Abstract
The properties, applications, and methods for producing titanium and vanadium diborides are considered. These diborides are oxygen-free, refractory metal-like compounds. As a result, they are characterized by high values of thermal and electrical conductivity. Their hardness is relatively high. Titanium and vanadium diborides exhibit significant chemical resistance in aggressive environments. Thus, these diborides have found application in current technology. They are used as surfacing materials when applying wear-resistant coatings on steel products. It is also possible to use vanadium diboride as a catalyst in organic synthesis and as an anode in renewable electrochemical current sources. The promising ceramics are B4C–TiB2 and B4C–VB2, which allow to obtain products based on boron carbide with high performance characteristics, in particular with increased crack resistance. Such composite ceramics are produced by hot pressing, spark plasma sintering, and pressureless sintering. The properties of refractory compounds depend on the content of impurities and dispersion. To solve the specific problem associated with the use of refractory compounds, it is important to choose the correct method for their preparation, as well as to determine the permissible content of impurities in the starting components. This leads to the presence of different methods for the synthesis of borides. The main methods for their preparation are: synthesis from simple substances (metals and boron); borothermal reduction of oxides; carbothermal reduction (reduction of mixtures of metal and boron oxides with carbon; metallothermic reduction of mixtures of metal and boron oxides; and carbide-boron reduction. Plasma-chemical synthesis (deposition from the vapor-gas phase) is also used to obtain diboride nanopowders. Each of these methods is characterized in the article.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Compounds of a number of transition metals (titanium and vanadium) with boron—their diborides—have a number of unique properties. These diborides are characterized by refractoriness, significant chemical resistance in various aggressive media, high values of hardness, and thermal and electrical conductivity. Thus, these diborides are increasingly used in industry and technology.
The significant hardness of titanium diboride makes it possible to use it as an abrasive providing high surface cleanliness when processing of ductile metals and alloys. The refractory properties of titanium diboride and cermets based on it are widely known, which are highly resistant to the action of many molten metals and alloys. This allows them to be used for the manufacturing of boats for vacuum evaporation of metals. Vanadium diboride plays an important role in the creation of wear-resistant coatings on steel products and in a completely new field—in the creation of high-capacity electrochemical current sources. It is possible to use this compound as a catalyst in organic synthesis.
The purpose of this work is to analyze information about the properties, applications, and methods for producing titanium and vanadium diborides.
MAIN PROPERTIES OF TITANIUM AND VANADIUM DIBORIDES
The state diagrams of the Ti–B and V–B systems [1, 2] are shown in Fig. 1a and Fig. 1b. The following borides were found in the Ti–B system: TiB, Ti3B4, and TiB2. Titanium diboride has a narrow area of homogeneity (65.6—67.9% B (at.) at 1730°C). With an excess of boron, a mixture of phases (TiB2 + B) is formed with a eutectic melting point of approximately 2080°C, and with an excess of titanium, two boride phases (Ti3B4 + TiB2) with a peritectic melting point of approximately 2200°C are formed. The following compounds are present in the V–B system: V3B2, VB, V3B4, and VB2. Vanadium diboride has a narrow area of homogeneity (approximately 66–68% B (at.)). With an excess of boron, a mixture of phases (VB2 + B) with a eutectic melting point of approximately 2000°C is formed, and with an excess of vanadium, two boride phases (V3B4 + VB2) with a melting point of approximately 2300°C are formed. With an increase in the vanadium content (to about 43% (at.) and higher), the VB2 phase in the V–B system is absent.
Titanium and vanadium diborides are characterized by high melting points and narrow areas of homogeneity. Therefore, during the synthesis, they are most likely to form in a powdery state. To obtain single-phase products (TiB2, VB2 diborides), an accurate calculation of the charge is required. Information about some properties of these compounds, taken from [2, 3], is given in Table 1. Titanium and vanadium diborides are thermodynamically very stable compounds, as evidenced by the high values of the formation heats from elements and the isobaric-isothermal potentials. The value of the thermal conductivity coefficients of these diborides is relatively large; the resistivity is small. Such values of these parameters are explained by the fact that titanium and vanadium diborides are metal-like refractory compounds [2]. The microhardness of these diborides is quite high. The resistance of these compounds to high-temperature oxidation is relatively high, which is due to the protective effect of the liquid film of B2O3 oxide formed on the surface of their particles (melting point is about 450°C [4]).
