Abstract
A low-waste combined technology for water softening, desalination, and deionization is proposed. The low-waste nature of the method is achieved through H-cationization of water using carboxylic polyacrylic ion exchange resin, followed by its deionization via reverse osmosis. If the requirements for desalinated water are high, such as when purified liquid is used for feeding supercritical pressure once-through boilers (SPB), it is additionally treated using ion exchange. The preconcentrate from reverse osmosis is processed through electrodialysis or electrolysis to yield acidic and alkaline solutions. These solutions are then used in conjunction with spent regeneration solutions from deep deionization ion exchange filters to regenerate the filter containing carboxylic polyacrylic ion exchange resin. The paper outlines the advantages of carboxylic cation exchange resins compared to sulfonated cation exchangers. A two-chamber H+,Na+-cation exchange filter operation circuit is proposed. Recirculation of the acidic portion of the spent regeneration solution from the BC storage reservoir through the H+Na+-cation exchange filter is planned to maximize the recovery of the cation exchange resin’s working capacity. The specifics of regenerating carboxylic cation exchange resin with acid solution in a fluidized bed mode are presented. The acidic solution storage tank should be constructed as a reservoir with a conical bottom and a cylindrical upper part. Such tank design enables its use as a gypsum particle crystallizer and settler. This circuit of separate water H+,Na+-cationization with a decarbonizer significantly expands technological capabilities. Intermediate water decarbonization decreases the alkalinity of the Na+-cation exchange filter effluent, thereby hindering the hydrolysis of the salt form of the cation exchanger. Water obtained through magnesium ionization is advisable to be directed into the softened water stream. To prevent an increase in liquid pressure drop across the filter and a sharp decrease in filtration rate, it is proposed to pass the alkaline regeneration solution from bottom to top.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Previously, we have examined combined systems for water softening, desalination, and deionization using ion exchange resins. New perspectives are opened by the utilization of membrane technologies along with ion exchange approaches. According to available information, such technical solutions have already been implemented in Ukraine in more than ten facilities. Thus, there are both necessary and sufficient conditions for the widespread adoption of the proposed technology.
ANALYSIS OF KNOWN TECHNICAL SOLUTIONS
In the known solutions [1–5], antiscalants are applied or water softening is performed using sulfonated cation exchange resins to prevent scaling on membranes. The former approach increases operational costs (the cost of antiscalant is approximately 100 UAH/kg; specific consumption ranges from 4 to 30 g/m3, adding 0.2–4.5 UAH/m3 to the cost of treated water and is associated with discharges of antiscalant into water bodies). The latter approach implies both the cost increase of the technology due to the use of sodium chloride for ion exchange regeneration and discharges of high-salinity effluents [1]. The analysis of existing technical solutions has demonstrated significant reagent expenses and harmful environmental impacts due to the discharge of both unused reagents and extracted salts from water. The reagent consumption and, consequently, the amount of discharged substances into the environment can be substantially cut by utilizing compounds obtained from waste or byproducts of their processing in water treatment technology. Therefore, the proposed solution in this study involves using extracted salts from water for the internal needs of the water treatment plant (WTP).
PROPOSED TECHNOLOGY
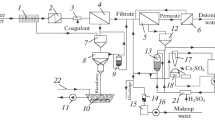
Figure 1 illustrates one of the versions of such a technological circuit. The incoming water from a surface source, which has significant turbidity and coloration, enters the clarifier 2, where it is freed from most suspended solids and coloring compounds. The clarifier efficiency is ensured by dosing it with one or a mixture of aluminum- or iron-based coagulants [1]. The coagulant dosage is determined experimentally based on laboratory tests of trial coagulation of suspensions at each significant change in the turbidity and/or coloration of the incoming water and is finally set during commissioning [1].
Schematic diagram of the desalination, softening, and deionization of surface water: (1) inlet water, (2) clarifier, (3) mechanical filter, (4) filter loaded with H+,Na+-carboxylic cation exchange resin, (5) reverse osmosis unit, (6) H‑cation exchange resin filter, (7) OH-anion exchange resin filter, (8) mixed-bed filter, (9) deionized water, (10) electrodialysis or electrolysis unit, (11) acidic water accumulation tank, (12) alkaline water accumulation tank, (13) softened water, (14) desalinated (drinking) water, and (15) discharge into sewage system.
