was dissolved in 4 M HNO3 up to 25 mL, then desiliconization was carried out with GKZh-11n (an aqueous solution of sodium methyl siliconate containing at least 5% Si). Extraction was performed from the obtained solution of metal nitrates using solutions of I in chloroform.
We used a scheme that consists in simulating a countercurrent continuous process by periodic 4-fold repetition of interphase contacts according to the cross-flow scheme (Fig. 2).
This scheme makes it possible to generate the concentration profile of the components over the cascade stages and, based on these data, to evaluate the extraction efficiency. When using the proposed simulation scheme, it was assumed that each contact of immiscible phases is equivalent to one ideal (theoretical) stage.
In cell 1, the initial solution is in contact with a fresh portion of the extractant, the extract is discarded, and the raffinate is extracted with a fresh portion of the extractant in cell 2. After that, the extract is fed into cell 1 of the second stage, and the raffinate is fed into cell 3 of the first stage, etc. Extracts flows from top to bottom, while raffinates move from left to right. The phase ratio at the contacts was 1 : 1 and did not change during the entire study.
The top row of operations (Fig. 2) represents four stages of cross-flow extraction (the initial mixture is extracted four times with the same amount of extractant). Accordingly, after these operations, the concentration of metals in the raffinate will be significantly less than the concentration of the final raffinate (after the fourth stage) when modeling a counter-flow cascade (exhaustive extraction). With an increase in the number of operations, the final concentration of metals in the raffinate increases.
The elemental composition of the aqueous phase was analyzed by ICP-MS inductively coupled plasma mass spectrometry by an Agilent 7500ce instrument (Agilent Technologies, USA). The composition of the organic phase was not analyzed.
In the course of the first experiment on the simulation of a counter-flow cascade, the initial aqueous phase was the solution after the eudialyte breakdown performed according to the procedure described above, and the initial organic phase was an 0.01 M solution of I in chloroform. During the second experiment, the raffinate from the first experiment was used as the initial aqueous phase, and a 0.05 M solution of I in chloroform was utilized as the initial organic phase. Stationary concentration profiles for the cascade stages are given in Tables 3 and 4. From the presented data, it can be seen that during extraction with a 0.01 M solution of I, almost complete extraction of one of the macrocomponents of the solution, zirconium, into the organic phase is achieved. Simultaneously, scandium almost completely and approximately half of calcium passes into the organic phase. At the same time, a significant extraction of a number of other macrocomponents: iron, titanium, as well as the total REE, is not detected.
Thus, the preliminary stage of extraction separation, which consists in the release of the process solution from a number of macrocomponents by extracting them into a dilute solution of I, allows effective purifying the solution from only one of the components, zirconium. Nevertheless, this should be recognized as an important result, since the high zirconium content in the technological solution significantly reduces the efficiency of REE extraction and can lead to a number of technological difficulties, for example, to the formation of third phases. The technological stage of purification from zirconium is necessary and can be designed within the framework of an unified hardware/technology design, followed by the stage of group separation of REE. As a result of the second experiment with four theoretical stages of extraction using phosphoryl ketone I at an 0.05 M concentration in chloroform, group separation of lanthanides along the Pr–Sm interface is possible, while light REE, uranium(VI) and thorium(IV), remain predominantly in the aqueous phase, and heavy REE pass into organic. After four stages of the counter-flow cascade, the elements of the La–Sm group, as well as uranium(VI) and thorium(IV), almost completely remain in the aqueous phase, while the heavy lanthanides pass into the organic phase. In this case, Tm and Lu are extracted quantitatively, Eu and Ho, by 95–97%, and Gd, Tb, Dy, Er, and Yb, by 80–90%. The content of Y in the aqueous phase after the second cycle decreases by almost 10 times (from 52 to 5 mg/L); its recovery reaches 88%.
Thus, the proposed new reagent of the class of phosphoryl ketones can be successfully used in the extraction processing of mineral and technogenic raw materials, making it possible to extract and concentrate REM, as well as to separate them from accompanying impurities, in particular, from radioactive uranium, thorium, and their decay products. Within one technological extraction stage it is possible to isolate a separate fraction of zirconium and scandium. In addition, it is possible to separate rare earth metals at the Nd–Sm interface with the production of concentrates containing heavy lanthanides, as well as concentrates of emanating uranium and thorium. This method allows separating components of rare metal raw materials of various origins and producing concentrates suitable for further processing.
REFERENCES
Kryukov, V.A., Yatsenko, V.A., Kryukov, Ya.V., Gornaya prom-st’, 2020, no. 5, pp. 68–84. https://doi.org/10.30686/1609-9192-2020-5-68-84
Kondrat’ev, V.B., Gornaya prom-st’, 2017, no. 4 (134), pp. 48–54.
Nikulin, A.A., Probl. nats. Strategii, 2014, no. 1, pp. 134–152.
Suglobov, D.N., Yakovlev, R.M., Myasoedov, B.F., Radiochemistry, 2007, vol. 49, no. 5, pp. 441–448. https://doi.org/10.1134/S106636220705001
Bobrov, E.A., Teplov, P.S., Gurin, A.V., Andrianova, E.A., Blandinskii, V.Yu., and Grol’, A.V., Vopr. Atom. Nauki i Tekhniki. Seriya: Fizika yader. Reaktorov, 2019, no. 3, pp. 28–38.
Alekseev, P.N., Innovatika Ekspertiza, 2016, no. 3(18), pp. 146–174.
