Abstract
TiC + Al binder metal-matrix composites are fabricated by self-propagating high-temperature synthesis (SHS) in reaction power mixtures of titanium, carbon (ash), and aluminum. It is established that stable combustion in a steady wave mode is possible with a content up to 50 wt % aluminum powder in a reaction mixture. Crushing of synthesized loose cakes and subsequent sieve scattering gave composite powders with a cloddy shape close to equilibrium. This shape is favorable for good looseness, which is necessary when using powders for surfacing and sputtering of wear-resistant coatings. Synthesis products are investigated by scanning electron microscopy and X-ray structural (XRS) and energy-dispersive X-ray (EDX) analysis. It is established that the average size of carbide inclusions in the composite structure decreases monotonically with an increase in the content of the aluminum powder inert in the thermal aspect in reaction mixtures. The lattice parameter of titanium carbide determined by the XRS method turned out much smaller than known values for equiatomic-composition carbide. Herewith, no dependence of the lattice parameter on the aluminum content is found for composites. Carbide inclusions in the composite structure are investigated by the EDX method, and it is established that the titanium content corresponds to its concentration in equiatomic-composition carbide. In addition to titanium and carbon, carbide contains up to 2.5 wt % dissolved aluminum, which can affect the carbide lattice parameter.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The fabrication of ternary alloys of the titanium–carbon–aluminum system is complicated by the large difference in properties of alloyed components. Metallic components have strongly differing melting points, notably, 660 and 1608°C for aluminum and titanium, correspondingly. Carbon does not melt upon heating but sublimes at 4200°C. The ability to form the alloy in a ternary system depends on the character of binary equilibrium diagrams. It follows from their consideration that titanium mixes well both with aluminum and with carbon in a liquid state and forms intermediate compounds in the solid state: one compound (titanium carbide) in the Ti–C system and several compounds in the Ti–Al system. One intermediate compound is also present in the Al–C binary system (Al4C3), which is formed upon holding carbon in the solid state, for example, graphite in the aluminum melt. This carbide is stable at least up to 2200°C. The components situating in compacts from the powder mixture interact in the solid phase at temperatures below the melting point of aluminum (tm Al = 660°C). In this case, the formation of all intermediate compounds stable at temperatures below 660°C (Al4C3, TiC, Ti3Al, TiAl, and TiAl3) and ternary intermediate compounds (Ti3AlC2, Ti2AlC, and Ti3AlC) is theoretically possible.
The actual phase composition of interaction products in ternary powder mixtures depends on the following factors:
(i) the content of components in a mixture;
(ii) kinetic parameters (the holding time at a concrete temperature);
(iii) thermodynamic functions (the Gibbs energy) of binary and ternary intermediate compounds.
The probability of formation of concrete intermediate compounds in mixtures of aluminum, titanium, and carbon is determined first and foremost by the ratio of free Gibbs energies and their temperature dependences, which are available in reference books. However, it should be noted that the data presented in various publications differ strongly.
The interaction of aluminum in powder mixtures with titanium and carbon at temperatures higher than tm Al abruptly intensifies due to spreading Al melt over the pores and switching-on the liquid-phase diffusion mechanism. This interaction consists, first and foremost, of the dissolution of titanium (and carbon at higher temperatures as well) in the aluminum melt with the formation of liquid Al solutions, which does not exclude the simultaneous formation of intermediate compounds in the form of diffusion layers on the surface of solid titanium and/or carbon. The appearance of nuclei of intermediate compounds in the saturated liquid solution in temperature intervals of stability of corresponding compounds is also possible.
The above description of the possible character of the interaction of components in a ternary system is based on known binary equilibrium diagrams, as well as on general notions on the factors affecting this interaction.
The data on phase equilibria in a ternary system are less detailed, but there are rather numerous results of studying the phase composition and structure of ternary alloys of various compositions, especially the so-called MAX phases Ti3AlC2 [1] and Ti2AlC [2]. These works were performed preferentially for the development of ternary materials for practical applications.
The most mass application of Al–Ti–C ternary alloys includes modifiers used for refining the structure of cast aluminum and its alloys. The results of studying such modifiers, as well as castings fabricated using it, are described in [3–9].
