The article presents the results of studies of the structure and non-metallic inclusions of metal surfaced from a flux-cored wire of the Fe – C – Si – Mn – Cr – Ni – Mo – V system. In the composition of the wire charge, instead of amorphous carbon, gas cleaning dust of aluminum production containing carbon and fluorine was introduced. Surfacing from a flux-cored wire with a diameter of 5 mm on plates made of St3 steel under AN-26S flux was carried out using a welding machine at a current of 420 – 520 A, a voltage of 28 – 32 V, a welding rate of 7.2 – 9 m/h with cooling at room temperature. The properties of two samples with different content of the carbon-fluorine-containing additive in a flux-cored wire have been studied. The grain size and martensite dispersion in the structure of the surfaced layer have been determined. The analysis of the chemical composition of the surfaced layer and non-metallic inclusions generated in it has been carried out. The hardness of the samples has been measured by the Rockwell method. It has been concluded that it is necessary to establish the optimal amount of the carbon-fluorine-containing additive in the flux-cored wire in order to avoid the formation of an increased amount of non-metallic inclusions in the surfaced layer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
The properties of the surfaced metal, other things being equal, largely depend on the carbon content in it. An increase in the carbon content, on the one hand, increases the hardness and wear resistance of the surfaced layer; on the other hand, it reduces the weldability and the crack resistance of the surfaced metal layer [1].
Earlier, as part of developing the direction for improving the compositions of surfacing materials of the Fe – C – Si – Mn – Cr – Ni – Mo – V system in SibGIU, a new composition of flux-cored wire using a carbon-fluorine-containing material and being the gas cleaning dust of aluminum production has been developed. The use of such a wire allows excluding the formation of pores, cavities, cracks and removing hydrogen in the surfacing [2,3,4,5,6,7,8,9, – 10]. The chemical composition of flux-cored wires obtained using gas cleaning dust of aluminum production and used for surfacing of mining equipment is protected by Russian Federation patents [11, 12].
In this study, the structure and chemical composition of non-metallic inclusions of the surfaced metal obtained using a flux-cored wire of the Fe – C – Si – Mn – Cr – Ni – Mo – V system are determined. In the wire charge composition, instead of amorphous carbon, an additive, i.e. gas cleaning dust of aluminum production containing carbon and fluorine, is introduced. In addition, the effect of increasing the concentration of this additive on the level of contamination of the surfaced metal with oxide non-metallic inclusions was determined.
The objective of this research is to study the microstructure and determine the composition of non-metallic inclusions in the surfaced layer obtained by the electric arc method using flux-cored wires of the Fe – C – Si – Mn – Cr – Ni – Mo – V system of the developed composition.
METHODS OF STUDY
The following powder-like materials were used as wire filler: silicon KR-1 according to GOST 2169–69, manganese MR-0 according to GOST 6008–82, chromium PKhA-1M according to TU 14-1-1474–75, nickel PNK-1L5 according to GOST 9722–97, molybdenum PM-1 according to TU 48-19-102–82, electrolytic vanadium VEL-1 according to TU 48-4-335–86 and iron powder PZhV-1 according to GOST 9849–86. Instead of amorphous carbon, gas cleaning dust from aluminum production of the following chemical composition was introduced (a carbon-fluorine-containing additive), wt.%: 12.5 – 30.2 Ctot; 21 – 46 Al2O3; 18 – 27 F; 8 – 15 Na2O; 0.4 – 6 K2O; 0.7 – 2.3 CaO; 0.5 – 2.5 SiO2; 2.1 – 3.3 Fe2O3; 0.07 – 0.9 MnO; 0.06 – 0.9 MgO; 0.09 – 0.19 S; 0.10 – 0.18 P.
Surfacing was carried out on plates made of St3 steel under AN-26S flux. Flux-cored wire with a diameter of 5 mm, made on a laboratory machine, was surfaced using an ASAW-1250 welding machine (tractor) in the following modes: I = 420 – 520 A, U = 28 – 32 V, welding rate vw = 7.2 – 9 m/h. After surfacing, the samples were cooled at room temperature.
The properties of samples 1 and 2, which differed from each other by the content of a carbon-fluorine-containing additive introduced instead of amorphous carbon into the composition of the flux-cored wire, were investigated.
