Abstract
In view of the shortcomings of traditional comprehensive utilization of high iron bauxite resources to produce alumina, such as high energy consumption and long process flow, this paper proposed a double-cycle process for alumina production. As a main component in high-iron bauxite, iron(Fe) has adverse effect on the recovery of aluminum. Removal of iron is essential for recovery of aluminum. Herein, this work reported an approach for efficient removal of iron from acid leaching liquor of high-iron bauxite. The extraction system of TBP and benzene was used to separate iron and aluminum ions from the acid leaching solution. The optimum extraction conditions were obtained as follows: the concentration of TBP was 30%, the extraction time was controlled for 3–4 min, the volume ratio of organic phase to aqueous phase (O/A ratio) was 1 : 1, and the concentration of hydrochloric acid was 1.5 mol/L at room temperature.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 INTRODUCTION
As an important industrial application material, alumina is mostly produced by Bayer process for the moment [1]. Because of the high-grade requirement of bauxite by Bayer process and the decreasing of global high-quality bauxite resources year by year, it is urgent to develop new non-traditional bauxite resources and propose a new feasible production process [2–4]. Among much non-traditional bauxite resources, high-iron bauxite resources have attracted widespread attention from academia and industry circles because of its large reserves and high useful components [5–10].
As the content of iron and aluminum in high-iron bauxite resources do not meet the industrial application requirements of single iron ore and bauxite, the development and utilization must be based on the recovery of iron and aluminum at the same time, and the separation of aluminum and iron is the first step for its comprehensive utilization [11–13]. There are two main methods to produce alumina from the high-iron bauxite (iron-aluminum symbiotic ore) resources. One is to produce alumina directly by using the Bayer process, and the other is to extract aluminum and iron from minerals step by step [14, 15]. In the process of using Bayer method to produce alumina, the iron element in high-iron bauxite completely enters into the red mud, so the excessive iron content will lead to the dissolution of red mud much higher than the same grade of low iron bauxite [16, 17]. The iron element in the red mud mainly exists in the form of weaker magnetic minerals such as goethite and hematite, which increases the difficulty of iron separation process in red mud. At present, there are many researches in this field of alumina in China and abroad, but the common problem that has been industrialized or studied in iron selection technology is the low iron recovery rate, mostly between 20 and 30%. According to the different sequence of alumina production and reduction iron-making process, the reduction smelting and sintering combined method can be divided into two ways, such as extracting iron before aluminum and extracting aluminum before iron. The greatest advantage of these methods is that it can extract the two main elements in high-iron bauxite, but the main disadvantage is that the process is longer, and the process energy consumption is higher, and the interaction between sintering process and smelting process will also lead to be difficult for controlling the production process. So it is difficult to be applied in the industrial production.
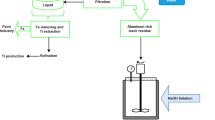
For the high-iron bauxite with aluminum silicon ratio close to 2, if extracted by Bayer method, the theoretical dissolution rate of alumina is only 50%. With the development of new materials and corrosion resistance technology. Acid method has greater advantages than alkali method in comprehensive utilization of valuable elements and energy consumption in treating with non-traditional bauxite resources. According to the characteristics of non-traditional bauxite resources and the problems in the comprehensive utilization methods of non-traditional bauxite resources, this study proposes a double-cycle process with the development of new material technology and anti-corrosion technology [18]. The double-cycle process is pressure leaching of hydrochloric acid—extraction and separation of leaching solution—direct pyrolysis of aluminum chloride solution for producing alumina. Firstly, high-iron bauxite is used as raw material to extract aluminum and iron elements in it by hydrochloric acid leaching. After leaching, the aluminum (and iron and other metal elements) in the minerals enters the leaching solution in the form of chloride. Secondly, the leaching solution is extracted and separated. After extraction, the aluminum ions exist in the aqueous phase. The organic phase is recycled after water reverse extraction. Metal ions and iron ions enter into the aqueous phase during the reverse extraction process to form a mixed solution with ferric chloride as the main component. Finally, the separated aluminum chloride and ferric chloride solutions are pyrolyzed directly to obtain alumina and ferric oxide as the main components. The HCl gas produced by pyrolysis is recycled. The leaching residue is mainly composed of silica, which can be treated with microwave to prepare silicon carbide. Compared with the existing alumina production technology using non-traditional bauxite resources, this method has the following advantages. The leaching rate of each valuable metal element in minerals and the utilization rate of valuable resources are high. Alumina and iron oxide are obtained by direct pyrolysis of aluminum chloride and ferric chloride solution. The process flow of the new method is simple and energy consumption is low. The acid and water used in the system are basically recycled to achieve zero discharge, while the main component of leaching slag is silicon oxide which can be directly used to produce products such as silicon carbide to achieve slag-free discharge. It is an ecological alumina production method which meets the requirements of environmental protection.