The clarification of water to meet the standards of its consumers (typically drinking water supply networks) is carried out in mechanical filter 3, loaded with quartz sand or crushed anthracite. If the clarified water meets the mineral composition standards [6, 7] or consumer requirements, it is entirely directed into the network. In cases where the mineral composition of water impurities does not meet such requirements or correction towards optimizing properties is desirable (e.g., drinking water with comprehensive physiological parameters and/or organoleptic qualities [6]), the water for desalination is divided into two streams: one stream goes into the water supply network, and another undergoes further treatment along with streams of softened water and water to be deionized.
The subsequent treatment of all water streams undergoing softening and deionization is conducted on an H+,Na+-cation exchange filter loaded with polyacrylic cationite. Any ion-exchange resins available in the market can be used as loading material. However, it is advisable within the territory of Ukraine to employ cation exchange resins conforming to either interstate standards [8] or Ukrainian standards, such as [9–11]. The advantage of polyacrylic cationites compared to sulfonic cationites, at first glance, lies in their high exchange capacity, which ranges from 3.8 to 4.5 mol/dm3 in substance equivalents, whereas for sulfocationites, it is 2.0 to 2.2 mol/dm3 [8–11]. However, such an advantage is, at least partially, illusory. When converting carboxylic cationite from the hydrogen form to the sodium form, the volume of the ionite layer increases by 50–100%. In contrast, the volume of the strong acid sulfocationite layer decreases by 10–15%. Therefore, the layer height, and consequently, the loading volume of commercial H-form carboxylic cationite in the filter should be 1.6–2.1 times smaller than those for sulfonic cationite. The largely positive effect of high exchange capacity can be utilized during the regeneration of polyacrylic cationites only with acids. In this case, the conversion of the ionite to the salt form during H-ionization of water leads to a swelling of the adsorbent by no more than 20% [2, 9–11]. When regenerated solely with acids, carboxylic cationites are capable of partially softening water by reducing its temporary hardness but are unable to adsorb cations of permanent hardness.
Significant advantages of carboxylic cationites over sulfonic cationites include:
(1) The ability of the regeneration of the cationite, including practically complete regeneration, with solutions of acids contaminated with salts (preliminary publications by one of the co-authors in this same journal). In the considered technological circuits of water desalination, softening, and deionization (Figs. 1 and 2), this enables far from complete decomposition of salts into acid and alkali in the electrolyzer and/or electrodialyzer, thereby decreasing electricity consumption for acid and alkali production, and facilitating the recirculation of the acid solution to increase the completeness of cationite regeneration;
Schematic diagram of the desalination, softening, and deionization of groundwater: (1) inlet water, (2) filter loaded with H+,Na+-carboxylic cation exchange resin, (3) reverse osmosis unit, (4) H-cation exchange resin filter, (5) OH-anion exchange resin filter, (6) mixed-bed filter, (7) electrodialysis or electrolysis unit, (8) acidic water accumulation tank, (9) alkaline water accumulation tank, (10) sewage system, (11) softened water, (12) desalinated (drinking) water, and (13) deionized water.
(2) High efficiency of cation exchange resin regeneration in a pseudoliquid layer (in a fluidized bed mode), which helps to avoid the formation of deposits inside the granules and on the surface of the ionite grains. The procedures required to implement this process are detailed in our published works;
(3) The significant affinity of polyacrylic cationites in their sodium form towards divalent ions during their adsorption from diluted electrolyte solutions at ionic strengths typical of fresh and slightly saline waters. All of these features are utilized to varying extents in the proposed technical solutions.
The fundamental technological systems for regenerating H+,Na+-cationite during the treatment of both surface and groundwater involve the recirculation of the acidic portion of the spent regeneration solution from the storage tank 8 through the H+,Na+-cation exchange filter (Figs. 1 and 2). This enables the achievement of equilibrium between the spent regeneration solution and the entire ionite layer, ensuring maximum restoration of the cationite’s working capacity. By varying the composition of the recirculation solution, we can alter the pH, acidity, or alkalinity of the softened water.
Let us consider the range within which we can change the mentioned parameters. In [13], potentiometric titration curves of the polyacrylic cationite Lewatit CNP 80 in the presence of 0.01 M and 2.0 M sodium chloride are presented (Fig. 1 of [13]). It follows from the figure that significant adsorption of sodium ions occurs at pH > 4 in the presence of 0.01 M sodium chloride. At pH >8, the ionite completely transitions into the salt form. The concentration of sodium chloride in the solution, 0.01 mol/dm3, corresponds to the salt concentration of fresh water. Therefore, during H+,Na+-cation exchange treatment of fresh waters softened water with pH values ranging from 4 to 8 can be obtained. According to the carbonic acid equilibrium in water, the lower limit is reached when there are no bicarbonate ions in the solution, while the upper limit is reached when dissolved carbonic acid is absent. This result aligns entirely with the known characteristic of carboxylic polyacrylic cationites. In their H+-form, they can only remove cations of temporary hardness from water.