Most aluminum-based modifiers contain 3–5 wt % titanium, while the carbon content varies in wide limits from 0.1 to 1.25%. The main phases that are present in the structure of aluminum-based modifiers are dispersed particles of titanium carbide and needlelike particles of the TiAl3 intermetallic compound. The powder modifiers with a high carbon (graphite) content also contain faceted inclusions of aluminum carbide. Modifiers were fabricated mainly by two methods, notably, casting from pure metals and/or master alloys [5–7, 9] and compaction of powder mixtures of the corresponding composition [4, 8], as well as by the combination of these two methods [3]. The phase composition and structure of modifiers naturally depended on their elemental composition and affected the final effect of refining the casting structure simultaneously with process parameters of introducing modifiers into the melt (the melt weight, holding time before pouring, and the presence and intensity melt stirring). Structure refining of castings substantially increases their tensile strength (from 105 to 137 MPa) and plasticity (from 8.4 to 13.2%) [8].
The main effect of structure refining during the introduction of modifiers is provided by the influence of dispersed titanium carbide particles, which are the crystallization centers. The role of titanium in master alloys is not reduced to the titanium source only during the formation of carbide particles. It is affirmed [3] that unreacted titanium, being in the liquid solution, increases supercooling and prevents the aluminum grain growth. The authors of [6, 7] also studied the modified effect when introducing modifiers complexly alloyed by carbon and boron. It is established [6] that the additional introduction of boron prevents the agglomeration of dispersed carbide particles, which introduce the main contribution to structure refining.
One more direction in studying the alloys of the Al–Ti–C system is being developed at Samara State Technical University. The authors of [10] studied the morphology and dispersity of TiC particles formed after the introduction of mixtures of titanium and graphite powders in the aluminum melt at 900°C in the proportion proportional to titanium carbide. The influence of flux additives based on haloid salts on the TiC particles was also considered. It is established that the interaction of titanium and carbon introduced into the melt in the form of powder mixtures rolled into the aluminum foil results in the formation of titanium carbide particles from 2–4 to 0.17–0.35 μm in size. This results in a cast composite with an aluminum matrix and content of reinforcing carbide particles of 10%. Judging by the structure, the TiC particles are strongly agglomerated, which lowers the effect of modifying the aluminum structure.
The Ti3AlC2 and Ti2AlC are of most interest among ternary compounds of the Ti–Al–C system. They are so-called MAX phases, possessing a unique combination of properties of metals and ceramics, notably, high electrical conductivity and thermal conductivity, plasticity, thermal shock resistance, mechanical treatment ability, scale resistance, and strength, as well as low density and thermal expansion coefficient. Numerous works are devoted to the investigation the fabrication methods and properties of MAX phases of the Ti‒Al–C system [1, 2, 11–18].
Two main synthesis methods of MAX phases are applied, notably, reactive sintering of powder mixtures of the target composition (free sintering [18], hot isostatic pressing (HIP) [2, 14, 17], and spark plasma sintering (SPS) [1, 14]), and self-propagating high-temperature synthesis (SHS) [2, 12, 15]. Attempts to fabricate the target product by mechanical synthesis immediately during the intense treatment of powder mixtures in planetary mills are also known [11, 16]. The use of power-saving SHS process seems to be promising to fabricate MAX phases, but the problem of fabricating the single-phase product in strictly nonequilibrium synthesis conditions is even more acute than with the help of methods based on reactive sintering. In all cases, side products are formed along with the target product. Titanium carbide is the main residual phase due to a large negative Gibbs energy.
In order to eliminate side phases in products formed by reaction sintering, the elemental composition of powder mixtures, temperature, and sintering time are varied. When using SHS, the possibility of varying the synthesis modes are limited, but, even in this case, along with the variation in the elemental composition of reaction mixtures, researchers attempt to regulate the final phase composition of the SHS product by preliminary heating or mechanical activation of reaction mixtures [13]. In the case of SHS with the subsequent application of pressure, the phase composition of synthesis products can be controlled when varying the delay time of pressure application after finishing the synthesis reaction [12].
SHS products in powder mixtures with a high aluminum content contain two main phases, notably, titanium carbide and unbound aluminum. Regularities of combustion and synthesis products in powder mixtures of titanium, carbon, and aluminum in the frontal combustion mode and heat explosion were investigated in [19–25]. The main problems in these works were the determination of the phase composition of synthesis products and lattice parameters of titanium carbide. However, the structure of synthesis products that can be used to fabricate metal-matrix composite powders was not studied. Such powders [26, 27] are successfully used for the surfacing [28, 30] and sputtering [29] of wear-resistant coatings.