In this paper, the separation of aluminum and iron from hydrochloric acid leaching solution was studied by solvent extraction. Compared with the traditional methods such as goethite, hematite and jarosite, there are many problems in removing iron from solution, such as complicated operation, large loss of valuable metals in iron slag, difficulty in recovery of precipitated iron slag and it is easy to cause secondary pollution [19]. Iron removal by solvent extraction has the advantages of high selectivity, less pollution and low energy consumption, which can better solve the problems in traditional methods.
Acidic organophosphorus esters, amine extractants, carboxylic acid extractants and neutral organophosphorus reagents have been studied in iron separation. The acidic organophosphorus ester P204 [20, 21] is a common reagent for iron extraction and is very effective for Fe(III) extraction, but the biggest disadvantage is difficult to trip Fe(III) from organic phase. Amine extractants have higher extraction rate and better selectivity when extracting iron. Compared with phosphates, amine systems with iron are easier to be stripped [22, 23]. The carboxylic acid extractants have the advantages of stable chemical performance, low price, high selectivity, etc. However, the pH needs to be strictly controlled when extracting Fe(III) from acid liquid, and it is difficult to strip Fe(III) from organic phase [24, 25]. The solvent extraction system usually studied is composed of neutral organic phosphorus and concentrated hydrochloric acid. Mishra et al. [25] reported the extraction and stripping efficiency of the extractants for iron in the order TBP < Cyanex921 < Cyanex923 and Cyanex923 < TBP < Cyanex921. The extracted species appeared to be H2FeCl4·2TBP/H2FeCl4·2Cyanex921/H2FeCl4·2Cyanex923. The mixture of 70% (v/v) TBP and 30% (v/v) MIBK [26–29] has been extensively analyzed because of its faster phase separation and efficient extraction capacity. Aiming at the effective separation of iron and aluminum, this paper intends to study the process conditions for extracting and removing iron from the mixed chloride solution of iron and aluminum.
2 EXPERIMENTAL REAGENTS AND PROCEDURES
2.1 Experimental Reagents
Tributyl phosphate (TBP) with analytical grade is produced by Sinopharm Chemical Reagent Co., Ltd. Its molecular formula is: \({{{\text{C}}}_{{{\text{12}}}}}{{{\text{H}}}_{{{\text{27}}}}}{{{\text{O}}}_{{\text{4}}}}{\text{P}}\). At 25.4°C, its relative density is 0.979 g/cm3. Its water solubility is 0.6 g/100 mL. Its molar mass is 266.32 g/mol. Its refractive index is 1.420. And it is a neutral organic solvent. Its structural formula is:

Benzene (analytical grade) was purchased from Shenyang Reagent No. 5 Factory. Kerosene and hydrochloric acid both were purchased from Shenyang Economic Development Zone Reagent Factory. AlCl3 and FeCl3·6H2O both were purchased from Shenyang Licheng Reagent Factory. The initial concentration of aluminum ions in staring solution was about 28 g/L, and the concentration of iron ions was about 23 g/L, which was consistent with previous acid leaching research results, by dissolving AlCl3 and FeCl3·6H2O in deionized water.
2.2 Experimental Procedures
All experiments were carried out by filling a 100 mL globe-shaped funnel with 20 mL aqueous phase and other volumes of organic phase at a constant temperature (Removal of the experiment of temperature studied on extraction effect) for predetermined periods of time. The different proportions of extractants and the effects of experimental parameters on the extents of Fe3+ removal and stripping were studied. Liquid separation was carried out when the organic phase and aqueous phase were clearly separated. The concentrations of Fe3+ and Al3+ in the extract liquid were determined by High Pispersion ICP full spectrum direct-reading plasma emission spectrometer via the dilution and volume determination process. And the extraction rate and distribution ratio were calculated by subtraction method.
