Abstract
High iron content is one of the challenges in utilizing the refractory bauxites in China. An improved method for treating the high-iron bauxite by roasting with (NH4)2SO4 was proposed, which offers a possible alternative method for utilizing the high-iron bauxite. The influences of the roasting time, roasting temperature, material ratio, and ore particle size on the extraction ratios of Fe and Al were studied, and the orthogonal test was used to optimize the reaction conditions. The optimized reaction conditions were proposed as follows: roasting temperature of 450 °C, roasting time of 120 min, material ratio of (NH4)2SO4 to ore of 2.5:1.0, and ore particle size below 80 μm. The roasting mechanism and kinetic parameters including the apparent activation energy and reaction rate constant were investigated. The results showed that the control step of the roasting process was the internal diffusion on the product layer and the apparent activation energy was 19.22 kJ mol−1 in the reaction temperature range. The kinetic equation was obtained finally.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Due to the rapid development of industrialization and urbanization, the demand for aluminum is growing exponentially [1]. As the main primary raw material, bauxite supplies more than 90% of material resources [2, 3]. China is the top producer and consumer of aluminum worldwide, and accounts for approximately 40% of the world’s aluminum production. However, the bauxite resources that can be utilized by the current industrial technology to produce Al2O3 are limited by its low commercial value [4,5,6,7]. The dependence on bauxite import is becoming more and more intensified.
Fortunately, the reserves of high-iron diasporic bauxite are abundant in China, such as in Guigang, Guangxi, China, where the high-iron bauxite is estimated to be more than 1.6 billion ton [8, 9]. The bauxite ores are mainly composed of aluminum with middle-high proportion, silicon with middle-low proportion and iron with high proportion [1, 5]. For a long time, attention is focused on the utilization of iron and aluminum from high-iron bauxite [2, 8]. However, there is no a technically feasible and economically reasonable countermeasure to deal with high-iron bauxite because of its complex structure and interwoven particles [10,11,12,13]. So far, most of high-iron bauxite ores have not been underutilized. Studies related to the effective, clean, and comprehensive utilization of high-iron bauxite ores are necessary and of great practical significance.
An improved process of roasting high-iron bauxite ore with ammonium sulfate was proposed for the effective utilization of high-iron bauxite ore. Iron and aluminum in ore were transformed into soluble sulfate and then separated from SiO2 by leaching and filtration. The excessive (NH4)2SO4 was recrystallized from SO3, NH3, and H2O, and the additional NH3 gas was absorbed by dilute H2SO4 to form (NH4)2SO4, or absorbed by carbonic acid to form NH4HCO3, which was used to precipitate Fe and Al. Iron and aluminum were selectively precipitated by adjusting the pH value of the solution based on the different chemical properties. Iron and aluminum were extracted and used, while ammonium sulfate was recycled.
As the significant step in effective utilization of high-iron bauxite ore, the roasting process is complex. Thus, in this work, the influences of material ratio of (NH4)2SO4 to ore, roasting time, roasting temperature, and ore particle size on the extraction ratios of Fe and Al were investigated in details, and the roasting kinetics was discussed.
2 Experimental
2.1 Materials and characterization
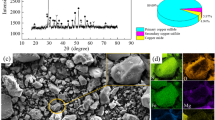
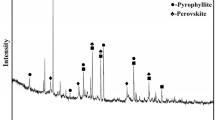
The high-iron bauxite ore used in experiments was obtained from Guangxi, China. The chemical compositions of the bauxite ore were chemically analyzed. The mineral phases and micromorphology are shown in Fig. 1. The (NH4)2SO4 from Sinapharm Chemical Reagent Co., Ltd. was of industrial grade, and the solvent was distilled water.
The main compositions in bauxite ore are Al2O3, Fe2O3, and SiO2, and the contents of Al2O3, Fe2O3, and SiO2 are 28.96, 43.85, and 10.49%, respectively. The sum of those three components reached 83.3 mass%, which is dominated and of utilization values. The main mineral phases in high-iron bauxite are gibbsite, hematite, goethite, quartz, and aluminum hydroxide. That is, iron mainly exists in the form of hematite and goethite, and aluminum mainly in the form of gibbsite and aluminum hydroxide.