APPLICATIONS OF TITANIUM AND VANADIUM DIBORIDES
Application of Titanium Diboride
Titanium diboride is promising for the manufacturing of wear-resistant, corrosion-resistant and heat-resistant products [5]. The TiB2–SiC composite with a sintering additive of 5% nickel (by weight), obtained at relatively low parameters of the hot pressing process (temperature 1700°C, pressure 32 MPa), had qualitative characteristics (ultimate bending strength 858 ± 87 MPa, crack resistance 8.6 ± 0.5 MPa m1/2, hardness 20.2 ± 0.9 GPa). Such high mechanical properties are explained by the fine homogeneous structure of the ceramic and the reinforcing effect of the sintering additive. Nickel also prevents the growth of TiB2 particle sizes [6]. Titanium diboride is used in the manufacturing of lightweight ceramic armor, wear-resistant products and cutting tools. This compound has a high resistance to the action of molten metals. Therefore, it is used in the manufacturing of crucibles and boats for vacuum evaporation of aluminum, as well as cathodes of aluminum electrolyzers, since it has a high electrical conductivity [7]. Micropowders of titanium diboride can be used for polishing and finishing works [2]. Surfacing compositions based on borides of refractory metals (including titanium diboride) provide high performance characteristics of surfacing [8]. A promising material for use is the B4C–TiB2 composite. It was found that the B4C–TiB2 system is quasi-binary and is described by a eutectic phase diagram. The composition of the eutectic corresponds to an alloy of about 75% B4C and about 25% TiB2 (mol); the temperature of the eutectic transformation is 2200 ± 40°С [9]. Information about the production of ceramics of B4C–TiB2 composition are known by three methods: (1) spark plasma sintering [10–16]; (2) hot pressing [17–19]; and (3) by sintering without applying pressure or pressureless sintering [20–22]. The starting materials were B4C + TiB2 [16, 22], B + C + Ti [11–15], B4C + TiO2 + C [17–21]. During spark plasma sintering, the process temperature was 1700–2000°C, the pressure was 60–100 MPa. The resulting ceramics had a hardness of 22–34 GPa and a crack resistance of 2.5–5.0 MPa m1/2. During hot pressing, the process temperature was higher (2050–2200°C), the pressure was 30–37 MPa. The resulting ceramics had a hardness of 24.5–29.5 GPa and a crack resistance of 3.9–4.8 MPa m1/2. In pressureless sintering, pre-pressing was carried out at a high (about 200 MPa) pressure. Sintering was carried out at temperatures of 2100–2150°C. The relative density of the obtained samples was relatively low (90–96%). The values of hardness (17–23 GPa) and crack resistance (no more than 4.6 MPa m1/2) were also low. It should be noted that in the instrumentation, the method of electrospark plasma sintering is more complicated than the method of hot pressing. In addition, using the preparation example of titanium diboride ceramics by the method of spark plasma sintering, it was established [23] that this process leads to instability of the phase composition (the second phase, TiB, is formed), and the obtained samples have significantly lower values of hardness (by about 30%) and elastic modulus (by about 20%) compared to the samples obtained by hot pressing. The pressureless sintering method requires significant pre-pressing pressure. The authors of [10] believe that the presence of titanium boride TiB2 prevents the growth of grains of boron carbide B4C, reduces the sintering temperature, and improves the mechanical properties of the resulting composite.
Application of Vanadium Diboride
Vanadium diboride is promising for the manufacturing of protective coatings on steel products [24]. The introduction of vanadium diboride into ceramics based on boron carbide makes it possible to activate the sintering process and obtain, by hot pressing, dense ceramics with high structural homogeneity at low temperatures of isothermal aging. Composite ceramics in a wide range of vanadium diboride concentrations (from 2.0 to 16.1% (vol.)) have higher values of hardness and flexing strength than monophase ceramics based on boron carbide. The resulting composite material B4C–VB2 is promising for the manufacturing of wear-resistant products [25, 26]. In [27, 28], the possibility of using vanadium diboride as a catalyst in the production of oxygen-containing organic compounds by liquid-phase oxidation of olefins (1-octene and cyclo-octene) with molecular oxygen was shown. Vanadium diboride (like a titanium diboride) can be used as an anode in renewable electrochemical current sources. Such a novelty is called “vanadium boride air cell”, which can be translated as “air-vanadium-boride element”. According to the principle of operation and structure, as well as by the composition of the electrolyte and the cathode, it is similar to the long-known zinc-air cell, in which electricity is produced by the oxidation of zinc. However, the capacity of such a source is much higher (3800 (mA h)/g, while the traditional MnO2–Zn source has 820 (mA h)/g) [29].
METHODS FOR PRODUCING TITANIUM AND VANADIUM DIBORIDES
The properties of refractory compounds depend on the degree of their stoichiometry (as applied to compounds of variable composition), impurity composition, and dispersion. Therefore, to solve a specific problem associated with the use of refractory compounds, it is important to choose the correct method for their preparation, as well as to determine the permissible content of impurities in the initial components. This leads to the presence of different methods for the synthesis of borides; the classification of methods is given in [30].
