Abstract
Potentialities have been studied for using flux-cored wire containing industrial wastes (dust taken from the gas-purification facilities of silicomanganese and aluminum production) in order to perform wear-resistant hardfacing. The hardfacing procedure has been carried out using a welding tractor under silicomanganese slag produced by the West Siberian Electrometallurgical Plant. The wear rate of the samples was determined using a 2070 SMT-1 machine. The method for determining wear rate is based on changing in the sample weight during disk-pad testing. The chemical composition of the hardfaced metal layer has been determined using an XRF-1800 X-ray fluorescence spectrometer and using a DFS-71 spectrometer according to atomic emission method. The hardness of the hardfaced layers was measured using a METH-DO hardness tester. The evaluation of the number of nonmetallic inclusions was performed according to GOST (State Standard) 1778–70 using an OLYMPUS GX-51 optical microscope. The manganese uptake coefficient was found at different ratios between components. This coefficient is associated with the reduction of manganese oxide of the manganese-containing flux (due to the carbon contained in the flux-cored wire). In the case of a significant excess of carbon in the flux-cored wire based on manganese-containing flux, the level of manganese uptake exceeds 100%. The process of manganese uptake is determined by the filling coefficient of the flux-cored wire, by the amount of the carbon-containing material in the charge mixture, and by the content of carbon in the arc coating itself. The hardfaced metal layer contains nondeforming silicates and point oxides. The contamination of the hardfaced metal layer by oxide-based nonmetallic inclusions is low. The presence of these nonmetallic inclusions does not affect to any significant extent the operational characteristics of the hardfaced layer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
In Russia and abroad, much attention is paid to the development of novel materials. One of the directions consists in the development of flux-cored wires for hardfacing [1–4]. Of interest are economically alloyed technological hardfacing materials [5–8] that provide the formation of a low-carbon martensite structure in the hardfaced metal layer (there is an effect of self-quenching during cooling). Upon using such economical hardfacing materials, a bainite-austenitic structure is formed in the hardfaced metal layer. Such a structure exhibits a higher wear resistance level than the structure obtained by hardfacing with the help by widely using a PP-Np-18Kh1G1M flux-cored wire containing expensive molybdenum [9–12].
The wires based on manganese and manganese-containing components play an important role in the wear resistance of the hardfaced layers. The distinctive feature of the low-carbon manganese-based hardfaced metal of the martensitic class consists in an increase in abrasive wear resistance, despite a decrease in hardness [13–16].
One of the promising directions in developing technologies for the formation of wear-resistant coatings and hardfaced layers employing the electric-arc coating method consists in the use of flux-cored wires containing metallurgical industry wastes served as fluxes. However, the development of this direction is hindered due to the lack of data concerning the dependences and regularities of the effect of various factors exerted on the coating structure and properties. That is why the studies wherein metallurgical production wastes are used as wire components [17–20] are of particular interest.
This work was aimed at developing a novel modification of flux-cored wire based on gas-purification dust taken from silicomanganese production facilities. To do this, we studied the potentialities of obtaining wear-resistant hardfaced layers with the use of flux-cored wires containing dust taken from gas-purification facilities of silicomanganese production (manganese oxide as a reduced component), as well as dust taken from gas-purification facilities of aluminum production (as a reducing agent).
MATERIALS AND METHODS
The flux-cored wire was obtained using a laboratory plant according to technology based on passing through dies. The sheath of the flux-cored wire was made of St3-grade steel tape, the wire diameter being of 6 mm. As the filler, we used dust taken from gas-purification facilities of aluminum and silicomanganese production. The chemical composition of aluminum production dust was as it follows (wt %): 21.00–46.23% of Al2O3; 18–27% of F; 8–15% of Na2O; 0.4–6.0% of K2O; 0.7–2.3% of CaO; 0.50–2.48% of Si2O; 2.10–3.27% of Fe2O3; 12.5–30.2% of Ctotal; 0.07–0.90% of MnO; 0.06–0.90% of MgO; 0.09–0.19% of S; 0.10–0.18% of P.
The gas-purification dust taken from silicomanganese production facilities contained the following concentrations of components(wt %): 2.43% of Al2O3; 1.32% of Na2O; 5.56% of K2O; 6.4% of CaO; 29.19% of SiO2; 0.137% of BaO; 7.54% of MgO; 0.23% of S; 0.04% of P; 1.067% of Fe; 27.69% of Mn; 2.687% of Zn; 3.833% of Pb. Table 1 shows the componential compositions of the wires under study, as well as the values of obtained filling coefficients and manganese uptake level.
The procedure of hardfacing was carried out with the use an ASAW-1250 welding tractor. The metal layer was hardfaced onto steel plates with a thickness of 14–16 mm and 10 × 500 mm in size. Hardfacing was carried out under a flux based on silicomanganese slag produced by the West Siberian Electrometallurgical Plant.