As far as the phase composition and structure of composite powders (dispersity and morphology of carbide inclusions, as well as the volume fraction of a metallic binder) affect the properties of coatings deposited with their application, it is of interest to investigate the synthesis products in powder mixtures of titanium, aluminum, and carbon. In connection with this, the goal and targets of our work were as follows:
(i) reveal the concentration limits for combustion of Ti–Al–C powder mixtures in a wave mode;
(ii) determine the phase and elemental composition of SHS products;
(iii) investigate the morphology of composite powders fabricated by crushing and sieving of porous cakes—synthesis products.
(iv) investigate the dependence of the carbide-phase dispersity in the composite structure on the composition of the reaction mixture.
EXPERIMENTAL
Reaction mixtures were prepared from powders of titanium (TPP-8, <160 μm, 99.4%), aluminum (PA-4, <100 μm, 99.3%), and technical carbon (ash of the P-803 brand). Powders were mixed for 4 h in a dry state and compacted into cylindrical samples ∅20 × 25 mm, the porosity of which was 35–38%. The charge composition of reaction mixtures and calculated values of the volume content of the aluminum binder (under the condition of the absence other phases in synthesis products excluding aluminum and titanium carbide) are presented in Table 1. The titanium-to-carbon ratio in reaction mixtures corresponded to titanium carbide of the equiatomic composition.
The synthesis was performed in a sealed reactor in argon with an excess pressure of about 0.5 atm. Combustion was initiated by heating an igniting pellet using a molybdenum coil. The cake was crushed with sieving into fractions.
Composite powders were investigated using equipment from the Nanotekh Joint Use Centers at the Institute of Strength Physics and Materials Science, Siberian Branch, Russian Academy of Sciences, and at Tomsk State University by X-ray structural analysis (an XRD-6000 diffractometer, CuKα radiation) and scanning electron microscopy (a EVO 50 and Philips SEM 515). The phase composition and structural parameters of the samples were studied using an XRD-6000 diffractometer in CuKα radiation. The primary diffraction data were processed using the PDF 4+ databases, as well as the POWDER CELL 2.4 full-profile analysis program. The quantitative determination of the phases was performed according to the Rietveld method. Unit-cell parameters were calculated by the least-squares method. Microstresses and coherent scattering regions (CSRs) were calculated from the physical broadening of X-ray peaks according to the Scherrer formula. Silicon was used as the standard for the diffractometer calibration.
RESULTS AND DISCUSSION
Phase Composition of Synthesis Products
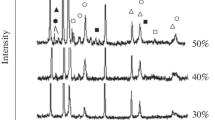
Synthesis in all compacts made of powder materials (Table 1) proceeded in a steady wave mode excluding the composition with the target content of the aluminum binder of 60 wt %, which we failed to ignite at room temperature. X-ray diffraction patterns of SHS powders are presented in Fig. 1, while the relative content of phases in synthesis products, which was determined from the sum of areas under the peaks of separate phases, is presented in Table 2. Lattice parameters of all phases, CSR size, and microdistortions of crystal lattices (Δd/d) are also determined from the results of processing X-ray diffraction patterns. No dependences of these structural characteristics of phases on the composition of reaction mixtures are found; they remained constant in the limits of spread, so they are not presented here.
The TiC lattice parameter turned out much smaller than the reference value for titanium carbide of the equiatomic composition (a = 0.4328 nm [24]). No explicit dependence of parameter a on the aluminum content in reaction powder mixtures is revealed in contrast with the data of [24], according to which the TiC lattice parameter in TiC–Al SHS composites turned out considerably smaller and decreased monotonically from 0.4322 nm at 10% Al binder to 0.4312 nm with its content of 40%. The possible reason for the lowering of this characteristic in [24] is the contamination of metallic components of reaction mixtures by oxygen during the prolonged (5 h) treatment of mixtures in a planetary mill.
In addition to the deficit of carbon [26] and dissolution of oxygen and/or nitrogen in the lattice [31], the variation in the TiC lattice parameter in metal- matrix SHS composites can be caused by the dissolution of a metallic binder in the component lattice [27, 32]. The relation of the titanium carbide lattice parameter in composites that we studied and elemental carbide composition will be discussed below.