The most common methods for the synthesis of borides are:
— synthesis from simple substances (metals and boron)
— borothermal reduction of oxides
— carbothermal reduction (reduction of mixtures of metal and boron oxides with carbon)
— metallothermic reduction of mixtures of metal and boron oxides
— carbide-boron reduction
Reactions of the synthesis of refractory compounds (including borides) from simple substances are always exothermic [3]. Sometimes, the heat release is so great that when initiated (most often by a red-hot spiral), the reaction then proceeds spontaneously. Such processes are called SHS-processes (self-propagating high-temperature synthesis processes). An important characteristic of SHS-processes is the heat (the heat effect ratio of the reaction to the mass of the charge). The process after initiation proceeds spontaneously with a thermicity value of at least 2400 kJ/kg of charge. At a lower thermicity, the heating of the charge is required, at a much higher—the inert additives are introduced into the charge. Under optimal conditions, almost complete conversion of the starting substances into the final ones occurs (the content of unreacted substances is usually no more than 0.01–0.20% (by weight)). Since there is no contamination during the synthesis, the purity of the product in terms of impurities is approximately equal to the purity of the reagents [31]. The disadvantage of such processes is the high cost of powders of simple substances.
In the metallothermic synthesis of borides, the reaction products must be treated (usually acidic) to remove the compounds (most often oxides) of the reducing metal, which is usually magnesium. With this treatment, metal borides can partially decompose, since they are unstable in acidic solutions [4]. Due to the low boiling point of magnesium (1090°C [32]) and significant heat release during the course of magnesiothermic processes, emissions of incandescent charge and reaction products are possible. Therefore, such processes are forced to be carried out only in sealed rectors at high argon pressure. It should also be considered that the price of magnesium is high; in powdered form, it is toxic [33].
A feature of borothermal synthesis of borides (which can be attributed to a disadvantage of the process) is the use of expensive elemental boron, and often in quantities exceeding the required stoichiometry. To remove boron oxides, the reaction products are usually treated with hot water.
In the carbothermal synthesis of transition metal borides, one of the reagents is boron oxide B2O3, the noticeable evaporation of which begins already at a temperature of 1200°C [34]. Since the temperatures of the boride synthesis by this method are significantly higher [2], losses of this compound occur, which leads to the need for careful adjustment of the composition of the charge. Carbothermal synthesis of borides is possible by the sol–gel method. A characteristic feature of sol–gel processes is a relatively low synthesis temperature, which is explained by the close contact of reagents in ultrafine charges [3]. The resulting products are in a nanodispersed state. The disadvantages of the sol-gel method are the use of toxic reagents in many synthesis processes, the complexity (duration and multistage) of the charge preparation process and, in some cases, incomplete reaction.
It is believed [2, 35] that carbide-boron synthesis of borides is most promising for large-scale production of these compounds. In the carbide-boron synthesis of borides, a very important requirement for one of the reagents (boron carbide) is its high purity and dispersion. Industrial micro-powders of this compound contain a significant amount of free carbon impurities [36]. Therefore, when calculating the charge, it is necessary to adjust its composition considering the free carbon content in boron carbide.
The method of synthesis of refractory borides by deposition from the vapor–gas phase is not widely used.
Producing of Titanium Diboride Synthesis from Titanium and Boron
The heat of titanium diboride formation from simple substances even at a temperature of 3000 K (473.53 kJ/mol) significantly exceeds its enthalpy at the same temperature (215.80 kJ/mol) [3]. In this regard, after the combustion initiation of a stoichiometric mixture of titanium and boron powders, the reaction will obviously proceed in the mode of self-igniting synthesis with significant heat release. It is also possible to obtain titanium diboride by mechanical activation [37]. The mechanical activation role of solid-phase reactions is to start an exothermic reaction, which then proceeds due to the release of heat. Explosive mechanochemical synthesis (EMS) of refractory compounds is carried out in a sealed mechanoreactor of an energy-stressed mill. As the initial components, one can use both powders and any material amenable to grinding. The initiation of the synthesis reaction is realized by high-energy mechanical activation of the initial components. In contrast to the SHS process, where the reaction is «ignited» from a powerful short-term (0.05–6 s) radiant source (a red-hot spiral), in the EMS, the energy transfer takes up to several tens of minutes. Thus, in the process of EMS, a gradual “pumping” of excess energy to the reacting components is carried out. When the critical values of this energy are reached, a reaction begins and proceeds according to explosive kinetics. In [38], the interaction of titanium