The chemical composition of the surfaced metal was determined by the x-ray fluorescence method on an XRF-1800 spectrometer and by the atomic emission method on a DFS-71 spectrometer. The hardness of the samples was measured by the Rockwell method in accordance with the requirements of GOST 9013–59.
Non-metallic inclusions were examined according to the standard procedure (GOST 1778–70) on unetched thin sections at ×100 magnification using an OLYMPUS GX-51 metallographic microscope. In order to determine the chemical composition of non-metallic inclusions in the surfaced layer, as well as the elements distribution in the inclusions, the samples were studied using a MIRA 3 LMH scanning electron microscope.
The microstructure of the surfaced metal was studied using an OLYMPUS GX-51 light microscope in a bright field in the magnification range of ×100 – ×1000 after etching the surface of the samples in a 4% nitric acid solution. The grain size was determined according to GOST 5639–82 at ×100 magnification. The martensite dispersion was assessed by comparing the structure with the standards of the corresponding scales and sizes of martensite needles according to GOST 8233–56. The length of the martensite needles was assessed using the SiamsPhotolab700 software package for metallographic studies.
RESULTS AND DISCUSSION
The chemical composition of the surfaced layers of metal obtained using the produced test wire and the results of determining its hardness are given in Table 1.
When examining the surface of samples 1 and 2, a small amount of one-type globular silicate inclusions was revealed. The size of the inclusions does not exceed 24.5 μm. A quantitative analysis of the chemical composition of two identified inclusions was carried out according to two spectra in each.
The results of the analysis of non-metallic inclusions identified in the samples are presented in Tables 2 – 6. The elements distribution on the selected area of all inclusions is shown in Figs. 1 – 4.
Analysis of the chemical composition of the Ø14 μm inclusion in sample 1 showed that the main components in it are Al, Si, Na, and F oxides. The bright phase in the inclusion consists of F, Ca, Mg, Na, and Al oxides (Fig. 1, Table 2). The Ø24 μm inclusion consists of Si, Al, F, Mn oxides; Ca and Mg are present in small amounts. The dark phase in the inclusion consists of Al and Mn oxides with a low Mg content (Fig. 2, Table 3).
Analysis of the chemical composition of the Ø13 μm inclusion in sample 2 showed that the main components in it are Al and Mg oxides with a low Mn content. The bright phase in the inclusion consists of Si, Ca, Al, F, Mg oxides and Mn traces (Fig. 3, Table 4). The Ø24.5 μm inclusion consists of Al, Si, Ca, F, Mn oxides, Na and Mg are present in small amounts. The dark component in the inclusion consists mainly of Al oxides, as well as a small content of Mg and Mn (Fig. 4, Table 5).
When examining the surfaced layer of samples 1 and 2, a significant amount of globular sulfide inclusions (the diameter of which is within 1 μm) uniformly distributed in the plane of the thin section was also revealed. Typical manganese sulfides are shown in Figs. 2 and 3, and their composition is presented in Tables 3 and 4 (spectrum 3 ).
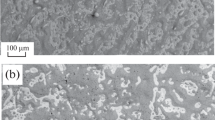
When studying the microstructure of the surfaced metal of samples 1 and 2 after etching, it was established that it has an identical dendritic (columnar) structure typical for cast metal and is martensite (Fig. 5).
The hardness of the surfaced metal, measured by the Rockwell method, was 45.0 – 50.7 HRC on sample 1 and 46.8 – 51.4 HRC on sample 2.
CONCLUSIONS
The chemical composition of non-metallic inclusions in metal surfaced from a flux-cored wire of the Fe – C – Si – Mn – Cr – Ni – Mo – V system with a carbon-fluorine-containing additive instead of amorphous carbon in its charge has been studied. It is shown that the inclusions mainly consist of Si, Al, F, Mn oxides; Ca and Mg are present in small amounts.
Metallographic analysis has shown that the microstructure of the surfaced layers consists of medium-acicular and coarse-acicular martensite formed within the boundaries of the former austenite grain, a small amount of residual austenite in the form of separate sections and thin interlayers of δ-ferrite. The structure is uniform and dendritic.
An increase in the content of the carbon-fluorine-containing component of the flux-cored wire charge and, accordingly, an increase in the carbon content in the surfaced layer promotes an increase in the hardness of the surfaced metal.