The composition of the slag was as it follows (wt %): 6.91–9.62% of A12O3; 22.85–31.70% of CaO; 46.46–48.16% of SiO2; 0.27–0.81% of FeO; 6.48–7.92% of MgO; 8.01–8.43 MnO; 0.28–0.76% of F; 0.26–0.36% of Na2O; 0.6–2.0% of K2O; 0.15–0.17% of S; 0.01% of P. The hardfacing mode was as it follows: the current strength amounting to 520 A; the voltage being of 28 V; the welding velocity being of 18 m/h. The chemical composition of the arc coating was determined using an XRF-1800 X-ray fluorescence spectrometer and using a DFS-71 spectrometer with the help of an atomic emission method (Table 2). The hardness of the arc coatings was measured using a MET-DU hardness tester [17, 18].
In order to determine the wear rate, we used a method based on changing in the sample weight in the course of disk-pad testing [17, 18]. The wear rate of the samples was determined using a 2070 SMT-1 machine. The wear rate testing was carried out at a load of 78.4 N and a rotation frequency of 20 rpm. The rotation frequency was measured by a tachogenerator mounted on the motor shaft. The number of revolutions for the shaft rotation was determined using a noncontact method. During testing, the sample interacted with a pad made of R18 grade steel.
The nonmetallic inclusions were assessed according to GOST (State Standard) 1778–70. The metallographic microsections were studied without etching using an OLYMPUS GX-51 optical microscope [17, 18].
RESULTS AND DISCUSSION
In this paper, we have considered the potentialities of using flux-cored wires containing dust taken from the gas purification facilities of aluminum production (as a reducing agent) and silicomanganese (manganese oxide as a reduced component) for wear-resistant hardfacing.
Tables 2 and 3 show the chemical composition of the electric-arc-induced coating and the composition of slag crusts. The hardness and the results of testing for wear rate are presented in Table 4.
The manganese uptake coefficient was determined as a ratio between the manganese content in the hardfaced metal layer to the total amount of introduced manganese. This coefficient is connected with the reduction of manganese oxide contained in the manganese-containing flux (owing to the carbon contained in the flux-cored wire). In the case of a significant excess of carbon in the flux-cored wire with manganese-containing flux, the manganese uptake exceeds 100% (Table 1).
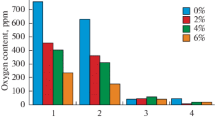
The process of manganese uptake is determined by the filling coefficient of the flux-cored wire, by the amount of carbon-containing material contained in the charge mixture, and by the content of carbon in the arc coating itself (Fig. 1). As a carbon-containing material, we used dust taken from the gas purification facilities of aluminum production [17–20].
The number of nonmetallic inclusions in the electric arc coating was determined according to GOST (State Standard) 1778–70. The assessment results for nonmetallic inclusions are presented in Table 5. Figure 2 shows nonmetallic inclusions in the arc coating: there are nondeforming silicates and point oxides. The contamination with oxide-based nonmetallic inclusions is insignificant. The presence of these nonmetallic inclusions does not exert any significant effect on the operational characteristics of the hardfaced layer [16‒20].
In order to process the results of the studies, statistical experimental data processing have been used, with the help of which curves for the effect of the chemical composition on the properties of the hardfaced layer have been plotted.
CONCLUSIONS
The potentialities of using for wear-resistant hardfacing flux-cored wires containing dust taken from gas purification of aluminum production (as a reducing agent) and silicomanganese (manganese oxide as a reduced component) are confirmed. Parameters and modes for wear-resistant hardfacing are established. The manganese uptake has been determined for different ratios between components. Nondeforming silicates and point oxides have been found in the hardfaced metal. The contamination of the hardfaced metal layer with oxide-based nonmetallic inclusions is low. The presence of nonmetallic inclusions does not significantly affect the operational characteristics of the hardfaced layer.
REFERENCES
Metlitskii, V.A., Flux-cored wires for arc welding and surfacing of cast iron, Weld. Int., 2008, vol. 22, no. 11, pp. 796–800. https://doi.org/10.1080/09507110802593646
Filippov, M.A., Shumyakov, V.I., Balin, S.A., Zhilin, A.S., Lehchilo, V.V., and Rimer, G.A., Structure and wear resistance of deposited alloys based on metastable chromium–carbon austenite, Weld. Int., 2015, vol. 29, no. 10, pp. 819–822. https://doi.org/10.1080/09507116.2014.986891
Liu, D.S., Liu, R.P., and Wei, Y.H., Influence of tungsten on microstructure and wear resistance of iron base hardfacing alloy, Mater. Sci. Technol., 2013, vol. 30, no. 30, pp. 316–322. https://doi.org/10.1179/1743284713Y.0000000359
Kejžar, R. and Grum, J., Hardfacing of wear-resistant deposits by MAG welding with a flux-cored wire having graphite in its filling, Mater. Manuf. Process., 2005, vol. 20, no. 6, pp. 961–976.