3 PRINCIPLE OF SOLVENT EXTRACTION AND RELATED CALCULATION FORMULA
3.1 Principle of Solvent Extraction
Tributyl phosphate (TBP) is a neutral phosphate extractant. Its molecular formula is (C4H9)3PO4. It is a colorless transparent liquid with high chemical stability and does not interact with acids and alkali. TBP was neutral during extraction. It forms a neutral extraction complex with metal atoms through the chlorine atoms of the bond. In hydrochloric acid medium, the following reactions occur in the extraction of iron by TBP [30–32]:
The partition coefficient of Fe3+ is DFe,
If Cl–, H+ remain unchanged, taking logarithm,
Drawing log DFe ~ log(TBP), as shown in Fig. 1.
The slope of the straight line is x, and the slope of the straight line is obtained from Fig. 1 which is 2.01, so x equals 2. So the extraction mechanism is as follows:
The stability of the extraction complex in the extracting process is related to the acidity of the solution and the concentration of Cl–. From the extraction reaction formula, the distribution ratio of iron ion is proportional to the fourth power of chloride concentration. But in the actual extraction process, the effect of Cl– concentration is more complex. The main reason is Fe3+ can form a variety of complexes with Cl–, such as \({{{\text{(FeC}}{{{\text{l}}}_{{\text{2}}}}{\text{)}}}^{ + }}\), \({\text{(FeC}}{{{\text{l}}}_{{\text{3}}}}{\text{)}}\), \({{{\text{(FeC}}{{{\text{l}}}_{{\text{4}}}}{\text{)}}}^{ - }}\).
The above reaction is reversible. The extraction effect depends on the acidity of hydrochloric acid in the system, the phase ratio and the medium, etc. Increasing the acidity of the solution will facilitate the extraction; when acidity is reduced, the iron will be stripped back to the aqueous phase.
3.2 Related Calculation Formula
In practical application, the extraction rate is a widely used parameter. When extract A reaches equilibrium in two incompatible phases, the extraction rate can be expressed as follows:
Where A1 is the total content of the extract in phase 1 and A2 is the total content of the extract in phase 2.
When the extract A reaches equilibrium in the two phase which are incompatible with each other, the ratio of the total concentration of A in phase 1 A1 to the total concentration of A2 in phase 2, called the distribution ratio, is expressed in D.
Where A1 is the total concentration of all the various chemical forms of solute A in phase 1, and A2 is the total concentration of all the various chemical forms of A in phase 2. D is a very important characteristic parameter in the study of extraction system. With the increase of D, extractive A is easier to enter phase 1. The value of D is related to the concentration of extract A, acidity of aqueous phase, concentration of organic phase extractant and other factors.
The extraction separation factor can be expressed as:
Where A1, A2, B1 and B2 are the equilibrium concentrations of substance A and substance B in phase 1 and phase 2, respectively. If β is greater than 1, the greater the β value, the easier the separation degree of the two substances. When β is less than 1, then the smaller the β value, the greater the separation degree of the two substances, and if equals 1, the two substances cannot be separated.
4 RESULTS AND DISCUSSION
From the above extraction principle, we can know that TBP system can extract and remove iron from acid medium. In the next step, TBP system is used for extraction and iron removal experiment.
In the experiment using TBP as the extractant, it is easy to have difficulty in stratification during the extraction process. Because the density of pure TBP is about 0.98 g/cm3, which is close to water. Therefore, benzene is used as the extraction diluent for the subsequent experiments.
4.1 Effect of TBP Concentration on Extraction Efficiency
In order to investigate the extraction effect of TBP + benzene system on iron, the effect of different TBP concentrations on the extraction performance of Fe3+ was studied.
The extraction experiments for investigating the effect of different TBP concentrations were carried out with the following conditions: The extraction temperature was 25°C, the initial aqueous phase was 20 mL, the hydrochloric acid concentration was 1.5 mol/L, the shaking time was 10 min, the concentration of aluminum ions in the initial aqueous phase was about 28 g/L, and the concentration of iron ions was about 23 g/L. The effect of TBP concentration on the extraction effect was shown in Fig. 2. Under the experimental conditions, TBP did not extract Al3+ from hydrochloric acid solution basically, and the corresponding distribution ratio was less than 0.1.