THEORETICAL BASIS OF A NEW TECHNOLOGY
In [14], Fig. 1 presents the potentiometric titration curves of six samples of polyacrylic cationites with a solution of calcium hydroxide. These potentiometric titration curves are slightly shifted towards the acidic side compared to a similar dependence for the H+–Na+ equilibrium. Noticeable adsorption of calcium ions occurs at pH 3.0–3.2. The ionites completely transit into the salt form at pH values of the equilibrium solution greater than 7.0 but significantly less than 8.0. For weak acid cationites, the exchange curves of H+–Na+, H+–Mg2+, and H+–Ca2+ ion exchangers from different manufacturers almost coincide ([14] and other published works). Furthermore, based on the potentiometric titration curves of carboxylic polyacrylic cationites at various ionic strengths of the solution, these values can be calculated in advance [14], as the exchanges of H+–Na+, H+–Mg2+, and H+–Ca2+ are satisfactorily described by the modified equation
or the generalized Henderson–Haselbalch equation
where K1 and K2 are imaginary equilibrium constants, corresponding to reactions (3) and (4), respectively; pMe is the negative decimal logarithm of the molar concentration of metal ions in the solution; α is the degree of conversion of the ionite from the acidic to the salt form, calculated as the ratio of the amount of adsorbed metal cation to the total exchange capacity of the cationite (regardless of the charge of the cation, ion adsorption is expressed in substance equivalents in moles), n is an empirical coefficient, the value of which deviates from ideality. Reactions (3) and (4) correspond to two adsorption mechanisms.
According to [14], unlike the H+–Ca2+, H+–Mg2+, and H+–Na+ equilibria, the resinate solution in exchanges Na+–Mg2+ and Na+–Ca2+ approaches ideality. Relatively small deviations of resinate solution properties from the ideal can be explained by the presence of two types of fixed ionogenic centers in the ion exchanger: paired and spatially separated. Spatially separated centers are formed during the polymerization of acrylic acid monomer through a “head-to-tail” mechanism,
and paired ones, through the “head-to-head” and “tail-to-tail” mechanisms,
Two types of reactions occur at ion exchange centers (3) and (4). Exchange centers (3) act as independent units. The exchange for them is described by the equation
Paired ionogenic groups react with singly charged cations as one unit,
Due to the close spatial arrangement of paired exchange centers, their selectivity for the adsorption of divalent cations is significantly higher than that of spatially separated centers. This effect specifically enables the thorough softening of water in the sodium form of polyacrylic cationites.
According to [14], the proportion of paired exchange centers in the total adsorption capacity of polyacrylic cationite Lewatit CNP 80, as determined from the investigation of the Na+–Ca2+ exchange equilibrium, amounts to 21 ± 8%. For the Na+–Mg2+ exchange, a value of 7 ± 6% was found. These data indicate significant discrepancies. To verify the reliability of such information and to obtain insights into the presence of the effects described above for any porous polyacrylic cationites, subsequent studies have investigated Na+–Ca2+ equilibria for Dowex MAC-3, Purolite C104, and Relite CNS cationites. To determine the reproducibility of experimental data, the isotherm of Na+–Ca2+ exchange for the same sample of Lewatit CNP 80 cationite was experimentally determined. The same exchange curves were then measured for the gel carboxylic cationite Amberlite IRC 86 and the macroporous Purolite C107 [15]. For the seven aforementioned cation exchangers, the Na+–Ca2+ exchange isotherms within the range of experimental data distribution coincide. Additionally, according to Figs. 2 and 3 of the same article, the potentiometric titration curves are satisfactorily described by the modified Nikolsky equation,
Operation of a two-chamber H+,Na+-cation exchange resin filter: (1) inlet water, (2) pre-included filter chamber, (3) decarbonizer, (4) pump, (5) main filter chamber, (6) softened water, (7) accumulation tank for spent water from the pre-included filter chamber, (8) pump, (9) pump, and (10) accumulation tank for spent water from the main filter chamber.
or the generalized Henderson–Haselbalch equation
where K3 and K4 are imaginary equilibrium constants, corresponding to Eqs. (7) and (8), respectively; pMe is the negative decimal logarithm of the molar concentration of magnesium or calcium ions in the solution; α is the degree of conversion of the ionite from the sodium to the salt form with a divalent metal ion, which is calculated as the ratio of the amount of adsorbed divalent metal cation to the total exchange capacity of the cationite.