The aluminum concentration in synthesis products increases monotonically with an increase in its content in reaction mixtures, while the titanium carbide content correspondingly decreases. Among other phases, the TiAl3 intermetallic compound is determinable, but its concentration is low and differs slightly for studied compositions.
Morphology of SHS Powders and Dispersity of the Carbide Phase
Granules of the composite powder (Fig. 2) have preferentially cloddy configuration without acute angles and edges. Such shape of granules formed during crushing is provided by the composite structure (Fig. 3), in which a plastic aluminum binder compensates the zero plasticity of the carbide phase. The rounded and close to equiaxial shape of granules promotes good fluidity, which is necessary to provide the stable supply with a constant rate of the powder from feeders applied in coating surfacing and sputtering technologies.
The size of carbide particles was evaluated from images of the surface of granules under large magnification (Fig. 3). As expected, the dispersity of the carbide phase decreases monotonically with an increase in the content of the aluminum powder inert in the thermal aspect in reaction mixtures (Fig. 4). Such a dependence is typical of SHS products with the structure of a metal-matrix composite based on titanium carbide [26, 27, 33], including those with an aluminum binder [23, 24].
Elemental Composition of the Carbide Phase
The elemental composition of carbide particles in SHS composites was determined by energy-dispersive X-ray spectroscopy (EDX) by the values in separate points on the surface of coarse carbide inclusions, which are formed in SHS products with 10 and 20% of the Al binder (Figs. 3a, 3b).
We failed to determine the elemental composition of fine carbide particles for compositions with a high aluminum content because of the insufficient locality of the electron-probe method. The composition of the surface layer of carbide inclusions averaged by local measurements (Fig. 5) with a thickness of several micrometers is presented in Table 3. It follows from its data that the titanium concentration in carbide coincides in the limits of spread with its content in the titanium carbide of the equiatomic composition (80 wt %). The carbon content is herewith much lower than 20 wt % for carbide of the equiatomic composition. In addition to titanium and carbon, aluminum is present in carbide, which supplements the summary carbon and aluminum content to 20 wt %.
The evaluation of the elemental composition of carbide (Table 3) agrees with the results of determining the TiC lattice parameter (Table 2), according to which values of a for titanium carbide in SHS products differ slightly from reference values [24] for equiatomic composition carbide. It is possible that the carbide lattice parameter is also affected by aluminum dissolved in a small amount.
CONCLUSIONS
(i) The composite powder fabricated by crushing SHS cakes synthesized from reaction mixtures of titanium, carbon (ash), and aluminum has a cloddy preferentially equiaxial shape favorable from the viewpoint of good looseness.
(ii) The size of carbide inclusions in an aluminum matrix decreases monotonically with an increase in the content of aluminum inert in the thermal aspect in powder mixtures and reaches the minimal value of 0.8 μm.
(iii) Carbide inclusions in the structure of the SHS composite contain dissolved aluminum in amounts reaching 2.5 wt %, while the lattice parameter of titanium carbide is close to the values for titanium carbide of equiatomic composition.
REFERENCES
Yang Chen, Jin Songzhe, Liang Baoyan, Liu Guojun, Duan Lianfeng, and Jia Shusheng, Synthesis of Ti3AlC2 by spark plasma sintering of mechanically milled 3Ti/xAl/2C powder mixtures, J. Alloys Compd., 2009, vol. 472, pp. 79–83.
Zhou Aiguo, Wang Chang-an, Ge Zhenbin, and Wu Lifeng, Preparation of Ti3AlC2 and Ti2AlC by self-propagating high-temperature synthesis, J. Mater. Sci. Lett., 2001, vol. 20, pp. 1971–1973.
Birol, Y., Grain refining efficiency of Al–Ti–C alloys, J. Alloys Compd., 2006, vol. 422, pp. 128–131.
Ding Haimin, Liu Xiangfa, Yu Lina, and Zhao Guoqun, The influence of forming processes on the distribution and morphologies of TiC in Al–Ti–C master alloys, Scr. Mater., 2007, vol. 57, pp. 575–578.
Gezer, B.T., Toptan, F., Daglilar, S., and Kerti, I., Production of Al–Ti–C grain refiners with the addition of elemental carbon, Mater. Des., 2010, vol. 31, pp. 30–35.
Nie Jinfeng, Ma Xiaoguang, Li Pengting, and Liu Xiangfa, Effect of B/C ratio on the microstructure and grain refining efficiency of Al–Ti–C–B master alloy, J. Alloys Compd., 2011, vol. 509, pp. 1119–1123.