Xiao, S.C., Zhao, J., Heng, X., Sheng, X.Y., Zhou, Z., and Yang, Y., Fusion Sci. Technol., 2015, vol. 68, no. 3, pp. 566–572. https://doi.org/10.13182/FST14-907
Irwanto, D. and Obara, T., J. Nucl. Sci. Technol., 2012, vol. 49, no. 2, pp. 222–229. https://doi.org/10.1080/00223131.2011.649080
Aksenov, S.M., Rastsvetaeva, R.K., Mitchell, R.H., and Chakrabarty, A., Crystallogr. Rep., 2014, vol. 59, no. 2, pp. 146–154. https://doi.org/10.1134/S1063774514020023
Chakrabarty, A., Pruseth, K.L., and Kumar Sen, A., J. Geol. Soc. India, 2011, vol. 77, pp. 12–16. https://doi.org/10.1007/s12594-011-0003-x
Amores-Casals, S., Gonçalves, A.O., Melgarejo, J.-C., and Molist, J.M., Minerals, 2020, vol. 10, no. 1, ID 5. https://doi.org/10.3390/min10010005
Masloboev, V.A. and Lebedev, V.N., Redkozemel’noe syr’e Kol’skogo poluostrova i problemy ego kompleksnoi pererabotki (Rare Earth Raw Materials of the Kola Peninsula and Problems of Its Complex Processing), Apatity: Izd-vo KNTs AN SSSR, 1991.
Zakharov, V.I., Skiba, G.S., Solov’ev, A.V., Lebedev, V.N., and Maiorov, D.V., Tsvet. Metally, 2011, no. 11, pp. 25–29.
Kaz’min, D.N. and Yakubenko, I.A., Global’naya Yader. Bezopasnost’, 2013, no. 4 (9), pp. 53–57.
Lysikov, A.V., Kuleshov, A.V., Samokhvalov, A.N. Patent RU 2362223.
Novikov, V.V., Bibilashvili, Yu.K., Mikheev, E.H., Grachev, A.F., Kalygin, V.V., Ovchinnikov, V.A., and Kobylyanskii, G.P., Atomic Energy, 2008, vol. 105, no. 4, pp. 262–269. https://doi.org/10.1007/s10512-009-9095-4
Dibrov, I.A., Chirkst, D.E., and Matveeva, T.E., Tsvet. Metally, 2002, no. 12, pp. 38–41.
Matveev, V.A., Maiorov, D.V., and Solov’ev, A.V., Tsvet. Metally, 2018, no. 1, pp. 5–28. https://doi.org/10.17580/tsm.2018.01.02
Lebedev, V.N. and Rudenko, A.V., Khim. Tekhnologiya, 2002, no. 12, pp. 27–30.
Lebedev, V.N., Russ. J. Appl. Chem., 2003, vol. 76, no. 10, pp. 1559–1563. https://doi.org/10.1023/B:RJAC.0000015712.10513.94
Mukaba J.-L., Eze, C.P., Pereao, O., and Petrik, L.F., Minerals, 2021, vol. 11, ID 1051. https://doi.org/10.3390/min11101051
Kosynkin, V.D., Selivanovskii, A.K., Fedulova, T.T., Smirnov, K.M., and Krylova, O.K., Tsvet. Metally, 2012, no. 3, pp. 31–34.
Safiulina, A.M., Matveeva, A.G., Evtushenko, A.V., Lizunov, A.V., Goryunov, E.I., Goryunova, I.B., Bodrin, G.V., Semenov, A.A., and Brel, V.K., Russ. J. Gen. Chem., 2015, vol. 85, no. 9, pp. 2128–2134. https://doi.org/10.1134/S1070363215090170
Treibal, R., Zhidkostnaya ekstraktsiya (Liquid Extracton), Moscow: Khimiya, 1966.
Al’ders, L., Zhidkostnaya ekstraktsiya (Liquid Extracton), Moscow: Izd. Inostr. Lit., 1962.
Safiulina, A.M., Matveeva, A.G., Dvoryanchikova, T.K., Sinegribova, O.A., Tu, A.M., Tatarinov, D.A., Kostin, A.A., Mironov, V.F., and Tananaev, I.G., Russ. Chem. Bull., 2012, vol. 61, no. 2, pp. 392–398. https://doi.org/10.1007/s11172-012-0055-0
Tatarinov, D.A., Mironov, V.F., Kostin, A.A., Baronova, T.A., and Buzykin, B.I., Russ. J. Gen. Chem., 2010, vol. 80, no. 7, pp. 1211–1213. https://doi.org/10.1134/S1070363210070297
Matveeva, A.G., Thu, A.M., Safiulina, A.M., Bodrin, G.V., Goryunov, E.I., Goryunova, I.B., Sinegribova, O.A., and Nifant’ev, E.E., Russ. Chem. Bull., 2013, vol. 62, no. 6, pp. 1309–1316. https://doi.org/10.1007/s11172-013-0184-0
Safiulina, A.M., Matveeva, A.G., Lizunov, A.V., Bodrin, G.V., Goryunov, E.I., Grigor’ev, M.S., Semenov, A.A., Brel, V.K., and Nifant’ev, E.E., Dokl. Chem., 2015, vol. 460, pt. 2, pp. 57–60. https://doi.org/10.1134/S001250081502007X
Funding
The work was carried out within the framework of State Assignment no. 075-00697-22-00 of the Ministry of Science and Higher Education of the Russian Federation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest.
Additional information
Translated from Radiokhimiya, No. 6, pp. 547–553, December, 2022 https://doi.org/10.31857/S0033831122060077
Rights and permissions
About this article
Cite this article
Safiulina, A.M., Semenov, A.A., Lizunov, A.V. et al. Recovery and Separation of Rare Metals during the Processing of Eudialyte Concentrate with New Reagents of a Series of Phosphoryl Ketones. Radiochemistry 64, 713–720 (2022). https://doi.org/10.1134/S1066362222060078
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1066362222060078