With tributyl phosphate as extractant, the extraction rate of Fe3+ decreased continuously with the increase of diluent under different O/A ratio, but the diluent could reduce the viscosity of tributyl phosphate, eliminate the emulsification in the extraction process, accelerate the oil-water stratified speed, and facilitate the extraction operation. Moreover, benzene and tributyl phosphate were completely miscible, it could be recycled for extraction after removing Fe3+ from the extraction phase by back extraction. As could be seen from Fig. 2, when O/A ratio was 1 and the TBP concentration was 30%, the extraction rate of Fe3+ reached more than 94%. If the concentration of tributyl phosphate was continuously increased, the extraction rate of iron was not significantly improved. Therefore, tributyl phosphate (containing diluent benzene) was used as extractant for subsequent experiments, and the TBP concentration was selected 30%.
4.2 Effect of Phase Ratio on Extraction Efficiency
The extraction experiments for investigating the effect of phase ratio were carried out with the following conditions: The extractant was 30% TBP-70% benzene, the extraction temperature was 25°C, the initial aqueous phase was 20 mL, the hydrochloric acid concentration was 1.5 mol/L, the shaking time was 10 min, the initial concentration of aluminum ions in aqueous phase was about 28 g/L, and the concentration of iron ions was about 23 g/L. The effect of phase ratio on the extraction effect was shown in Figs. 3 and 4.
From Figs. 3 to 4, it could be seen that with the increase of extraction phase ratio O/A, the extraction rate of Fe3+ increased gradually, but with the further increase of O/A, the increase of extraction rate was not obvious, and the load of subsequent extractant recovery was greatly increased. When O/A ratio ≥ 1, the extraction rate of Fe3+ reached about 95% while the extraction rate of Al3+ remained basically unchanged, less than 1%. The distribution ratio of iron was much larger than that of aluminum, which indicated that iron and aluminum could be separated well. At O/A ratio of 1 : 1, the distribution ratio of iron and the separation factor also reached the maximum. It could be seen that when O/A ratio was 1 : 1, it was conducive to the separation of iron and aluminum from leaching solution. Therefore, for single-stage extraction experiments, the extraction phase ratio O/A could be controlled at 1 : 1.
4.3 Effect of Aqueous Acid Concentration on Extraction Efficiency
The aqueous acid concentration has a significant influence on the extraction process. Therefore, a series of experiments were carried out with the following conditions: the volume ratio of organic phase to aqueous phase(O/A) equaled 1 : 1, the extractant was 30% TBP–70% benzene, the extraction temperature was 25°C, the initial aqueous phase was 20 mL, shaking time was 10 min, the initial concentration of aluminum ions in aqueous phase was about 28 g/L, and the concentration of iron ions was about 23 g/L. The effect of the aqueous phase concentration on the extraction effect was shown in Figs. 5 and 6.
Figure 5 showed that the extraction rate of Fe3+ increases almost linearly with the increase of hydrochloric acid concentration, while the extraction rate of Al3+ did not change significantly. When the concentration of hydrochloric acid was less than 0.5 mol/L, the extraction rate of Fe3+ was lower, below 60%. However, when the concentration of hydrochloric acid was more than 1.5 mol/L, the extraction rate of Fe3+ was more than 95%, and basically tended to be stable. This was because the free Fe3+ was difficult to be extracted by the solvent, the more hydrochloric acid was added, the more Fe3+ was complexed, the higher the extraction rate. The experiment proved that hydrochloric acid not only had strong complexation ability with Fe3+, but also the residual hydrochloric acid in the extraction phase could be recovered by simple distillation method and recycled.
As shown in Fig. 6, with the increasing of aqueous acid concentration, the iron distribution ratio and separation factor of iron and aluminum increased gradually while the aluminum distribution ratio was very small and had no obvious change, so it could be seen that iron and aluminum could be separated well. When the concentration of hydrochloric acid was 1.5 mol/L, the separation factor of iron and aluminum reached the maximum value of 340, iron and aluminum could achieve effective separation.
According to the experimental results, the concentration of hydrochloric acid of 1.5 mol/L was selected for subsequent experiments.
4.4 Effect of Extraction Time on Extraction Efficiency
The extraction experiments for investigating the effect of the extraction time were carried out with the following conditions: The concentration of HCl in aqueous phase was 1.5 mol/L, volume ratio of organic phase to aqueous phase O/A equaled 1 : 1, the extractant was 30% TBP–70% benzene, the extraction temperature was 25°C, the initial aqueous phase was 20 mL, the initial concentration of aluminum ions in aqueous phase was about 28 g/L, and the concentration of iron ions was about 23 g/L. The effect of extraction time on the extraction effect was shown in Figs. 7 and 8.
