Abstract
Make-up water is treated at thermal power stations (TPS) with high-pressure or superhigh pressure boilers using membrane processes implemented in ultrafiltration, microfiltration, or reverse-osmosis (RO) units. Among the criteria of the efficiency of reverse osmosis units is the amount of highly mineralized effluents (or concentrate). At present, the RO concentrate is disposed of at TPSs by discharging it into an industrial sewage system in accordance with the applicable standards on the salt content limit of waste water, routing it into a district heating network, or returning it into recirculation water supply systems, decreasing as far as possible the volume of the discharged concentrate which is to be reused, for example, in regeneration of Na-cation exchangers installed upstream of the reverse-osmosis unit. The adsorption process is proposed for treatment of the reverse-osmosis concentrate using sludge from the makeup water treatment. The characteristics of carbonate sludge are presented. The regularities of adsorption of sulfate- and chloride-anions from the RO concentrate by carbonate sludge are described. An adsorption isotherm was obtained. The mechanism of adsorption on a sorption material is proposed. The effect of pH on adsorption of sulfate- and chloride-ions by a sorption material was investigated. A system is proposed for the treatment of the concentrate from the RO units at the Kazan Cogeneration Power Station TETs-2 to remove sulfate- and chloride anions using a three-stage adsorption method with a counter-current injection of the sorbent, namely, the carbonate sludge. The calculated values of the consumption of the sorption material required to achieve the desired residual concentration of sulfate- and chloride-anions in the treated water are presented. The economic effectiveness from implementation at the Kazan TETs-2 of the adsorption treatment of the RO concentrate by carbonate sludge to remove sulfate- and chloride-ions is estimated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
At present, the make-up water at thermal power stations (TPS) with high-pressure and superhigh-pressure boilers is treated by demineralization in series-connected reverse-osmosis and ion-exchange units. This process has been implemented at the Nizhnekamsk TETs-1, the Zainskaya district power station (GRES), and the Kazan TETs-2.
Among the criteria of the efficiency of reverse-osmosis (RO) units is the amount of highly mineralized effluents (or concentrate). The concentrate is disposed of using the following methods [1]:
(1) Discharge of the concentrate into an industrial sewage system in accordance with the established requirements for the salt content of effluents;
(2) Delivery of the concentrate into a district heating network or its return into water-recirculation systems; and
(3) Reuse of the concentrate, for example, for regeneration of Na-cation exchangers in water treatment systems with RO units.
The latter can be done only with a certain chemical composition of the source water if the following relationship  (here, \({{C}_{{{\text{N}}{{{\text{a}}}^{{\text{ + }}}}}}}\) is the sodium concentration; Ht and Alо are the total hardness and the total alkalinity, respectively) is met. In this case, the content of impurities (such as \(\text{SO}_{4}^{{2 - }},\,\text{SiO}_{3}^{{2 - }},\,\text{CO}_{3}^{{2 - }}\) ions) should be such that low-soluble salts of calcium and magnesium would not form in the ion-exchange bed of Na-cation exchangers.
(here, \({{C}_{{{\text{N}}{{{\text{a}}}^{{\text{ + }}}}}}}\) is the sodium concentration; Ht and Alо are the total hardness and the total alkalinity, respectively) is met. In this case, the content of impurities (such as \(\text{SO}_{4}^{{2 - }},\,\text{SiO}_{3}^{{2 - }},\,\text{CO}_{3}^{{2 - }}\) ions) should be such that low-soluble salts of calcium and magnesium would not form in the ion-exchange bed of Na-cation exchangers.
Application of the RO concentrate treatment procedure is examined by an example of the water-treatment plant (WTP) at the Kazan TETs-2. The RO concentrate is proposed to be treated by an adsorption method using sludge formed during water pretreatment in WTP using lime-softening and coagulation processes.