Information necessary for calculations of the values of pK and n for the H+–Ca2+, H+–Mg2+, H+–Na+, Na+–Mg2+, and Na+–Ca2+ equilibria is reported in [13–15] and other publications.
RESULTS AND DISCUSSION
Regeneration of Carboxyl Cation Exchanger
Special attention should be paid to the regeneration of carboxylic cationites. Preliminary regeneration with an acid solution is advisable to be carried out in a fluidized bed mode. This eliminates the expansion stage of the ionite, clarification of water during expansion, and decreases the hydraulic load on the distributor and mechanical filter, as the neutral portion of the spent regeneration solution is discharged. During acid regeneration, it is expedient to carry out the regeneration in a direct-flow mode, by supplying the solution from top to bottom with recirculation through the acid solution storage tank. This tank should be constructed as a reservoir with a conical bottom and cylindrical upper part. Such a design enables the tank to be used as a crystallizer and settling tank for gypsum particles if they are formed. For this purpose, the H+,Na+-cation exchange filter is supplied to the lower part of the tank. The expansion of the conical part upwards ensures the classification of suspended gypsum particles by size. Larger particles accumulate in the lower part of the tank and are removed together with its blowing. Finer suspension, classified by size, serves as nuclei for gypsum crystallization, gradually enlarging and settling downwards. In the cylindrical part of the tank, nuclei for gypsum crystallization form, which gradually settle downwards due to enlargement. The residence time of the recirculation solution in the BC tank should be at least 30 min. This, as experience shows, is sufficient to remove the saturation of the solution with calcium sulfate.
Another operational feature of the H+,Na+-cation exchange unit for water is related to its hardware design. Due to the significant swelling of carboxylic cationites when transitioning from the acidic form to the sodium form, the height of their loading in the form of the exchangeable product (H-form) into filters is much less than that of sulfonic cationites. This prevents fully leveraging the advantages of weakly dissociated ionites in the acidic form. However, if sodium and hydrogen-cation exchanges are conducted separately, technological capabilities are significantly expanded.
Technological Solution for the Separate H+,Na+-Ionization of Water
The schematic diagram for the separate H+,Na+-ionization of water is shown in Fig. 3. In addition to ion exchange filters, it includes decarbonizer 3.
According to design standards [1], each ionization stage should include at least three filters: one in operation, one designated for regeneration, and one spare filter that can be repaired. Since two filter housings are in operation simultaneously during separate H+,Na+-ionization of water, the installation should comprise no fewer than six units. There may be two decarbonators: one in operation and one spare. Intermediate water decarbonization lowers the alkalinity of the Na+-cation exchange filter effluent, preventing hydrolysis of the salt form of the cation exchanger. The main housing is taken offline for regeneration due to the breakthrough of hardness ions. Such breakthrough is caused by magnesium ions. Therefore, if the magnesium hardness of the treated water is not regulated, the filter can be taken offline for regeneration based on the breakthrough of calcium ions. The water obtained through magnesium ionization should be directed into the softened water stream. Both housings of the filter designated for regeneration are rinsed with an acidic solution from tank 7. After a brief intermediate flushing with feed water, the main filter housing is additionally regenerated with an alkaline solution from tank 10, accompanied by a significant increase in liquid pressure drop across the filter and a sharp decrease in filtration rate. To avoid this effect, the alkaline regeneration solution should be passed from bottom to top. Simultaneously, there is recirculation of the alkaline solution from tank 10 through the tank after a neutral portion of the filtrate is discharged into the sewer. At this stage of regeneration, ionite layer compaction may occur due to the strong swelling of the cation exchanger (by 10–30%), preventing expansion of the ionite layer.
The main decrease in the salt content of water is achieved through its demineralization in the reverse osmosis unit (Figs. 1 and 2). The quality of the permeate from membrane units depends on the properties of the membranes and their service life. As experience shows, during the first year of operation, the salt content of the permeate increases almost by an order of magnitude and then stabilizes at a level of 2–4 μmol/dm3.
Thus, on one of the first reverse osmosis demineralization units put into operation in Ukraine (with a capacity of 1040 m3/h of deionized water, including 700 m3/h of deeply deionized water), during the initial months of operation, the salt content of the permeate was maintained at a level of 0.3–0.4 μmol/dm3. Subsequently, it began to increase and stabilized within the specified limits over the next 1–2 years. This confirms our conclusion regarding the feasibility of using equipment 6 and 7 (Fig. 1) or 4 and 5 (Fig. 2) in the technological scheme alongside the FSD filter.