It could be seen from Fig. 7 that the mass transfer rate of Fe3+ extracted by Tributyl phosphate was very fast, and it took only 4 min to reach the chemical equilibrium, at which time the extraction rate had reached 95%.
As observed in Fig. 8, with the increasing of extraction time, the iron distribution ratio increased gradually and tended to be stable after 4 min, while the aluminum distribution ratio was small and did not change significantly. And the iron-aluminum separation factor increased at first and then decreased, reaching a maximum of 264 at 4 min.
In actual industrial production, the speed of extraction reaction represented the time of the whole production process, and shortening the reaction time was equivalent to bring greater economic benefits. In the case of treating equal amount of raw materials, the improvement of reaction speed could not only reduce equipment investment, but also reduced operating costs and equipment energy consumption, and ultimately reduced production costs.
4.5 Effect of Temperature on Extraction Efficiency
The extraction experiments for investigating the effect of temperature were carried out with the following conditions: concentration of hydrochloric acid was 1.5 mol/L, volume ratio of organic phase to aqueous phase(O/A) equaled 1 : 1, the extractant was 30% TBP-70% benzene, the initial concentration of aluminum ion was about 28 g/L, concentration of iron ion was about 23 g/L. The effect of temperature on the extraction effect was shown in Figs. 9 and 10.
It could be seen from Fig. 9 that the temperature had little effect on the extraction rate of Fe3+ and Al3+. When the temperature increased from 25 to 55°C, the extraction rate of Fe3+ increased from 94.85 to 96.12%, with little change.
As shown in Fig. 10, with the increase of temperature, the distribution ratio of iron and aluminum gradually increased, but the separation factor of iron and aluminum decreased almost in a straight line. Obviously, the increase of temperature was not conducive to the separation of iron and aluminum. Because the temperature was too high, TBP was easy to be oxidized. Therefore, the room temperature was selected for subsequent experiments.
4.6 Stripping Experimental Results
The organic phase recovery method of this study was to use deionized water, dilute hydrochloric acid and sodium hydroxide as stripping agent. Iron ions in the extraction phase were separated by stripping method while the extractant was recycled. The effects of water, dilute hydrochloric acid and sodium hydroxide aqueous solution on the stripping effect were investigated. The results showed that the performance of several stripping reagent for recovering organic phase reagent was basically the same. Considering the cost, deionized water was the suitable stripping reagent because it was cheap and easy to obtain. When the initial organic phase volume was 20 mL, the shaking time was 5 min, O/A ratio was 1 : 1, the stripping rate of iron was more than 95%.
5 CONCLUSIONS
This study investigates extraction and separation of iron and aluminum from hydrochloric acid solution containing iron and aluminum by TBP extraction system. The effects of TBP concentration, extraction time, aqueous acid concentration and O/A ratio on the separation performance of iron and aluminum were investigated. The following conclusions can be drawn:
(1) The extraction rate can reach more than 95% when the TBP concentration was 30% in the TBP–benzene system.
(2) Because the mass transfer process of Fe3+ extraction with tributyl phosphate is very fast, the extraction time should be controlled within 3–4 min.
(3) Compared with the effect of O/A on extraction efficiency, it has a certain saturation effect on extraction rate of Fe3+: when O/A ≥ 1, the extraction rate of Fe3+ reaches about 95%. If the O/A ratio is further increased, the extraction rate will no longer increase. Therefore, for single-stage extraction, the extraction ratio O/A can be controlled at 1 : 1.
(4) The extraction rate of Fe3+ increases almost linearly with the increasing of hydrochloric acid concentration. When the concentration of hydrochloric acid is less than 0.5 mol/L, the extraction rate is lower, below 60%. However, when the concentration of hydrochloric acid is more than 1.5 mol/L, the extraction rate of Fe3+ is more than 95%, and basically tends to be stable.
(5) The extraction rate of iron does not change significantly with the temperature increasing from 25 to 55°C, so the experiment is carried out at room temperature.
REFERENCES
Cardenia, C., Balomenos, E., and Panias, D., Iron recovery from bauxite residue through reductive roasting and wet magnetic separation, J. Sustainable Metall., 2019, vol. 5, pp. 9–19. https://doi.org/10.1007/s40831-018-0181-5
Zhang, W.G., Zhang, T.A., Lv, G.Z., Zhang, X.H., Zhu, X.F., Wang, Y.X., and Wang, L., Study on effective extraction of Al and Fe from high-iron bauxite through “calcification-carbonization” method, in Light Metals, Springer, 2016, pp. 15–18.