and boron during the implementation of the SHS process was investigated with the following composition of the charge, % (by weight): 95Ti–5B; 90Ti–10B; 85Ti–15B; 82Ti–18 B. The calculated boron content in titanium boride TiB is 18.6% (by weight), in titanium diboride TiB2 is 31.4% (by weight). The reaction products, as expected, were a mixture of boride phases (TiB and TiB2) in a titanium matrix. A number of publications [39–44] provide information on the synthesis of titanium diboride during mechanical activation. Mechanical activation was carried out in an argon atmosphere. Sometimes, the process time was very long. Thus, in [39], the reagents were titanium and amorphous boron. The TiB2 phase began to appear after 180 h of mechanical activation, and the Ti phase disappeared after 280 h of mechanical processing. In [40], the mechanical activation time was 60 h. Mechanical activation, combined with the transmission of electric discharges through the charge, intensified the process of boride formation (according to X-ray diffraction analysis, the reaction products consisted of TiB and TiB2 phases after 10 min of treatment); however, the process obviously required more complex equipment [41]. The authors believe that the reason for the rapid completion of the process is the additional heating of the charge when electric spark discharges, which create a high temperature, are passed through it. The time of mechanical activation was also short (10 min) in [42]. The probable reason was an additional operation—heat treatment in a vacuum furnace at 1000°C for 1 h. The resulting product, according to X-ray phase analysis, is single-phase (TiB2). Powders consist of particles less than 1 micron in size, collected in aggregates ranging in size from 5 to 20 microns, which is the reason for the small values of the specific surface area (2.7 m2/g). Mechanical activation is accompanied by wear of the grinding media. For example, for this reason, the iron content in the reaction products was significant (1.55% (by weight)) [43]. The time of mechanical activation was very short in [44]. Titanium diboride was obtained after 2 h by processing the charge in a planetary ball mill in an argon atmosphere at a ball to charge weight ratio of 17 : 1. The particle size of the objective compound was 1.8 μm.
Borothermal Reduction
In [45], a mixture of titanium oxide TiO2 and amorphous boron was preliminarily subjected to mechanical activation for 25 h. The process was carried out in vacuum. An additional stage was the heat treatment of the mechanically activated powder at 1050°C. Initially, the interaction of TiO2 oxide and boron forms titanium (III) borate TiBO3 and one of the lower titanium oxides Ti2O3. Next, the formation of the target product—TiB2 and a by-product—lower boron oxide В2О2 occurs. This oxide was removed by treatment with hot water. The particle size of the titanium diboride powder was 0.5—1.5 μm; the particles are predominantly aggregated. Chen et al. [46] provided information on the preparation of titanium diboride using an exothermic additive (a mixture of sodium and sulfur to form Na2S sulfide). The reagents were taken in a molar ratio TiO2 : B = 1 : 4. The process was carried out in an autoclave. The autoclave was heated to 150°C and kept at this temperature for 2 h. A multi-stage enrichment procedure followed. The reaction products were treated sequentially with absolute ethanol, distilled water, hydrochloric acid, distilled water, and absolute ethanol, and then dried in a vacuum oven at 60°C for 8 h. The authors note that during the acid treatment of the reaction products, toxic hydrogen sulfide is released during the decomposition of sodium sulfide. The enriched product according to X-ray phase analysis was single-phase (TIB2), with an average particle size of approximately 100 nm.
Carbothermal Reduction
The process is described by the total reaction
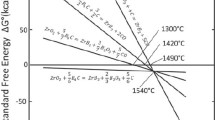
According to the results of thermodynamic calculations performed in [47], it was found that in the TiO2–B2O3–C system with a stoichiometric composition of the charge for reaction (6), the interaction begins with the dissociation of titanium oxide TiO2 with the formation of Ti4O7 in the temperature range of 720–800 K. Titanium carbide is formed by the reaction of Ti4O7 and carbon at temperatures of 830–850 K, and then it reacts with boron oxide B2O3 to form TiB2 diboride. Above 880 K, titanium diboride is the only condensed phase. In [48], experiments were carried out in an argon atmosphere at its pressure of 0.1 MPa and temperatures of 940, 1100, 1200, 1400, 1500, and 1600°C at a molar ratio of reagents TiO2 : B2O3 : C = 1 : 2 : 8 (boron oxide and carbon was present in the charge in amounts exceeding stoichiometric for reaction (6)). The exposure time in all cases was 45 min. The reaction products consisted only of titanium diboride at temperatures above 1400°C. In [49], a charge with a molar ratio of reagents TiO2 : B2O3 : C = 1 : 2 : 5.4 (boron oxide and carbon were present in the charge in amounts exceeding stoichiometric for reaction (6)) was preliminarily subjected to high-energy grinding for 1, 48, 100, and 200 h. Next, the powder mixture was subjected to heat treatment at temperatures of 600–1400°C for 1 h under vacuum (1–50 Pa). X-ray diffraction analysis revealed that the process of boride formation proceeds through the formation of intermediate phases: Ti3O5, Ti2O3, Ti4O7, TiO and TiBO3. At high-energy