The experimental investigations were performed using sludge with a moisture content of 3% at the Kazan TETs-1. The dried sludge was a free-flowing fine material ranging in color from light-yellow to brown. Sludge with 0.09–0.50 mm grains was used in the investigation. The chemical composition of the sludge (%) measured by the roentgenographic qualitative phase analysis using a Brukner D8 ADVANCE diffractometer is given below:
Calcite CaCO3 | 73 |
Brucite Mg(OH) | 8 |
Portlandite Ca(OH)2 | No more than 1 |
Quartz SiO2 | 0.4 |
Other substances | 17.6 |
The full chemical composition of the sludge (ion concentration in wt %) is presented below:
Са2+ | 76.56 ± 11.3 |
Fe3+ | 0.38 ± 0.15 |
Mg2+ | 9.7 ± 2.2 |
Cu2+ | 0.04 ± 0.014 |
Ni2+ | 0.008 ± 0.003 |
Zn2+ | 0.033 ± 0.013 |
Mn2+ | 1.05 ± 0.407 |
Cr3+ | 0.001 ± 0.0003 |
Pb2+ | 0.002 ± 0.0003 |
Cd2+ | 0.22 ± 0.08 |
Hg2+ | Traces |
\({\text{CO}}_{3}^{{2 - }}\) | 71.7 ± 10.6 |
\({\text{SO}}_{4}^{{2 - }}\) | 5.7 ± 0.85 |
OH– | 10.03 ± 3.61 |
\({\text{SiO}}_{3}^{{2 - }}\) | 0.52 ± 0.11 |
\({\text{PO}}_{4}^{{3 - }}\) | None |
Organic part | 12 |
Gaseous chromatography-mass spectrometry with electronic ionization carried out using a DFS (FisherSchCu) mass-spectrometer revealed the functional groups of humic substances –ОН, –NH, –CH3, –CH2, aromatic bonds С=С, carboxyl groups –CООН–, and alcohol groups ОН– on the sludge surface.
Previous studies considered cleaning of TPS effluents of dissolved oil products using sludge with a moisture content of 2.88 wt %, a bulk density of 560 kg/m3, a total pore volume of 0.2 cm3/g, and an ash content of 89 wt % as a sorption material.
The data in Table 1 demonstrate that the sludge does not increase secondary pollution of water body.
The carbonate sludge is presented in [3] as a sorption material for cleaning of industrial effluents of heavy metal ions (Fe3+, Cr3+, Zn2+, Cu2+, Ni2+). This paper considers the potential for treatment of the RO concentrate with carbonate sludge to remove sulfate- and chloride-ions. The adsorption capacity of the sludge with respect to these anions is determined experimentally.
The laboratory studies for determining the efficiency of adsorption of sulfate- and chloride ions were performed with the standardized test solutions of Na2SO4 and NaCl with an initial concentration of 300 mg/dm3 (Table 2).
An isotherm of absorption of sulfate- and chloride ions with carbonate sludge was plotted using the variable sample weight method. The concentration of sulfate ions was determined using a PE-5400 UF spectrophotometer in accordance with [4], and the concentration of chloride-ions was measured by the method of mercurimetric titration with a diphenylcarbazide indicator.
We placed 100 mL of the standardized test solution of Na2SO4 into seven conical flasks, and 100 mL of the standardized test solution of NaCl was placed into another seven conical flasks. The concentration of both solution was 300 mg/dm3. Sludge samples of different weight between 0.01 and 2.0 g were added into each flask. The solutions were mixed and then shaken for 24 h in a laboratory shaker. The sorption material was separated from the solution using a paper filter after that, and the residual concentration of sulfate- and chloride ions in the filtrate was measured. The adsorption А, mg/g, was calculated by the formula

where Сinit and Сeq are the initial and the equilibrium concentrations, respectively, of sulfate- or chloride-ions, mg/dm3; V is the standardized solution volume, dm3; m is the sludge sample weight, g.