2.2 Procedure
Experiments were performed in a resistance wire heating furnace under air atmosphere. The ground high-iron bauxite ore and ammonium sulfate were homogeneously mixed in a medicine grinder, and then put into a corundum crucible. The corundum crucible was placed in the furnace and heated to the setting temperature and then held for a period of time. When the roasting ended, the sample was taken out and leached in 80 °C water for 60 min and filtered [8]. The contents of Fe and Al both in solution and filter residue were examined by titration method. It is noteworthy that the material ratio of (NH4)2SO4 to ore was the mass ratio of (NH4)2SO4 dosage to the stoichiometric consumption of (NH4)2SO4 that reacted with Al2O3 and Fe2O3 in bauxite ore.
At kinetics experiments, 25 g high-iron bauxite ore was used in each test. The mixed material was put into a series of corundum crucibles, which were placed in the roasting furnace and then heated. When the temperature reached the desired temperature, the time device was started. The crucibles were taken out one by one at specified time and cooled rapidly in order to reduce the measurement error. The roasted material was leached in 80 °C water for 60 min and filtered. The contents of Fe and Al both in solution and filter residue were examined by titration method.
The extraction ratio of Fe or Al was calculated using Eq. (1).
where η is the extraction ratio of Fe or Al, %; M is the molecular mass of Fe or Al; c1 is the concentration of Fe or Al, mol L−1; v1 is the volume of Fe or Al, L; m0 is the mass of bauxite ore, g; and w0 is the mass fraction of Fe or Al in bauxite ore, %.
3 Results and discussion
3.1 Effect of roasting time on extraction ratios of Fe and Al
The influence of roasting time on the extraction ratios of Fe and Al was investigated with ore particle size below 80 μm, roasting temperature of 450 °C and ammonium sulfate-to-ore ratio of 3.0:1.0, as shown in Fig. 2. The extraction ratios of Fe and Al increase rapidly when the roasting time varies from 20 to 120 min before reaching a plateau. That is, a thermostatic time of 120 min is sufficient for extracting Fe and Al. A longer roasting time will waste not only time but also energy. 120 min is considered to be suitable.
3.2 Effect of roasting temperature on extraction ratios of Fe and Al
The influence of roasting temperature on the extraction ratios of Fe and Al was studied with ore particle size below 80 μm, roasting time of 120 min and ammonium sulfate-to-ore ratio of 3.0:1.0, as shown in Fig. 3.
The extraction ratios of Fe and Al increase gradually when the roasting temperature extends from 250 to 450 °C before reaching a plateau. From the kinetics point of view, high temperature can promote the movement of reaction molecules, thus facilitating the multi-phase reactions. When the high-iron bauxite ore reacted with ammonium sulfate or decomposed at 450 °C for 120 min, the extraction ratios of Fe and Al rose up to 98.5% and 98.8%, respectively. Thus, the roasting temperature of 450 °C was chosen in the following experiments.
3.3 Effect of material ratio of (NH4)2SO4 to ore on extraction ratios of Fe and Al
The influence of material ratio of (NH4)2SO4 to ore on the extraction ratios of Fe and Al was studied with ore particle size below 80 μm, roasting time of 120 min and roasting temperature 450 °C. Figure 4 shows the relationships between extraction ratios of Fe and Al and material ratio of (NH4)2SO4 to ore. The extraction ratios of Fe and Al increase gradually with the material ratio of (NH4)2SO4 to ore varying from 1.0:1.0 to 2.5:1.0; then, the extraction ratios of Fe and Al are stabilized. When (NH4)2SO4 is insufficient, the reaction between ore and (NH4)2SO4 is inadequate. Increasing the dosage of (NH4)2SO4 can intensify the contact between (NH4)2SO4 and its decomposition with bauxite ore, thereby promoting the reactions.