grinding times of 48, 100, and 200 h, the products containing only the TiB2 phase were obtained at temperatures of 1300, 1300, and 1200°C, respectively. The particle size of titanium diboride was in the range of 2–5 μm. The authors note that in the absence of high-energy grinding, similar results were obtained at a much higher (1500°C) temperature. [50] reported the use of a new source of boron—partially dehydrated boric acid HBO2. For this, boric acid was heated at 120°C for 8 h. The effect of different boron sources (H3BO3, HBO2, and B2O3), different amounts of HBO2 in the charge (42.9–46.7% (by weight)), and heat treatment temperatures (1400–1800°C) on the composition and microstructure of the reaction products was studied. The best results (containing only the TiB2 phase, a powder with a particle size of about 10 μm) were achieved by holding a charge in argon with an HBO2 content of 46.7% (by weight) in argon for 30 min at a temperature of 1700°C. In [51], resin was used as a carbon source (its characteristics were not given). A charge was used with a molar ratio of reagents TiO2 : B2O3 : C = 1 : 2 : 5 (boron oxide and carbon were present in the charge in amounts exceeding stoichiometric for the reaction (6)). The charge components were mixed in a planetary ball mill in acetone medium for 2 h. Then, the mixture was dried at 70°С, and finally heated in argon at 500°С to decompose the resin. The reaction mixture was heat treated at 1500°C in argon for 20 min. After synthesis, the reaction products were ground for 12 h in methanol to break down agglomerates and remove unreacted boron oxides. The authors believe that when the charge is heated, titanium carbide is initially formed. Then, it interacts with boron oxide to form titanium diboride. This is evidenced by the presence of two phases (TiC and TiB2) in the reaction products after heat treatment for 10 min. The content of only one phase (TiB2) was recorded by X-ray phase analysis after heat treatment for 20 min. The titanium diboride particles were approximately 2 microns in size; they are aggregated. After grinding in a planetary mill for 12 h, the aggregates were destroyed and the particle size decreased to about 80 nm. It is noteworthy that in [48–51], the charge contained a twofold excess of boron oxide as compared with the stoichiometry required for reaction (6). The most probable reason is the significant evaporation of this compound at synthesis temperatures [34]. The synthesis of titanium diboride nano-powder by the sol–gel method is described in [52]. The reagents were tetrabutyl titanate Ti(OCH2–CH2–CH2–CH3)4, boric acid, and sucrose. Initially, boric acid and sucrose were dissolved in distilled water with stirring. The solution was then slowly mixed with a titanium precursor (tetrabutyl titanate) to form a gel. The gel was dried at 120—140°С for 24 h until a dry residue was formed. The samples were heated for 2 h in an argon atmosphere. At temperatures of 1000, 1100, and 1200°C, the reaction products contained two phases: TiC and TiB2. Only one titanium diboride was obtained at 1300°C. The particle size was 3–5 μm, the particles are aggregated. Practically in all works devoted to this method [47, 48, 50–52], it is reported that titanium carbide is initially formed. Further, with an increase in temperature, it is transformed into diboride according to the most probable reaction of interaction with boron oxide B2O3. At optimal temperatures, the reaction products contain only one phase, TiB2, according to X-ray phase analysis.
Metallothermic Reduction
The calciothermic reduction of a TiO2/B mixture (the molar ratio TiO2 : B = 1 : 2) was studied in [53]. The reaction mixture consisted of titanium oxide, amorphous boron, and calcium. Calcium was introduced into the charge with an excess of 20%. The reaction was carried out with mechanical activation of the charge in a planetary ball mill in an argon atmosphere for 1–5 h. According to the results of thermogravimetric analysis, the reaction began at a temperature of 836°C. To remove the reaction by-products (CaO and CaB6), the resulting powders were treated with an acetic acid solution. The particle sizes of titanium diboride were in the range of 1–2 μm. The process of magnesiothermic synthesis is described by the total reaction
Boron oxide [54–58] or boric acid [59] were the sources of boron in magnesiothermic reduction. The reaction was initiated by local heating of the charge with a red-hot spiral [54, 59], its mechanical activation [55], and heating in a furnace [56–58]. The process of magnesiothermic synthesis is characterized by significant heat release. The calculated thermicity for reaction (7) is 3848 kJ/kg of charge [54]: to reduce it, it is advisable to introduce inert additives (sodium, potassium and calcium chlorides) into the charge [54, 55, 58, 59]. In [54], a mixture of 50% KCl, 25% NaCl, and 25% CaCl2 (by weight) was added to the reaction mixture at the molar ratio of the reagents TiO2 : B2O3 : Mg = 1 : 2 : 5.5 (magnesium was introduced in a small excess to compensate for its losses during evaporation). The salt mixture was preheated to 850°C for complete melting and then ground after cooling. The content of the salt additive was 0—60% (by weight) from the mass of the reaction charge. After stirring for 1 h, the charge and the salt mixture were tableted and the compressed samples were heated in an argon medium until the reaction began. After the end of the