Lyu, G.Z., Zhang, T.A., Zhao, A.C., and Wang, H.X., Effects of acid-leaching and extraction on separation characteristic of valuable elements from high-iron bauxite, J. Northeast. Univ., Nat. Sci., 2013, vol. 34, pp. 1442–1445.
Zhang, Z.M., Lu, G.Z., Chen, Y.C., Zhang, T.A., Chao, X., Chen, Y., Wang, Y.X., and Liu, Y., Alumina extraction from kaolinite via calcification-carbonation process, Russ. J. Non-Ferrous Met., 2020, vol. 61, pp. 248–256.
Zhang, W.G., Zhang, T.A., Lyu, G.Z., and Guo, F.F., Dissociation behavior of Al–Fe in the “calcification-carbonization” process for high-iron bauxite, J. Northeast. Univ., Nat. Sci., 2018, vol. 39, pp. 351–356.
Wu, Y., Bai, H., Xin, H.X., and Xu, Y.J., Study on separation of iron and aluminum of high iron bauxite by reduction roasting, Light Metals, Springer, 2019, vol. 12, pp. 15–19. https://doi.org/10.13662/j.cnki.qjs.2019.12.004
Tian, D., Shen, X.Y., Zhai, Y.C., Xiao, P., and Webley, P., Extraction of iron and aluminum from high-iron bauxite by ammonium sulfate roasting and water leaching, J. Iron Steel Res. Int., 2019, vol. 26, pp. 578–584. https://doi.org/10.1007/s42243-018-0128-x
Zhang, L.H., Rational development and utilization of resources to realize sustainable development, Nonferrous Met. (Beijing, China), 2009, vol. 5, pp. 525–529.
Chen, Q., Guan, H.Q., and Xiong, H., Development and utilization of bauxite and alumina resources in the world, Min. Investment, 2007, vol. 1, pp. 27–33.
Meyer, F.M., Availability of bauxite reserves, Nat. Resour. Res., 2004, vol. 13, pp.161–172. https://doi.org/10.1023/B:NARR.0000046918.50121.2e
Xu, B. and Li, S.J., Research status of separation of aluminum and iron from high-ferric bauxite, Min. Eng., 2014, vol. 12, pp. 17–20. https://doi.org/10.16672/j.cnki.kygc.2014.02.006
Li, G.H., Dong, H.G., Xiao, C.M., Fan, X.H., Guo, Y.F., and Jiang, T., Mineralogy and separation of aluminum and iron from high ferrous bauxite, J. Cent. South Univ. (Sci. Technol.), 2006, vol. 37, pp. 235–240.
Yuan, S., Xiao, H.X., Yu, T.Y., Li, Y.J., and Gao, P., Enhanced removal of iron minerals from high-iron bauxite with advanced roasting technology for enrichment of aluminum, Powder Technol., 2020, vol. 372, pp. 1–7. https://doi.org/10.1016/j.powtec.2020.05.112
Papassiopi, N., Vaxevanidou, K., and Paspaliaris, I., Effectiveness of iron reducing bacteria for the removal of iron from bauxite ores, Miner. Eng., 2010, vol. 23, pp. 25–31. https://doi.org/10.1016/j.mineng.2009.09.005
Anand, P.A., Modak, J.M., Natarajan, K.A., Biobeneficiation of bauxite using bacillus polymyxa: calcium and iron removal, Int. J. Miner. Process., 1996, vol. 48, pp. 51–60. https://doi.org/10.1016/s0301-7516(96)00013-0
Kuys, K., Ralston, J., and Smart, R., Surface characterization, iron removal and enrichment of bauxite ultrafines, Miner. Eng., 1990, vol. 3, pp. 421–435. https://doi.org/10.1016/0892-6875(90)90036-B
Zwingmann, N., Jones, A.J., Dye, S., Swash, P.M., Gilkes, R.J., et al., A method to concentrate boehmite in bauxite by dissolution of gibbsite and iron oxides, Hydrometallurgy, 2009, vol. 97, pp. 80–85. https://doi.org/10.1016/j.hydromet.2009.01.006
Zhao, A.C., Basic Study on Bauxite with High Iron Content by Acid Process, Shenyang: Northeastern Univ., 2013.