According to the results of the studies performed, it has been established that with an increase in the carbon-fluorine-containing additive content in the composition of a flux-cored wire, an increase in the concentration of non-metallic inclusions containing F, Na and Al in the surfaced layer occurs. In its turn, it can adversely affect the physical and mechanical properties of the surfaced layer. It is necessary to optimize the concentration of the carbon-fluorine-containing additive or use refining additives to reduce the contamination of the surfaced layer with non-metallic inclusions.
The research was performed within the framework of the state assignment (subject code 0809-2021-0013).
References
B. E. Paton (ed.), Technology of Electric Fusion Welding of Metals and Alloys [in Russian], Metallurgiya, Moscow (1974).
I. V. Osetkovskiy, N. A. Kozyrev, A. I. Gusev, et al., “Influence of the cobalt additive on the flux cored wire of system Fe – C – Si – Mn – Cr – Ni – Mo – V,” Key Eng. Mater., 736, 63 – 67 (2017).
N. A. Kozyrev, N. V. Kibko, A. A. Umanskii, et al., “New C – Si – Mn – Cr – V – Mo powder wires for roller surfacing,” Steel Transl., 46(10), 711 – 717 (2016).
A. I. Gusev, N. A. Kozyrev, I. V. Osetkovskiy, et al., “Quality of metal deposited flux cored wire with the system Fe – C – Si – Mn – Cr – Mo – Ni – V – Co,” Key Eng. Mater., 736, 23 – 28 (2017).
A. I. Gusev, N. A. Kozyrev, I. V. Osetkovskiy, et al., “Quality of metal deposited flux cored wire with the system Fe – C – Si – Mn – Cr – Mo – Ni – V – Co,” IOP Conf. Ser.: Mater. Sci. Eng., 253, 1 – 6 (2017).
A. I. Gusev, N. A. Kozyrev, A. A. Usoltsev, et al., “Study of the properties of flux cored wire of Fe – C – Si – Mn – Cr – Mo – Ni – V – Co system for the strengthening of nodes and parts of equipment used in the mineral mining,” IOP Conf. Ser.: Earth Env., 84, 1 – 8 (2017).
N. A. Kozyrev, D. A. Titov, S. N. Starovatskaya, et al., “Influence of introducing a carbon-fluorine-containing additive and nickel into the charge for the production of flux-cored wire of the C – Si – Mn – Cr – V – Mo system,” Izv. Vysh. Uchebn. Zaved., Chern. Metallurg., 57(4), 34 – 37 (2014).
A. I. Gusev, N. A. Kozyrev, N. V. Kibko, et al., “Influence of introduction of tungsten and chromium on properties of metal surfaced with a cored wire of the Fe – C – Si – Mn – Mo – Ni – V – Co system,” Zagot. Proizvod. Mashinostr., 17(2), 56 – 60 (2019).
N. A. Kozyrev, N. V. Kibko, A. A. Umanskii, and D. A. Titov, “Influence of nickel and carbon-fluorine containing additives on the structure and properties of deposited steel 25Kh5FMS,” Aktual. Probl. Mashinostr., No. 3, 54 – 59 (2015).
N. A. Kozyrev, A. A. Usoltsev, A. N. Prudnikov, et al., “Study of properties of cored wire based on ferrochrome gas-cleaning dust,” Chern. Metallurg., Byul. Nauchn. Tekhn. Ekonom. Inform., 75(3), 365 – 372 (2019).
N. A. Kozyrev, A. I. Gusev, G. V. Galevskii, et al., Russian Federation Patent 2641590, IPC 8 V23 K35/36, Cored Wire [in Russian], Siberian State Industrial University, Novokuznetsk (2016).
A. A. Umanskii, N. A. Kozyrev, A. R. Mikhno, et al., Russian Federation Patent 2726230, IPC 8 V23 35/368, Cored Wire [in Russian], Siberian State Industrial University, Novokuznetsk (2020).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Metallovedenie i Termicheskaya Obrabotka Metallov, No. 6, pp. 29 – 35, June, 2022.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Osetkovskiy, I.V., Yunusov, A.M., Kozyrev, N.A. et al. Investigation of the Microstructure of Surfaced Layer from Flux-Cored Wire of the Fe – C – Si – Mn – Cr – Ni – Mo – V System. Met Sci Heat Treat 64, 321–327 (2022). https://doi.org/10.1007/s11041-022-00808-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11041-022-00808-8