Abstract
The crystal stock defect was formed in growing for a long time (22 h) of plate type Ib large diamond single crystals from the NiMnCo−C system at high pressure and high temperature (HPHT). The existence of crystal stock defect has great influence on the synthesis of high quality and large size diamond single crystal. In this paper, the effects of crystal growth rate and catalyst components on diamond crystal stock defects are studied. Firstly, the results show that the growth rate of diamond crystals is closely related to the generating of diamond crystal stock. This defect can be eliminated as the growth rate of diamond crystal synthesized with NiMnCo catalyst is reduced to less than 3 mg/h. Secondly, the composition of the catalyst also affects the formation of the crystal stock. High viscous FeNi catalyst is used to grow high quality large single crystals of diamonds at a growth rate of 3.18 mg/h for a long time (22 h). Thirdly, the effect of catalyst composition on diamond crystal stock was analyzed by testing the micro-morphology and nitrogen content of diamond. It can be shown from the results by scanning electron microscopy (SEM) that the surface of crystals synthesized with low-viscosity catalyst is rough, while it synthesized with high-viscosity catalyst is smooth. It was found from the test results of micro Fourier-transform infrared spectroscopy (FTIR) that the content of nitrogen in the crystals synthesized with FeNi catalyst was much lower than that in the crystals synthesized with NiMnCo. FeNi catalyst can effectively avoid the crystal stock, and is more suitable for the growth of large-size and high-quality diamond single crystal. Changing the composition of the catalyst is an effective way to avoid the crystal stock phenomenon.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 INTRODUCTION
As a kind of functional material with various ultimate properties, diamond has been widely concerned by scholars in the industry all over the world. In 1955, General Electric Company of the U. S. first synthesized diamond with graphite and nickel under HPHT, which initiated a new era of synthetic diamond [1]. Due to the limitation of the size of artificial diamond, in the early years, only the superhard as one of characteristics for diamonds was used most commonly in industry, so that many excellent properties were not fully developed and used. In 1970, the technology of the temperature gradient method (TGM) under HPHT for synthesizing diamond large single crystal, which was also known as gem-grade diamond single crystal and whose particle size was greater than 1 mm was invented [2]. Thus, the application fields of diamond are broadened, such as cutting tool, high precision machining, the heat sink of high power laser, the window material for infrared spectroscopy, the anvils and so on [3, 4]. High efficiency synthesis of high quality large diamond single crystals will promote the technological progress in related fields.
Large diamond single crystals with different crystal shapes have diverse applications. The plate shape of large diamond crystals can be used to manufacture cutting tools for precision parts and window materials for high-energy synchrotron radiators and so on. Studies by Sumiya and Reza have shown that the type Ib large diamond single crystal with (100) crystal surface as the main crystal surface under a certain pressure has a narrow growth temperature range and a low temperature portion in the crystal growth temperature range [5, 6]. This indicates that it is difficult to control the plate crystal shape of large diamond single crystals. For this reason, we have studied in detail the growth of different crystal shapes of type Ib large diamond single crystals [7]. On the basis of this study, we have studied growth control of plate diamond large single crystal. In the experimental process of synthesizing plate type Ib diamond large single crystal with NiMnCo as catalyst, the phenomenon of crystal stock was found. In a short period of time (11 h), high-quality large single crystal of plate diamond can be synthesized, while the crystal stock phenomenon often occurs in the course of the long period of growth (more than 22 h), which seriously affects the growth of the plate shape for large diamond single crystal. Therefore, the reasons for this crystal stock phenomenon are discussed in terms of the growth rate and the composition of catalyst for diamonds in this paper. Then the way to avoid the occurrence of crystal stock was proposed. This will provide a technical basis for the growth of high-quality plate type Ib large diamond single crystals, and help to improve its production efficiency.
2 EXPERIMENTAL
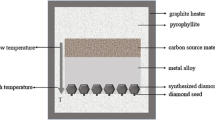
Large diamond single crystals were synthesized by TGM under HPHT in a China-type cubic high pressure apparatus (CHPA) (SPD-6 × 1200). The assembly diagram of diamond synthesis is shown in Fig. 1. The samples were synthesized under conditions of pressure at approximately 5.6 GPa and temperature at 1260–1280°C. Axial temperature gradient is the main driving force of diamond growth. In this experiment, the crystal growth rate was adjusted by changing the temperature gradient. The temperature was kept the same for achieving the repeatability of assembly for all the samples, thereby ensuring that except temperature gradient and catalyst components, other the conditions relating to the growth rate were the same.