3.4 Effect of ore particle size on extraction ratios of Fe and Al
The influence of ore particle size on the extraction ratios of Fe and Al was studied at roasting time of 120 min, roasting temperature of 450 °C and material ratio of (NH4)2SO4 to ore of 2.5:1.0. Figure 5 shows the relationships between extraction ratios of Fe and Al and ore particle size. The extraction ratios of Fe and Al increase rapidly with the ore particle size decreasing from 120 to 80 μm, and then, the extraction ratios of Fe and Al are stabilized. Additional energy consumption was needed if grinding the ore particles to smaller size.
3.5 Orthogonal test
To optimize the reaction conditions of roasting high-iron bauxite ore using (NH4)2SO4, the L9(34) tabulation including four factors and three levels was chosen based on the single factor experiments, as shown in Table 1. The results of the orthogonal test are listed in Table 2.
From Table 2, the order of those factors affecting the extraction ratios of Fe and Al was material ratio of (NH4)2SO4 to ore, ore particle size, roasting temperature, and roasting time. The optimized reaction conditions were obtained as follows: roasting temperature of 450 °C, roasting time of 120 min, material ratio of (NH4)2SO4 to ore of 2.5:1.0, and ore particle size below 80 μm. The extraction rations of Fe and Al were both higher than 96% in the verification experiments under the optimized reaction conditions.
Scholars [14,15,16,17] suggest that the roasting process was complex, involving the decomposition of (NH4)2SO4, the reactions between oxides of Fe and Al and (NH4)2SO4 and its decomposition product, as well as the decompositions of sulfuric acid double salts. From the experimental results and the studies [14,15,16,17], the main chemical reactions were concluded as below:
The recrystallization of (NH4)2SO4 and the additional NH3 absorption reactions were:
4 Roasting kinetics analysis
As known, (NH4)2SO4 can be decomposed as NH3 and NH4HSO4, and the NH4HSO4 will decompose into NH3 and H2SO4; thus, the reaction process is a liquid–solid reaction. Therefore, the roasting process can be investigated by the shrinking unreacted model when the ore particles are regarded as sphere. The relationships of iron and aluminum extraction ratios to roasting temperature in the selected range are plotted in Fig. 6. The extraction ratios of Fe and Al increase with the roasting temperature increasing. And at a certain temperature, the extraction ratios of Fe and Al increase with prolonging the roasting time. When the mixed material was roasted at 450 °C for 120 min, the extraction ratios of Fe and Al rose to 90% and 93%, respectively.
Figure 6 was analyzed on the basis of the shrinking-core model. The experimental data were substituted into the Crank–Ginsting–Braunshtein’s kinetic equation [18,19,20]:
where α is the extraction ratio of iron or aluminum; k is the apparent rate constant; and t is the reaction time. The corresponding relationships between the value of left side of Eq. (2) and the roasting time are plotted in Fig. 7. It was obvious that the value of 1 + 2(1 − α) − 3(1 − α)2/3 has significantly linear correlation with the roasting time, and the correlation coefficients R2 were higher than 0.99. All of those indicated that the roasting process is fitted to the Crank–Ginsting–Braunshtein’s kinetic equation, which proves that the reaction rate is controlled by the internal diffusion on the solid product layer in the roasting process.
According to the Arrhenius expression,
where A is the frequency factor; E is the apparent activation energy; R is the mole gas constant; and T is the absolute temperature. The relationships between lnk and 1/T are shown in Fig. 8. It can be seen that the approximation of the apparent activation energy could be obtained by the slope of the straight line.
The apparent activation energy calculated from Fig. 8a, b was 20.43 kJ mol−1 and 19.44 kJ mol−1, respectively. Similarly, the k value obtained from Fig. 8 was 0.05635 and 0.05608, respectively. The average E and k values were 19.94 kJ mol−1 and 0.05622, respectively. The results verified that the reaction rate was controlled by the internal diffusion.
Thus, in the experimental temperature range, the kinetic equation of roasting process could be described as follows:
5 Conclusions
-
1.