Chen, J.Y., Yu, S.Q., and Wu, Z.C., Separation and Utilization of Iron in Hydrometallurgy, Beijing: Metallurgical Industry Press, 1990.
Sheng, W., Wang, Y., and Fu, X., Extraction separation of Fe(III) from crude Al2(SO4)3 by extractant P538, Chin. J. Appl. Chem., 2002, vol. 19, pp. 464–467.
Ma, Y.Q., Wang, X.W., Wang, M.Y., Jiang, C.J., Xiang, X.Y., and Zhang, X.L., Separation of V(IV) and Fe(III) from the acid leach solution of stone coal by D2EHPA/TBP, Hydrometallurgy, 2015, vol. 153, pp. 38–45. https://doi.org/10.1016/j.hydromet.2015.02.003
Luo, F., Li, D., and Wei, P., Synergistic extraction of zinc(II) and cadmium(II) with mixtures of primary amine N1923 and neutral organophosphorus derivatives, Hydrometallurgy, 2004, vol. 73, pp. 31–40. https://doi.org/10.1016/j.hydromet.2003.07.006
Li, M.Y., He, Z.M., and Zhou, L., Removal of iron from industrial grade aluminum sulfate by primary amine extraction system, Hydrometallurgy, 2011, vol. 106, pp. 170–174. https://doi.org/10.1016/j.hydromet.2010.12.018
Van der Zeeuw, A.J., Metals extraction with carboxylic acids em dash 1. Composition of complexes with nickel, cobalt(II) and Fe(III), Hydrometallurgy, 1979, vol. 4, pp. 21–37.
Mishra, R.K., Rout, P.C., Sarangi, K., and Nathsarma, K.C., A comparative study on extraction of Fe(III) from chloride leach liquor using TBP, Cyanex 921 and Cyanex 923, Hydrometallurgy, 2010, vol. 104, pp. 298–303. https://doi.org/10.1016/j.hydromet.2010.07.003
Reddy, B.R. and Sarma, P.V.R.B., Extraction of iron(III) at macro-level concentrations using TBP, MIBK and their mixtures, Hydrometallurgy, 1996, vol. 43, pp. 299–306. https://doi.org/10.1016/0304-386X(95)00117-Y
Saji, J. and Reddy, M.L.P., Liquid–liquid extraction separation of iron(III) from titania wastes using TBP–MIBK mixed solvent system, Hydrometallurgy, 2001, vol. 61, pp. 81–87. https://doi.org/10.1016/S0304-386X(01)00146-3
Sarangi, K., et al., Separation of iron(III), copper(II) and zinc(II) from a mixed sulphate/chloride solution using TBP, LIX 84I and Cyanex 923, Sep. Purif. Technol., 2007, vol. 55, pp. 44–49. https://doi.org/10.1016/j.seppur.2006.10.021
Mehdi, I., Zahra, A., and Hossein, K.H., Solvent extraction of copper using TBP, D2EHPA and MIBK, Russ. J. Non-Ferrous Met., 2018, vol. 59, pp. 605–611.
Wu, Z.L., Removal of iron by TBP extraction in hydrochloric acid system, Nonferrous Met. (Smelting Part), 1987, vol. 4, pp. 37–39.
Yi, X.T., Huo, G.S., and Tang, W., Removal of Fe(III) from Ni–Co–Fe chloride solutions using solvent extraction with TBP, Hydrometallurgy, 2020, vol. 192, pp. 1–6. https://doi.org/10.1016/j.hydromet.2020.105265
Lee, M., Lee, G., and Sohn, K.Y., Solvent extraction equilibria of FeCl3 with TBP, Mater. Trans., 2004, vol. 4, pp. 1859–1863. https://doi.org/10.2320/matertrans.45.1859
ACKNOWLEDGMENTS
The present study was financially supported by the National Natural Science Foundation of China (no. U1710257), Scientific and Technological Innovation Programs of Higher Education Institutions in Shanxi (no. 2019L0656) and Doctoral Research Foundation of Taiyuan University of Science and Technology, China (no. 20142001).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflicts of interest.
About this article
Cite this article
Aichun Zhao, Ting’an Zhang Study on Extraction and Separation Performance of Iron and Aluminum from Acid Leaching Solution of High-Iron Bauxite. Russ. J. Non-ferrous Metals 63, 37–45 (2022). https://doi.org/10.3103/S1067821222010023
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1067821222010023