The growth rate of diamond is proportional to the temperature gradient along the axis in the growth cell. Namely, the growth rate of diamond is controlled by adjusting the structure of the growth cell. The synthetic pressure is determined from the relationship between the cell pressure and the oil press load, which is established based on the pressure-induced phase transition of bismuth (Bi), barium(Ba) and thallium (Tl) etc. (accuracy 0.1 GPa). The temperature is determined from the relationship between the temperature and the input power, which is calibrated using a Pt6%Rh−Pt30% Rh thermocouple (accuracy 1°C) [8]. By measuring the temperature located at the top and the bottom of the catalyst in the growth cell center, the average temperature gradient in the growth cell of diamond can be calculated.
The catalysts used in the high pressure experiment were alloy NiMnCo (70 : 25 : 5 wt %) and FeNi (64 : 36 wt %). A particle size of about 0.5 mm of high quality diamond was selected as the seed to grow along the (100) crystal orientation. The collected samples were treated in boiling mixture of H2SO4 and HNO3 to remove remnant graphite and catalyst. Then, the collected samples were cleaned in boiling deionized water. The morphology, color and surface micro-morphology of large diamond single crystals were analyzed by optical microscopy and SEM. The content of N in diamond single crystals synthesized with different catalysts was determined by micro FTIR. The growth rate of diamond crystals was calculated by the ratio of the mass of diamond and the time of diamond synthesis. The mass of diamonds was weighed with a high precision electronic balance (accuracy 0.01 mg).
3 RESULTS AND DISCUSSION
3.1 The Phenomenon of Crystal Stock
The plate shape of diamond single crystals was synthesized with NiMnCo catalyst under high temperature of 1268°C and a high pressure of 5.6 GPa. The growth time of both crystals was 11 and 22 h, respectively. The experimental results were seen in Table 1. The corresponding diamond crystal optical micrographs were seen in Fig. 2.
It can be seen from Table 1 and Fig. 2 that the quality of the diamond single crystal synthesized with NiMnCo catalyst in a short time is good, and the diamond crystal having a growth time of more than 20 h appears the phenomenon of crystal stock. In the process of synthesizing large diamond single crystal by TGM under HPHT, the reason of producing the crystal stock is generally due to the growth rate is too high and the characteristics of catalyst components action [9, 10]. At constant pressure and temperature, the ability of diamond crystal seeds to absorbing carbon atoms is fixed. When too many carbon atoms are diffused from the carbon source at the high temperature, the excess carbon atoms will form new diamond crystals in the form of spontaneous nuclei, thus forming diamond crystal stock. In addition, the catalytic alloys have a great influence the crystal growth. The growth characteristics of synthetic diamond crystals with different catalysts are different. The convection mode of carbon atoms in the catalyst melt varies with the viscosity and the structure of the catalyst. The impurity composition and the aggregation mode of the catalyst melt also are different, so the possibility that diamond crystals grown in different catalysts have the crystal stock defect is different.
3.2 Effect of Crystal Growth Rate on Crystal Stock Formation
The temperature gradient is the main driving force for the growth of large diamond single crystals by hydrostatic catalyst method. As shown in Fig. 3, there is a temperature difference in the synthetic chamber of diamond. The concentration of carbon atoms at the high temperature end (carbon source) is high, while that at the low temperature end (seed crystal) is low, which causes the diffusion of carbon atoms from carbon source to seed crystal. The temperature gradient directly affects the growth rate of crystal. The temperature gradient is positively correlated with the crystal growth rate. The temperature gradient can be adjusted by changing the geometrical structure of the synthetic cavity, and the temperature gradient can be reduced by increasing the height of the crystal bed. In the process of synthesizing diamond crystal by TGM, the crystal growth is affected not only by the axial temperature gradient, but also by the radial temperature gradient, but the radial temperature gradient is obviously smaller than the axial temperature gradient. Under the combined action of axial and radial temperature gradients, there will be a large temperature gradient in the growth region of (111) crystal surface with slant on the side, which is prone to spontaneous nucleation due to excessive local concentration of carbon atoms, leading to the emergence of crystal stock.