Li, R., He, D.Y., Zhou, Z., Wang, Z.J., and Song, X.Y., Wear and high temperature oxidation behavior of wire arc sprayed iron based coatings, Surf. Eng., 2014, vol. 30, no. 11, pp. 784–790. https://doi.org/10.1179/1743294414Y.0000000331
Ma, H.R., Chen, X.Y., Li, J.W., Chang, C.T., Wang, G., Li, H., Wang, X.M., and Li, R.W., Fe-based amorphous coating with high corrosion and wear resistance, Surf. Eng., 2017, vol. 33, no. 1, pp. 56–62. https://doi.org/10.1080/02670844.2016.1176718
Lim, S.C., Gupta, M., Goh, Y.S., and Seow, K.C., Wear resistant WC–Co composite hard coatings, Surf. Eng., 1997, vol. 13, no. 3, pp. 247–250. https://doi.org/10.1179/sur.1997.13.3.247
Zhuk, Yu., Super-hard wear-resistant coating systems, Mater. Technol., 1999, vol. 14, no. 3, pp. 126–129. https://doi.org/10.1080/10667857.1999.11752827
Hardell, J., Yousfi, A., Lund, M., Pelcastre, L., and Prakash, B., Abrasive wear behavior of hardened high strength boron steel, Tribol.-Mater., Surf. Interfaces, 2014, vol. 8, no. 2, pp. 90–97. https://doi.org/10.1179/1751584X14Y.0000000068
Deng, X.T., Fu, T.L., Wang, Z.D., Misra, R.D.K., and Wang, G.D., Epsilon carbide precipitation and wear behaviour of low alloy wear resistant steels, Mater. Sci. Technol., 2016, vol. 32, no. 4, pp. 320–327. https://doi.org/10.1080/02670836.2015.1137410
Kirchgaßner, M., Badisch, E., and Franek, F., Behaviour of iron-based hardfacing alloys under abrasion and impact, Wear, 2008, vol. 265, nos. 5–6, pp. 772–779. https://doi.org/10.1016/j.wear.2008.01.004
Patsekin, V.P. and Rakhimov, K.Z., Proizvodstvo poroshkovoi provoloki (Production of Cored Wire), Moscow: Metallurgiya, 1979.
Tekhnologiya elektricheskoi svarki metallov i splavov plavleniem (Technology of Electrical Welding of Metals and Alloys by Melting), Paton, B.E., Ed., Moscow: Metallurgiya, 1974.
Geller, Yu.A., Instrumental’nye stali (Tool Steel), Moscow: Metallurgiya, 1975.
Teplyashin, M.V. and Komkov, V.G., Effect of alloying elements on wear resistance in alloys for electric slag hardfacing of hammer mills, Uch. Zametki Tikhookean. Gos. Univ., 2013, vol. 4, no. 4, pp. 1554–1561.
Teplyashin, M.V., Komkov, V.G., and Starienko, V.A., Development of a sparingly doped alloy for restoration of hammer mill bits, Uch. Zametki Tikhookean. Gos. Univ., 2013, vol. 4, no. 4, pp. 1543–1549.
Kozyrev, N.A., Kryukov, R.E., Kibko, N.V., and Nepomnyashchikh, A.S., Development of a new wear-resistant cored wire for hardfacing of armor buckets for mining equipment, Naukoemkie Tekhnol. Razrab. Ispol’z. Miner. Resur., 2018, no. 4, pp. 288–292.
Kozyrev, N.A., Usol’tsev, A.A., Prudnikov, A.N., and Kryukov, R.E., Study of properties of cored wire based on ferrochrome gas-cleaning dust, Chern. Metall., Byull. Nauchno-Tekh. Ekon. Inf., 2019, vol. 75, no. 3, pp. 365–373. https://doi.org/10.32339/0135-5910-2019-3-365-372
Kozyrev, N.A., Kryukov, R.E., Usol’tsev, A.A., Umanskii, A.A., and Sokolov, P.D., The development of the new cored wires for surfacing. The new cored wires with the use of the carbon and fluorine containing materials for the repair of the mill, Chern. Metall., Byull. Nauchno-Tekh. Ekon. Inf., 2018, no. 1, pp. 77–86.
Osetkovsky, I.V., Kozyrev, N.A., Kryukov, R.E., Usoltsev, A.A., and Gusev, A.I., Development of a wear-resistant flux cored wire of Fe–C–Si–Mn–Cr–Ni–Mo–V system for deposit welding of mining equipment parts, Proc. Int. Sci.-Res. Conf. on Knowledge-Based Technologies in Development and Utilization of Mineral Resources (KTDMUR2017), June 6–9, 2017, Novokuznetsk, 2017, vol. 84, no. 012017. https://doi.org/10.1088/1755-1315/84/1/012017
ADDITIONAL INFORMATION
Authors ORCID ID. N.A. Kozyrev (0000-0002-7391-6816), A.A. Usol’tsev (0000-0001-6220-7910), L.P. Bashchenko (0000-0003-1878-909X).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated by O. Polyakov
About this article
Cite this article
Kozyrev, N.A., Kryukov, R.E., Usol’tsev, A.A. et al. Development of a Novel Flux-Cored Wire Based on Silicomanganese Gas-Purification Dust. Steel Transl. 51, 847–852 (2021). https://doi.org/10.3103/S0967091221120068
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0967091221120068