The optimized reaction conditions for roasting high-iron bauxite with (NH4)2SO4 were obtained as roasting temperature of 450 °C, roasting time of 120 min, material ratio of (NH4)2SO4 to bauxite of 2.5:1.0, and ore size of less than 80 μm. Among them, the material ratio of (NH4)2SO4 to bauxite was the most predominant factor.
-
2.
The shrinking-core model was used to analyze the kinetics of roasting process. The control step of the roasting process was the internal diffusion on the solid product layer. The kinetic equation is obtained, and the apparent activation energy was 19.22 kJ mol−1.
References
Z.N. Lou, Y. Xiong, X.D. Feng, W.J. Shan, Y C. Zhai, Hydrometallurgy 165 (2016) Part 2, 306–311.
J.Y. Jin, Z.Y. Li, Y. Wu, Appl. Mech. Mater. 624 (2014) 3–7.
H.X. Xin, Theoretical and technological research on exacting valuable elements from high-iron bauxite, Northeastern University, Shenyang, 2014.
Z.G. Liu, M.S. Chu, Z. Wang, W. Zhao, J. Tang, High Temp. Mater. Process. 36 (2017) 79–88.
T. Lu, C.A. Pickles, S. Kelebek, High Temp. Mater. Process. 31 (2012) 139–148.
X.B. Li, Y.L. Wang, Q.S. Zhou, T.G. Qi, G.H. Liu, Z.H Peng, H.Y. Wang, Trans. Nonferrous Met. Soc. China 27 (2017) 2715–2726.
C.A. Pickles, T. Lu, B. Chambers, J. Forster, Can. Metall. Quart. 51 (2012) 424–433.
H.X. Xin, Y. Wu, S.M. Liu, Y.C. Zhai, Chin. J. Nonferrous Met. 24 (2014) 808–813.
J.H. Chen, Q.F. Wang, Q.Z. Zhang, E.J.M. Carranza, J.Q. Wang, J. Geochem. Explor. 188 (2018) 413–426.
R. Neumann, A.N. Avelar, G.M. da Costa, Miner. Eng. 55 (2014) 80–86.
C.H. Yeh, G. Zhang, Int. J. Miner. Process. 124 (2013) 1–7.
W.T. Hu, H.J. Wang, C.L. Ji, C.Y. Sun, H.D. Yu, Y.H. Zhang, J. Cent. South Univ. 10 (2012) 3755–3762.
N. Zwingmann, A.J. Jones, S. Dye, P.M. Swash, R.J. Gilkes, Hydrometallurgy 97 (2009) 80–85.
Y. Sun, X.Y. Shen, Y.C. Zhai, Int. J. Miner. Metall. Mater. 22 (2015) 467–475.
H.M. Shao, X.Y. Shen, Y. Sun, Y. Liu, Y.C. Zhai, Int. J. Miner. Metall. Mater. 23 (2016) 1133–1140.
H.M. Shao, X.Y. Shen, H.T. Zhu, Y.C. Zhai, Chin. J. Nonferrous Met. 27 (2017) 138–144.
X.F. Song, J.C. Zhao, Y.Z. Li, Z. Sun, J.G. Yu, Front. Chem. Sci. Eng. 7 (2013) 210–217.
L.L. Sui, Y.C. Zhai, Trans. Nonferrous Met. Soc. China 24 (2014) 848–853.
R.C. Wang, Y.C. Zhai, Z.Q. Ning, Int. J. Miner. Metall. Mater. 21 (2014) 144–149.
R.C. Wang, Y.C. Zhai, X.W. Wu, Z.Q Ning, P.H. Ma, Trans. Nonferrous Met. Soc. China 24 (2014) 1596–1603.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 51774070), the National Basic Research Program of China (2014CB643405), and the Fundamental Research Funds for the Central Universities of China (150204009).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Tian, D., Shen, Xy., Zhai, Yc. et al. Extraction of iron and aluminum from high-iron bauxite by ammonium sulfate roasting and water leaching. J. Iron Steel Res. Int. 26, 578–584 (2019). https://doi.org/10.1007/s42243-018-0128-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42243-018-0128-x