When the (100) crystal surface of the seed is used as the growth surface to synthesize the large diamond single crystal, the diagram of the growth for the plate cubic crystal on seed shown in Fig. 4c is synthesized at low temperature area of V shape for diamond growth. The crystal shape of the selected seed is generally hex-octahedron. The diamond crystal grows with (100) face on top of seed crystal as the main growth surface, at the same time, it grows with slant (111) surface on the top side of seed crystal, too. The plate crystal shape of diamond crystal for growth time 11 or 22 h is formed gradually under the action of the (111) fast growing and the (100) slow growing in the area low temperature of V growth shape by the law of “fast surface submerged, slow surface exposed” [11]. The early phase of crystal growth is shown in Fig. 4a, the crystal mainly has (100) and (111) crystal face growing at the same time, and the atomic stacking modes of (100) and (111) crystal surfaces are different. (100) carbon atoms on the crystal face combine with two adjacent carbon atoms on the same crystal face, and two dangling bonds bond with other carbon atoms; (111) carbon atoms on the crystal face combine with three adjacent carbon atoms on the same crystal face, and just one dangling bond can bond with other carbon atoms [12]. Therefore, the (100) crystal surface can absorption added free carbon atoms from the catalyst. When diamond crystals are synthesized at low temperature area, (100) crystal face normal growth rate is slow, (111) crystal surface normal growth rate is fast. When the growth time is short (11 h), the crystal surface of (111) still has time to receive carbon atoms, so there is no crystal stock. With the extension of crystal growth time, the area of crystal (111) face gradually decreases, then the thickening speed of (111) crystal face accelerates. The carbon atoms in the growth area of (111) do not have enough time to fully diffuse, and the carbon atom concentration will be too high in the local area, thus stable spontaneous crystal nucleus of diamond will appear. When the crystal grows for a long time, the crystal stock in the form shown in Fig. 4b is produced.
Therefore, in the experiment, the height of the bed for crystal seed placement was gradually increased and the crystal growth rate was reduced. Diamonds are synthesized with NiMnCo catalyst at different growth rates by selecting the (100) surface of seed as the growth surface. The experimental results of diamond synthesis grown at 5.6 GPa pressure and 1268°C for 22 h are shown in Table 2.
There are optical micrographs of diamond crystal growing at different growth rates in Fig. 5. It can be seen from Figs. 5a, 5b that when the diamond single crystals were synthesized with NiMnCo catalyst, whose growth rate exceeds 3 mg/h and the time is longer, the synthetic diamond appears crystal stock phenomenon. This is because the temperature gradient in the synthesis cavity is too high, the carbon atoms diffuse too fast in the catalytic melt, and the ability of the crystal (111) surface to absorption carbon atoms is limited. The excess carbon atoms precipitate out in the form of spontaneous nucleus, and the crystal and spontaneous nucleus are connected together to form a crystal stock in the course of the growth process. It can be seen from Fig. 2b that the spontaneous nucleus competes for carbon atoms with the target crystal, resulting in the crystal growth of failing to achieve the desired effect and seriously affecting the crystal yield and quality of large single crystal diamond. With the continuous decrease of the crystal growth rate to 2.93 mg/h, the carbon atom concentration in the catalyst melt was reduced to that can be synthesis for a long time, and the high-quality large single crystal of plate diamond is synthesized in a long time.
3.3 The Influence of Catalyst on Crystal Stock
In the process of diamond growth, the alloy catalyst in the synthetic cavity is in the melting state, and the carbon atom is dissolved into the alloy catalyst. Under a certain temperature gradient, the oversaturated carbon precipitates out as the diamond phase, and the diamond begins to grow on the seed surface. If the viscosity coefficient of the alloy catalyst becomes large, the resistance of the carbon atom increases upon diffusion. The carbon convection velocity in the synthesis chamber is reduced, and the carbon deposition rate is reduced, which slows the growth rate of the crystal [13]. Table 3 lists the viscosity coefficients of some metal catalyst elements [14].
According to the Table 3, the viscosity coefficient of Fe element is relatively large, so iron-based alloy catalyst can be considered. In the course of the experiment, NiMnCo catalyst was replaced with FeNi catalyst. At different growth rates, the results of large diamond single crystals at 22 h after synthesis is shown in Table 4. There are the corresponding optical micrographs of diamond crystals in Fig. 6.