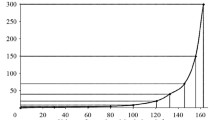
Isotherms for sulfate- and chloride ions (Fig. 1) belong to the H-type according to the Smith classification and describe the chemosorption process. The enthalpy Δh, kJ/mol, and the Gibbs energy ΔG, kJ/mol, were calculated at various temperatures using the plotted adsorption isotherms:
Δh | ΔG | |
\({\text{SO}}_{4}^{{2 - }}\) | +62.3 | –21.5 |
Cl– | +65.4 | –20.08 |
Higher values of the enthalpy can be explained by formation of a strong bond between sulfate- and chloride anions and functional groups in the sludge.
The effect of pH on the adsorption of sulfate- and chloride-anions is shown in Fig. 2. It has been found that high absorption capacity is attained in a range of рН = 3–9 that enables water to be cleaned in an acidic or weakly alkaline environment.
Based on the experimental data, a procedure is proposed for treatment of the RO concentrate to remove sulfate- and chloride ions at the Kazan TETs-2.
Make-up water for the boiler and heat network is processed in the water treatment plant of the Kazan TETs-2 [5]. The source water is pretreated using the inline coagulation process. Aluminum oxychloride is used as a coagulant in acidification of the treated water with sulfuric acid. Make-up of the heating network is performed with partly demineralized water (permeate) from the RO unit. To make up the boilers, demineralized water after additional treatment of the permeate in H-cation and OH-anion exchangers is used.
Quality indices of the source water, permeate, and RO concentrate are given in Table 3.
It is proposed to use a multistage adsorption unit with sequential or countercurrent metering of a sorbent as a process unit for treatment of the RO concentrate to remove sulfate and chloride ions with a sorption material.
Static adsorption is performed by vigorous mixing of the treated water with a sorbent for a certain time followed by separation of the sorbent from water by settling or filtration [6]. The adsorption process is carried out in one or, more often, several stages. Single-stage cleaning of the sorbent is employed at low initial concentrations of sorbent impurities when a small amount of the sorbent is required or if the sorbent is inexpensive and readily available. In the multistage sorption by metering new portions of the sorbent into water, a certain difference in the concentration of the extracted substance between the water and the sorbent is maintained all the time, thereby increasing the adsorption rate and decreasing consumption of the sorption material.
Multistage adsorption can be done with sequential or countercurrent metering of the sorbent. In the first case, the sorption material is metered into water in portions at each treatment stage; in the second case, it is introduced only at the last treatment stage and then is pumped from each subsequent stage to the previous one.
To select the most-efficient and cost-effective option for the adsorption treatment of RO concentrate, the total consumption of the sorption material, mtot, and the consumption in each stage, mst, were calculated for the following input data:
Adsorption rate, mg/g, for | |
Sulfate ion | 130.44 |
Chloride ion | 113.702 |
Initial concentration in concentrate, mg/dm3: | |
\({\text{SO}}_{4}^{{2 - }}\) | 334.57 |
Сl– | 81.39 |
Final concentration in permeate, mg/dm3: | |
\({\text{SO}}_{4}^{{2 - }}\) | 0.28 |
Сl– | 0.11 |
Concentrate flow, dm3/h | 20 000 |
The predictions presented in Table 4 demonstrate that the three-stage adsorption with counter-current metering of the carbonate sludge is the most efficient and cost-effective method for treatment of RO concentrate to remove sulfate- and chloride ions.
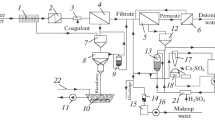
The schematic diagram of an adsorption unit with counter-current metering of carbonate sludge and its regeneration with hot water is shown in Fig. 3.
RO concentrate enters the Stage I reactor 1 with a vane mixer, into which pump 10 feeds carbonate sludge from Stage II receiving tank 9. The concentrate is mixed vigorously with the carbonate sludge in the reactor. The prepared suspension is routed to Stage I thin-layer settling tank 2 for separation of the waste carbonate sludge and removal of water after the initial treatment to Stage II. The waste carbonate sludge is directed to reactor 11 for regeneration with hot water.