process, the samples were washed with distilled water to remove the salts. This was followed by grinding and acid enrichment. Since all borides are unstable to the action of acids [4], the enrichment with nitric acid was carried out stepwise, and in small portions. X-ray phase analysis revealed the presence of the following compounds in the reaction products: TiB2, MgO, Mg2TiO4 and Mg3B2O6. The last three were effectively removed by acid enrichment. The addition of the salt mixture in an amount of 60% (by weight) of the reaction mixture led to a decrease in the reaction start temperature from 785 to 500°C and the maximum combustion temperature from 1316 to 687°C. In addition, the inert salt diluent led to the formation of deagglomerated particles, which favorably affected the efficiency of acid cleaning. In [55], the effect of mechanical activation parameters on the formation of titanium diboride nanopowders was studied. The results showed that the synthesis process is significantly influenced by the ratio of the mass of balls to the mass of the load and the addition of an inert diluent (NaCl). When the ratio of the mass of the balls to the mass of the load 10 : 1, 15 : 1, and 20 : 1, the time to the start of the reaction was 73, 34, and 40 min, respectively. When the ratio of the mass of balls to the mass of the load was 15: 1, the addition of 5% (by mass) NaCl to the reaction mixture increased the time before the start of the reaction to 60 min. In all cases, the reaction products were TiB2, MgO, and Mg2TiO4. During treatment with an 18% HCl solution at 60°C for 30 min, the unwanted compounds were completely removed. The enriched product (TiB2) had an average particle size of about 140 nm according to scanning electron microscopy. In [56], on the basis of thermodynamic calculations and experimental data, a possible mechanism for the formation of titanium diboride was proposed, which consists in the sequential course of reactions
In [57], the optimal parameters of acid treatment of the reaction products were determined. X-ray phase analysis revealed the presence of TiB2, MgO, Mg2TiO4, and Mg3B2O6 compounds in them. The best results were achieved upon treatment with hydrochloric acid with a concentration of 9.3 M for 30 min at a suspension ratio of S : L = 1 : 5. The resulting titanium diboride was porous agglomerated particles with a particle size of 1 to 30 μm and a developed surface (~23 m2/g). In [58], the synthesis was carried out in a liquid eutectic LiCl/KCl melt at a temperature of 700°C. The use of a salt melt led to the formation of homogeneous and ultrafine particles (size 8.1 ± 1.4 nm) of titanium diboride. It was shown in [59] that preliminary mechanical activation of the charge practically does not affect the size of TiB2 particles, and the addition of an inert diluent (NaCl) reduces their average size from 31 to 27 nm. In a number of works [54, 55, 57, 59], the presence of undesirable (yield-reducing) by-products of the reaction (Mg3B2O6 and Mg2TiO4) is noted. However, they are easily removed during acid treatment. To initiate the reaction, it is possible to carry out partial oxidation of magnesium when it interacts with water [60]. A feature of this process is the use of boron as a reagent (and not boron oxide). The molar ratio of the reagents is as follows: TiO2 : B : Mg : H2O = 1 : 3 : 12 : 10. Thus, boron was taken in a 1.5-fold excess. Powdered reagents were stirred in an autoclave, after which distilled water was added to the mixture. The autoclave was closed, heated to a temperature of 150°C and held for 2 h. The authors noted that due to the release of hydrogen, the pressure in the reactor is very high (49.15 MPa). The reaction products were treated with hydrochloric acid (0.2 M), distilled water, and ethanol and then dried in a vacuum oven at 60°C for 8 h. The resulting product was single-phase (TiB2) and consisted of spherical particles of approximately 200 nm in size. Magnesiothermic synthesis can also be attributed to the process using magnesium diboride MgB2 as a reducing agent (and at the same time a source of boron) [61]. The titanium source was TiCl3 chloride. The reagents in a molar ratio TiCl3 : MgB2 = 1 : 1.5 (boron was taken in a 1.5-fold excess) were mixed in a helium medium. Then, they were subjected to heat treatment in vacuum at a temperature of 850°C for 18 h. After the process was completed, the products were ground and treated with water or methanol to remove the magnesium chloride. The resulting product was single-phase (TiB2) and consisted of aggregated nanosized particles. The yield was at 80%. It is also reported that titanium may be added to the charge for the beneficial use of excess boron.
When titanium diboride is prepared by the metallothermic method, aluminum can be used as a reducing agent [62]. In the studied process, at the first stage, boron oxide was dissolved in titanium isopropoxide Ti[OCH(CH3)2]4 and isopropyl alcohol C3H8O. The resulting solution was mixed for 2 h at 90°C. The formed precipitate was dried for 24 h. Then aluminum powder was added to it. This charge was mechanically activated in an argon atmosphere for 15 h. The reaction products were treated with a NaOH solution (10M) to remove alumina and unreacted aluminum. After decantation, the precipitate was washed with deionized water and dried in air at 100°C. According to scanning electron microscopy, the average particle size of the resulting titanium diboride was about 30 nm.