There are optical micrographs of diamond crystals growing at different growth rates in Fig. 6. It can be seen from Fig. 6a that when the FeNi catalyst is used to synthesize the single crystal of diamond, when the crystal growth rate reaches 3.71 mg/h and the growth time is 22 h, the synthetic diamond appears crystal stock phenomenon. This verifies the previous result that excessive growth rate is prone to the crystal stock. It was found from the comparison of micrograph analysis for diamonds in Figs. 5 and 6 that after replacing the NiMnCo catalyst with FeNi catalyst, the crystal growth rate reached 3.18 mg /h when the crystal bed height was 5.0 mm, and the crystal growth still did not appear the phenomenon of crystal stock after a long time. The microstructure of the crystal surface synthesized by the two catalysts was analyzed with the help of SEM. The results of the electron microscope analysis are shown in Fig. 7.
It can be seen from the SEM images of Fig. 7 that the surface of diamond crystal grown with NiMnCo catalyst is rough and the growth pattern is obvious, and the surface of diamond crystal grown with FeNi catalyst is smooth and perfect. This shows that under the same temperature gradient, the carbon precipitation rate in the high viscosity catalyst is slow, and the diamond atoms can be regularly precipitated on the crystal growth surface. The use of alloy catalysts with high viscosity coefficient can relatively reduce the precipitation rate of carbon atoms and prevent the occurrence of crystal stock to a certain extent. Diamond synthesized with NiMnCo catalyst is easy to appear crystal stock when the crystal growth rate exceeds 3 mg/h. Although the growth rate of diamond crystal grown with FeNi catalyst under the same temperature gradient is lower than that of NiMnCo catalyst, FeNi catalyst can still synthesize high-quality single crystal at the growth rate above 3 mg/h. Therefore, FeNi catalyst is more suitable for the growth of large-size plate shape of diamond single crystal.
The composition and aggregation mode of impurities in the catalyst melt will have a significant influence on the morphology and growth of the synthesized crystal. In the process of diamond synthesis, excessive impurities will also lead to the generation of crystal stock. The impurities often cause local aggregation of temperature and thus form energy aggregation. The presence of impurities is conducive to the formation of non-spontaneous nucleation and may cause the generation of crystal stock. The main impurities in the process of diamond synthesis are the catalyst and the nitrogen element in the air. The content of nitrogen (Nc) in plate diamond single crystals synthesized with NiMnCo and FeNi catalysts was measured by micro Fourier-transform infrared (FTIR) spectroscopy. The FTIR absorption spectra of the synthetic diamond crystals are shown in Fig. 8. It was reported that Nc was proportional to the intensities of absorbance at 1130 and 1344 cm–1 in an ideal spectrum, and determined from the equation Nc = (25.0 ± 2)a (1130 cm–1) [15]. It was calculated that the content of nitrogen in the diamond synthesized with NiMnCo catalyst was 362.3 ppm, and that in the diamond synthesized with FeNi catalyst was 55.4 ppm. The Nc in diamond synthesized with FeNi catalyst is lower than that synthesized with NiMnCo catalyst. This is because the solubility of nitrogen element in iron-based catalyst is higher than that in nickel-based catalyst [16], so the content of nitrogen in crystal is obviously different. Therefore, FeNi catalyst can be used to grow large single diamond crystal without the crystal stock at a high rate.
4 CONCLUSIONS
The reasons for the crystal stock formation were explored during the plate shape of type Ib large diamond single crystals growing for long time. Conclusions can be drawn as follows.
(i) When the diamond single crystals were synthesized with NiMnCo catalyst, the plate diamond single crystal, whose growth rate exceeds 3 mg/h and its growth time is longer, is prone to appear crystal stock defects. The crystal growth rate can be controlled by adjusting the temperature gradient of crystal growth to grow high-quality large-size plate diamond single crystal.
(ii) The use of high viscosity FeNi catalyst can relatively reduce the rate of carbon precipitation, so that the synthesized crystal surface is smooth, suitable for the growth of high-quality type Ib plate diamond large single crystal for a long time.
(iii) The content of impurity nitrogen in diamond crystal synthesized with FeNi catalyst is significantly lower than that synthesized with NiMnCo catalyst. FeNi can be used to effectively avoid the crystal stock caused by impurities. Changing the composition of the catalyst is an effective way to avoid the crystal stock phenomenon.
REFERENCES
Linares, R. and Doering, P., Properties of large single crystal diamond, Diamond Relat. Mater., 1999, vol. 8, no. 8, pp. 909–915.