Wang Enzhao, Gao Tong, Nie Jinfeng, and Liu Xiangfa, Grain refinement limit and mechanical properties of6063 alloy inoculated by Al–Ti–C (B) master alloys, J. Alloys Compd., 2014, vol. 594, pp. 7–11.
Lia Xiaoteng and Hao Hai, The influence of carbon content on Al–Ti–C master alloy prepared by the self-propagating high-temperature synthesis in melt method and its refining effect on AZ31 alloy, J. Alloys Compd., 2015, vol. 623, pp. 266–273.
Yang Huabing, Gao Tong, Wang Haichao, Nie Jinfeng, and Liu Xiangfa, Influence of C/Ti stoichiometry in TiCx on the grain refinement efficiency of Al–Ti–C master alloy, J. Mater. Sci. Technol., 2017, vol. 33, pp. 616–622.
Luts, A.R., Amosov, A.P., Ermoshkin, And.A., Ermoshkin, Ant.A., Nikitin, K.V., and Timoshkin, I.Yu., Self-propagating high-temperature synthesis of highly dispersed titanium-carbide phase from powder mixtures in the aluminum melt, Russ. J. Non-Ferr. Met, 2014, vol. 55, no. 6, pp. 606–612.
Shahin, N., Kazemi, Sh., and Heidarpour, A., Mechanochemical synthesis mechanism of Ti3AlC2 MAX phase from elemental powders of Ti, Al and C, Adv. Powder Technol., 2016, vol. 27, pp. 1775–1780.
Stolin, A.M., Vrel, D., Galyshev, S.N., Hendaoui, A., Bazhin, P.M., and Sytschev, A.E., Hot forging of MAX compounds SHS-produced in the Ti–Al–C system, Int. J. Self-Propag. High-Temp. Synth., 2009, vol. 18, no. 3, pp. 194–199.
Hendaoui, A., Vrel, D., Amara, A., Langlois, P., Andasmas, M., and Guerioune, M., Synthesis of high-purity polycrystalline MAX phases in Ti–Al–C system through mechanically activated self-propagating high-temperature synthesis, J. Eur. Ceram. Soc., 2010, vol. 30, pp. 1049–1057.
Zhou Aiguo, Wang Chang-an, and Huang Yong, A possible mechanism on synthesis of Ti3AlC2, Mater. Sci. Eng., A, 2003, vol. 352, nos. 1–2, pp. 333–339.
Hendaoui, A., Andasmas, M., Amara, A., Benaldjia, A., Langlois, P., and Vrel, D., SHS of high-purity MAX compounds in the Ti–Al–C system, Int. J. Self-Propag. High-Temp. Synth., 2008, vol. 17, no. 2, pp. 129–135.
Potanin, A.Yu., Loginov, P.A., Levashov, E.A., Pogozhev, Yu.S., Patsera, E.I., and Kochetov, N.A., Effect of mechanical activation on Ti3AlC2 MAX phase formation under self-propagating high-temperature synthesis, Eurasian Chem.-Technol. J., 2015, vol. 17, pp. 233–242.
Tzenov, N.V. and Barsoum, M.W., Synthesis and characterization of Ti3AlC2, J. Am. Ceram. Soc., 2000, vol. 83, no. 4, pp. 825–832.
Yoshida, M., Hoshiyama, Y., Ommyoji, J., and Yamaguchi, A., Microstructural evolution during the formation of Ti3AlC2, Mater. Sci. Eng., C, 2010, vol. 173, nos. 1–3, pp. 126–129.
Liu Zhiwei, Rakita Milan, Xu Wilson, Wang Xiaoming, and Han Qingyou, Ultrasound assisted combustion synthesis of TiC in Al–Ti–C system, Ultrason. Sonochem., 2015, vol. 27, pp. 631–637.
Chaubey, A.K., Prashanth, K.G., Ray, N., and Wang Zhi, Study on in-situ synthesis of Al–TiC composite by self-propagating high temperature synthesis process, Mater. Sci., 2015, vol. 12, no. 12, pp. 454–461.
Li, Y.X., Hu, J.D., Liu, Y.H., Yang, Y., and Guo, Z.X., Effect of C/Ti ratio on the laser ignited self-propagating high-temperature synthesis reaction of Al–Ti–C system for fabricating TiC/Al composites, Mater. Lett., 2007, vol. 61, pp. 4366–4369.