In [63], the interaction of titanium chloride TiCl3 with lithium borohydride LiBH4 and lithium hydride LiH was studied by the reaction
The synthesis of titanium diboride was carried out by mechanical activation of the reaction mixture in an argon medium. The enrichment was carried out sequentially by treatment with water, ethanol and acetone. The single-phase product (TiB2) had a particle size of 15–60 nm.
Carbide-Boron Reduction
Total reaction of the process
This method is considered the most promising for the large-scale production of titanium diboride [7]. In [64] titanium dioxide, boron carbide according to GOST 5744–85 [65] (grain size not specified) and soot PM-50 GOST 7885–86 [66] were used as reagents. The content of impurities in such a carbide can be significant. For example, for boron carbide micropowders of the M40, M28, M20, and M14 grain sizes, the free carbon content should be no more than 10% (by weight). The author directly points out that due to the discrepancy between the composition of the boron carbide used and the calculated one (that is, B4C), when compiling the charge formulation, it is necessary to recalculate the amount of soot added to the charge. According to the results of the experiments, the optimal parameters of the process were found: exposure for 60 min at a temperature of 1900°C in a hydrogen medium. Information about the dispersion is not provided.
The preparation of doped titanium diboride is described in [67]. Optimal process parameters: exposure for 60 min at a temperature of 1800°C. The average particle size of the resulting compound after grinding for 5 h was 1.3 μm. The shape of the particles in this case, of course, became fragmentary.
In [68], petroleum coke with an average particle size of 18 μm was used as a carbon material. The reagents were stirred in a planetary mill for 4 h in an almost stoichiometric ratio for the reaction (12). The experiments were carried out in a deep vacuum (4 × 10–5 mbar ≈ 0.004 Pa). At a temperature of 1820°C, the boride formation reaction proceeded almost completely, as evidenced by a weight loss of 44.6–44.9% (by weight) (calculated value 44.44% (by weight)) and the presence of only the TiB2 phase in the reaction products; the content of impurities is 0.5% O; 0.5% N; 0.6% C (by weight). After grinding, the average particle size of titanium diboride was approximately 1 μm. Titanium isopropoxide can be used as a precursor of titanium dioxide and carbon in this method [69]. In experiments, it was hydrolyzed to form first titanium hydroxide Ti(OH)4, and then highly dispersed titanium oxide TiO2. Next, boron carbide was added to this suspension. The suspensions were mixed for 105 min and then dried under vacuum at 140°C. Heat treatment of the dry residue in argon for 1 h at a temperature of 1200°C yielded a single-phase product, TiB2. The particle sizes of titanium diboride were 0.2–0.5 μm.
In [70], titanium dioxide, boron carbide, and soot were used as reagents. The influence of various amounts of boron carbide (22.0–26.8% by weight), temperature (1400–1900°C), and heat treatment time (15–90 min) on the composition and microstructure was studied. The process was carried out in an argon atmosphere. At a temperature of 1800°C, a boron carbide content in the charge of 25.3% (by weight) and a holding time of 30 min, titanium diboride with an average particle size of about 1 μm was obtained. It should be noted that the calculated content of boron carbide in the charge as applied to the reaction (12) is 22.2% (by weight). Therefore, under optimal synthesis conditions, its content in the charge exceeded the required stoichiometry.
In [71], titanium diboride was obtained in a crucible-type induction furnace in an argon atmosphere using nanofibrous carbon characterized by a significant specific surface area (approximately 150 m2/g) and a low content of impurities (approximately 1% (by weight)) [72], also highly dispersed boron carbide with an impurity content (approximately 1% (by weight)) [73]. At heat treatment temperatures of 1600 and 1700°C, regardless of the time of the experiment, the weight loss (43.6–43.8% (by weight)) practically coincided with the calculated one (44.0%), which was an obvious proof of the completion of the process. Thus, the optimal parameters of the process are: temperature 1600°С; heat treatment time 20 min. The content of impurities does not exceed 2.5% (by weight), the average particle size of the diboride obtained at a temperature of 1600°C, determined by the “geometric” method [74], was 7.4 μm.
Deposition from the Vapor–Gas Phase
Titanium Diboride mixture (73.85–75.54% (by weight)) and titanium nitride (10.23–11.31% (by weight)) was obtained in a nitrogen-hydrogen plasma stream by reducing of titanium dioxide with propane-butane in the presence of boron [75]. The average particle size was 36 nm. The preparation of this compound in argon plasma is described in [76]. At the molar ratio B : Ti = 1 : 2 in the initial mixture, the reaction products contained the phases TiB2, TiB, and Ti. The particle size was 20–30 nm.