Water after the initial treatment with the carbonate sludge enters Stage II of the unit, i.e., reactor 3 with a vane mixer where pump 8 feeds carbonate sludge from Stage III receiving tank 7. The suspension is then routed to Stage II thin-layer settling tank 4 for separation of the waste carbonate sludge and removal of the water after second treatment to the last Stage III.
Water after second treatment with carbonate sludge enters Stage III of the unit, i.e., Stage III reactor 5 with a vane mixer where pump 14 feeds fresh carbonate sludge from tank 13 where the sludge is fed after its regeneration in settling tank 12. Then the suspension is routed to Stage III thin-layer settling tank 6 for separation of the carbonate sludge from water and removal of the treated (clarified water) after the third treatment.
The application of the proposed scheme for adsorption treatment of the RO concentrate for removal of sulfate- and chloride-ions with carbonate sludge at the Kazan TETs-2 will reduce the environment protection cost by 412 000 rubles/year.
CONCLUSIONS
(1) The adsorption isotherms plotted on the basis of the experimental data belong to the H-type according to the Smith classification and describe the chemosorption process. Thermodynamic and kinetic characteristics of the process are as follows: the Gibbs energy is between –21.5 and –20.08 kJ/mol and the enthalpy ranges from 62.3 to 65.4 kJ/mol.
(2) In the developed three-stage adsorption unit for treatment of RO concentrate to remove sulfate- and chloride-ions, the carbonate sludge injected in a counter-current manner is regenerated with hot water. The consumption of the sorbent fed into each treatment stage for attainment of the required residual concentration of sulfate- and chloride-ions in the treated concentrate was, respectively, 209.48 and 79.5 g/dm3.
(3) The implementation of the adsorption process for treatment of the RO concentrate with carbonate sludge at the Kazan TETs-2 will cut down the costs for ensuring environmental safety of the Kuibyshev reservoir in the Republic of Tatarstan where treated effluents/waste water are discharged by 412 000 rubles/year.
REFERENCES
I. A. Malakhov, A. A. Askerniya, I. I. Borovkova, G. I. Malakhov, V. A. Rogovoi, V. Yu. Lebedev, and N. N. Velichkina, “Technology for deep demineralization of makeup water at thermal power stations with utilization of wastewaters,” Therm. Eng 53, 596–599 (2006).
SanPin 2.1.4.1074-01. Potable Water (Minzdrav Rossii, Moscow, 2002).
T. G. Lupeiko, E. M. Bayan, and M. O. Gorbunova, “Use of carbonate-containing industrial waste for treatment of aqueous solutions to remove nickel(II) ions,” J. Appl. Chem. 77, 83–87 (2004).
MU 08-47/250. Thermal Waters. Methods of Sulfates Mass Concentration Estimation (With Change No. 1) (Sib-Strim, Tomsk, 2010).
Modernization of the Water Treatment Unit at “Kazanskaya TETs-2” Thermal Power Plant. http://www1. ta-tgencom.ru/.
Sewerage of Populated Places and Industrial Enterprises. Designer’s Handbook, Ed. by V. N. Samokhin (Stroiizdat, Moscow, 1981) [in Russian].
ACKNOWLEDGMENTS
The work was performed within the scope of the basic part of the state assignment in the field of scientific activities (no. 13.6384.2017/BCh).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by T. Krasnoshchekova
Rights and permissions
About this article
Cite this article
Nikolaeva, L.A., Minneyarova, A.R. Adsorption Treatment of Reverse-Osmosis Concentrate from Water-Treatment Units at Thermal Power Stations. Therm. Eng. 66, 372–376 (2019). https://doi.org/10.1134/S0040601519050069
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0040601519050069