CONCLUSIONS
Thus, the following results were obtained in the study:
— Analysis of existing technical solutions indicates significant reagent costs and their detrimental environmental impact due to the disposal of both unused reagents and extracted salts from water;
— Reagent consumption and, consequently, the amount of discharge into the environment can be significantly decreased by using compounds formed as waste or byproducts of their processing in water treatment technology;
— The proposed low-waste combined technology for water softening, desalination, and deionization presented in the study provides suitable conditions for implementing such an approach. The low-waste nature of the method is achieved through H+,Na+-ionization of the feed water using carboxylic polyacrylic ion-exchange resin, followed by its deionization via reverse osmosis. The reverse osmosis preconcentrate is further processed through electrolysis or electrodialysis to produce acidic and alkaline solutions, which are utilized in conjunction with spent regeneration solutions from deep deionization ion-exchange filters for regenerating the filter containing carboxylic polyacrylic ion-exchange resin;
— The advantages of using carboxylic cation exchange resins over sulfonated cation exchange resins have been discussed based on their physicochemical and technological characteristics;
— A circuit for operating a two-chamber H+,Na+-cation exchange filter in the proposed technology has been presented;
— The technology demonstrates significant potential for practical application, as evidenced by the operation of over ten water treatment plants equipped with reverse osmosis units in Ukraine alone.
REFERENCES
DBN V.2.5-74:2013. Water Supply. External Networks and Structures, Kyiv, 2013.
Dow Water Solutions DOWEX™ Ion Exchange Resins Water Conditioning Manual, Lenntech: Dow Chemical, 1995.
Goh, P.S. and Ismail A.F., A review on inorganic membranes for desalination and wastewater treatment, Desalination, 2018, vol. 434, no. 5, pp. 60–80. https://doi.org/10.1016/j.desal.2017.07.023
Ayol, A., Demiral, Y.O., and Güneş, S., Efficient treatment of domestic wastewaters by using a dynamic membrane bioreactor system, J. Membr. Sci. Res., 2021, vol. 7, no. 1, pp. 55–58. https://doi.org/10.22079/JMSR.2020.120244.1330
Choi, J. Dorji, P., Shon, H.K., and Corrigendum, S.H., Applications of capacitive deionization: Desalination, softening, selective removal, and energy efficiency, Desalination, 2019, vol. 468, no. 10, pp. 118–130. https://doi.org/10.1016/j.desal.2019.114096
DSTU 7525:2014. Drinking Water. Requirements and Methods of Quality Control, Kyiv, 2014.
GKD 34.20.507-2003: Rules for Technical Operation of Electrical Stations and Networks, Kyiv, 2003.
GOST (State Standard) 20298-74: Ion-Exchange Resins. Cation Exchangers. Specifications, Moscow, 1991.
TU U 02071045-001-98: Cation Exchange Resins. Cation Compounds. Requirements for the Quality of Cations of the Purolite Company. Introduction, 1997.
TU U 02071045-001-98: Cation Exchange Resins. Cationites. Requirements for the Quality of Cations of the Rohm and Haas Company. Introduction, 1998.
TU U 00013579-001-99: Cation-Exchange Resins. Cations. Requirements for the Quality of Cations of the Dow Chemical Company. Introduction, 1999.
Crittenden, J.C., Trussell, R.R., Hand, D.W., Howe, K.J., Tchobanoglous, G., Water Treatment: Principles and Design, New York: Wiley, 2012, 3rd ed.
Mamchenko, O.V. and Valuyskaya, Ye.A., A model of acidbase equilibrium for weakly dissociated ion exchangers, J. Water Chem. Technol., 1998, vol. 20, no. 5, pp. 10–18.
Mamchenko, A.V. and Valuiskaya, E.A., Analysis of potentiometric titration curves for carboxyl ion exchangers in terms of the exchange equilibrium theory, J. Water Chem. Technol., 1998, vol. 20, no. 9, pp. 1–10.
Mamchenko, A.V. and Valuiskaya, E.A., H+Mg2+ exchange on carboxylic ion exchanges from the viewpoint of the exchange equilibria theory, J. Water Chem. Technol., 1999, vol. 21, no. 1, pp. 1–14.
Funding
This work was supported by ongoing institutional funding. No additional grants to carry out or direct this particular research were obtained.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The autors of this work declare that they have no conflicts of interest.
Additional information
Publisher’s Note.
Allerton Press remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Mamchenko, O.V., Pakhar, T.A. Combined Technology of Water Softening, Desalination, and Deionization. J. Water Chem. Technol. 46, 125–131 (2024). https://doi.org/10.3103/S1063455X24020103
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1063455X24020103