Song, M.S., Huang, B., Huo, Y.Q., Zhang, S.G., Zhang, M.X., Hu, Q.D., and Li, J.G., Growth of TiC octahedron obtained by self-propagating reaction, J. Cryst. Growth, 2009, vol. 311, pp. 378–382.
Li, Y.X., Hu, J.D., Liu, S.Y., Wang, H.Y., Yang, Y., and Guo, Z.X., Laser igniting synthesis of powders with Al, Ti and C powders, J. Laser Appl., 2006, vol. 18, no. 2, pp. 113–116.
Song, M.S., Huang, B., Zhang, M.X., and Li, J.G., Study of formation behavior of TiC ceramic obtained by self-propagating high-temperature synthesis from Al–Ti–C elemental powders, Int. J. Refract. Met. Hard Mater., 2009, vol. 27, pp. 584–589.
Li, Y.X., Hu, J.D., Wang, H.Y., Guo, Z.X., and Chumakov, A.N., Thermodynamic and lattice parameter calculation of TiCx produced from Al–Ti–C powders by laser igniting self-propagating high-temperature synthesis, Mater. Sci. Eng., A, 2007, vol. 458, pp. 235–239.
Pribytkov, G.A., Krinitsyn, M.G., and Korzhova, V.V., Investigation of products of SHS in powder mixtures of titanium and carbon containing an excess of titanium, Perspekt. Mater., 2016, no. 5, pp. 59–68.
Pribytkov, G.A., Korzhova, V.V., Baranovskii, A.V., and Krinitsyn, M.G., Phase composition and structure of composite powders of titanium carbide with a bundle of P6M5 steel obtained by the SHS method, Poroshk. Metall. Funkts. Pokrytiya, 2017, no. 2, pp. 64–71.
Pribytkov, G.A., Krinitsyn, M.G., Firsina, I.A., and Durakov, V.G., Hardness and abrasive wear resistance of electron beam coatings “titanium carbide–titanium binder”, cladded with synthesized composite powders, Vopr. Materialoved., 2017, no. 4, pp. 52–61.
Pribytkov, G.A., Kalita, V.I., Komlev, D.I., Korzhova, V.V., Radyuk, A.A., Baranovsky, A.V., Ivannikov, A.Yu., Krinitcyn, M.G., and Mikhailova, A.B., Structure and wear resistance of plasma coatings sputtered using TiC+HSS binder composite powder, Inorg. Mater.: Appl. Res., 2018, vol. 9, no. 3, pp. 442–450.
Pribytkov, G.A., Baranovskii, A.V., Firsina, I.A., Durakov, V.G., and Krinitsyn, M.G., Hardness and abrasive wear resistance of electron beam coatings deposited by SHS composite powders “TiC + steel P6M5”, Uprochnyayushchie Tekhnol. Pokrytiya, 2017, no. 10, pp. 446–452.
Zuev, L.V. and Gusev, A.I., Influence of nonstoichiometry and ordering on the period of the basic structure of cubic titanium carbide, Fiz. Tverd. Tela, 1999, vol. 41, no. 4, pp. 1134–1141.
Zhang, W.N., Wang, H.Y., Wang, P.J., Zhang, J., He, L., and Jiang, Q.C., Effect of Cr content on the SHS reaction of Cr–Ti–C system, J. Alloys and Compd., 2008, vol. 465, pp. 127–131.
Rogachev, A.S. and Mukas’yan, A.S., Gorenie dlya sinteza materialov: vvedenie v strukturnuyu makrokinetiku (Combustion for the Synthesis of Materials: An Introduction to Structural Macro-Kinetics), Moscow: Fizmatlit, 2012.
ACKNOWLEDGMENTS
We thank V.P. Krivopalov for help in synthesizing powders.
Funding
This study was performed within the frame of the Fundamental Research Program of the State Academies of Sciences for 2013–2020, line of research III.23.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Translated by N. Korovin
About this article
Cite this article
Pribytkov, G.A., Krinitsyn, M.G., Korzhova, V.V. et al. Structure and Phase Composition of SHS Products in Powder Mixtures of Titanium, Carbon, and Aluminum. Russ. J. Non-ferrous Metals 61, 207–215 (2020). https://doi.org/10.3103/S106782122002011X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S106782122002011X