Zang, C.Y., Li, M., and Chen, L.J., Growth and characterization of large, high quality cubic diamond crystals, Chin. Sci. Bull., 2012, vol. 57, no. 14, pp. 1733–1738.
Chen, S.T., Tsai, M.Y., Lai, Y.C., and Liu, C.C., Development of a micro diamond grinding tool by compound process, J. Mater. Process. Technol., 2009, vol. 209, no. 10, pp. 4698–4703.
Zhang, G.F., Zhang, B., Deng, Z.H., and Tan, Y.Q., An experimental study on a novel diamond whisker wheel, CIRP Ann. Manuf. Technol., 2010, vol. 59, no. 1, pp. 355–360.
Sumiya, H., Superhard diamond indenter prepared from high-purity synthetic diamond crystal, Rev. Sci. Instrum., 2005, vol. 76, no. 2, 026112.
Abbaschian, R., Zhu, H., and Clarke, C., High pressure-high temperature growth of diamond crystals using split sphere apparatus, Diamond Relat. Mater., 2005, vol. 14, nos. 11–12, pp. 1916–1919.
Wang, J.Z., Li, S.S., Su, T.C., Hu, M.H., Gao, G.J., and Guo, M.M., Shape controlled growth for type Ib large diamond crystals, Acta Phys. Sin., 2018, vol. 67, no. 16, 16181.
Gong, C.S., Li, S.S., Zhang, H., Su, T.C., Hu, M.H., Ma, H.A., Jia, X.P., and Li, Y., Study on synthesis and electrical properties of slab shape diamond crystals in FeNiMnCo–C–P system under HPHT, Int. J. Refract. Met. Hard Mater., 2017, vol. 66, pp. 116–121.
Sumiya, H., Toda, N., and Satoh, S., Growth rate of high-quality large diamond crystals, J. Cryst. Growth, 2002, vol. 237, no. 1, pp. 1281–1285.
Dannefaer, S., Defects in diamond, Phys. Status Solidi C, 2010, vol. 4, no. 10, pp. 3605–3613.
Strong, H.M. and Chrenko, R.M., Diamond growth rates and physical properties of laboratory-made diamond, J. Phys. Chem., 1971, vol. 75, no. 12, pp. 1838–1843.
Zhang, J.Q., Ma, H.A., Jiang, Y.P., Liang, Z.Z., Tian, Y., and Jia, X., Effects of the additive boron on diamond crystals synthesized in the system of Fe-based alloy and carbon at HPHT, Diamond Relat. Mater., 2007, vol. 16, no. 2, pp. 283–287.
Xiao, H.Y., Qin, Y.K., Li, S.S., Ma, H.A., and Jia, X.P., Effects of catalyst stickiness on the growth of high quality diamond single crystal in carat grade, Jingangshi Yu Moliao Moju Gongcheng, 2011, vol. 31, no. 6, pp. 26–28.
Sung, C.M. and Tai, M.F., Reactivities of transition metals with carbon: Implications to the mechanism of diamond synthesis under high pressure, Int. J. Refract. Met. Hard Mater., 1997, vol. 15, no. 4, pp. 237–256.
Zhang, Y.F., Zang, C.Y., Ma, H.A., Liang, Z.Z., Zhou, L., Li, S.S., and Jia, X.P., HPHT synthesis of large single crystal diamond doped with high nitrogen concentration, Diamond Relat. Mater., 2008, vol. 17, no. 2, pp. 209–211.
Kanda, H., Ohsawa, T., Fukunaga, O., and Sunagawa, I., Effect of solvent metals upon the morphology of synthetic diamonds, J. Cryst. Growth, 1989, vol. 94, no. 1, pp. 115–124.
Funding
The authors greatly acknowledge the financial support by Project for Key Science and Technology Research of Henan Province, China (182102210311), Natural Science Foundation of Henan Province (grant nos. 182300410279 and 182300410248), Program for Innovative Research Team (in Science and Technology) in the University of Henan Province (19IRTSTHN027).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Guangjin Gao, Li, S., Hu, M. et al. The Crystal Stock Phenomenon in the Process of the Plate Shape for Type Ib Large Synthetic Diamond Single Crystals. J. Superhard Mater. 42, 401–408 (2020). https://doi.org/10.3103/S1063457620060039
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1063457620060039