Preparation of Vanadium Diboride Synthesis from Vanadium and Boron
The mixture preparation of vanadium borides (V3B2, VB, V5B6, V3B4, V2B3, and VB2) by the SHS process was studied in [77]. To obtain homogeneous mixtures, powders of vanadium and boron in different molar ratios were stirred for 12 h. The reactor was filled with argon. To maintain stable combustion, a mixture with a molar ratio of V : B = 1 : 2 was preheated to 300°C. With this ratio of reagents, a single— phase product, VB2 diboride, was obtained.
In [78], it was reported on the preparation of a nanosized powder of vanadium diboride from a mixture in a molar ratio V : B = 1 : 2 (that is, stoichiometric composition). Mechanical activation was performed for 4 h.
Borothermal Reduction
In [79], the process was carried out according to the total reaction
Vanadium diboride was formed at a temperature of approximately 1300°C. Noteworthy is the significant consumption of boron.
Carbothermal Reduction
The preparation of vanadium diboride layers by electron beam treatment of charges with a molar ratio V : B : C : O = 2 : 4 : 3 : 3 on the surface of carbon steel samples in high vacuum (10–3–10–2 Pa) was described in [41, 80, 81].
Metallothermic Reduction
The total reaction of the magnesiothermic process using vanadium and boron oxides
The process was investigated in [82]. The charge was heated at a temperature of 1150°C in an argon flow. Vanadium diboride was obtained with a twofold excess of boron oxide and an approximately one and a half-fold excess of magnesium over stoichiometry. The content of impurities in the finished product was 0.6% (by weight). In magnesiothermic synthesis, other reagents can be used: vanadium chloride VCl4 and sodium borohydride NaBH4 [83]. These reagents, together with magnesium, were loaded into an autoclave and kept in it for 8 h at a temperature of 650°C. Due to the release of hydrogen, the pressure in the autoclave during the synthesis can reach 12.4 MPa. The particle size of vanadium diboride is 50–100 nm.
The process with the use of magnesium diboride MgB2 as a reducing agent (and at the same time as a source of boron) can also be attributed to the magnesiothermic process [61]. The process was carried out in a vacuum. The source of vanadium was chloride VCl3. The reagents in the molar ratio VCl3 : MgB2 = 1 : 1.5 (boron was taken in a 1.5-fold excess) were mixed in a helium medium. Then they were subjected to heat treatment in a vacuum at a temperature of 850°C for 18 h. After the process was completed, the products were ground and treated with water or methanol to remove the magnesium chloride. The resulting product was single-phase (VB2) and consisted of aggregated nanosized particles. The yield was at 80%. In [63], the interaction of titanium chloride VCl3 with lithium borohydride LiBH4 and lithium hydride LiH was studied by the reaction
The synthesis of vanadium diboride was carried out by mechanical activation of the reaction mixture in an argon medium. The enrichment was carried out by sequential treatment with water, ethanol and acetone. The single-phase product (VB2) had a particle size of 15–60 nm.
Carbide-Boron Reduction
Meerson and Samsonov mentioned the possibility of obtaining vanadium diboride by this method [84]. Process parameters and product characteristics were not provided. In [85], vanadium diboride was obtained in a crucible-type induction furnace in an argon atmosphere using nanofibrous carbon characterized by a significant (approximately 150 m2/g) specific surface area and a low (approximately 1% (by weight)) impurity content [72], with using finely dispersed boron carbide with an impurity content of about 1% (by weight) [73]. At heat treatment temperatures of 1600 and 1700°C and a heat treatment time of 20 min, the experimental weight loss (37.0–38.6% (by weight)) practically coincided with the calculated one (36.2% (by weight)), which was an obvious proof of completeness of process. Thus, the optimal parameters of the process are: temperature 1600°C, time 20 min. The content of impurities does not exceed 2.5% (by weight), the average particle size of the diboride obtained at a temperature of 1600°C, determined by the “geometric” method [74], was 8.9 μm.
Deposition from the Vapor–Gas Phase
Vanadium diboride with a basic substance content of 90.04–92.22% (by weight) was obtained in a nitrogen–hydrogen plasma stream during the reduction of V2O3 oxide with propane–butane in the presence of boron [75]. The average particle size was 36 nm.
In most of the cited works on the synthesis of titanium and vanadium diborides [38–43, 60–62, 69, 70, 77–79, 82–84], information on the content of impurities in the target product was not given.
CONCLUSIONS
Information on refractory oxygen-free metal-like compounds, titanium and vanadium diborides, is presented. Their properties and applications are considered. Titanium and vanadium diborides are characterized by high thermal and electrical conductivity, significant hardness, and chemical inertness. For these reasons, such compounds have found application in a variety of areas. Methods for the preparation of these compounds are described and analyzed, and the features of these methods are indicated. In most works on the synthesis of titanium and vanadium diborides, their preparation from simple substances by carbothermal, metallothermic and carbide-boron processes is considered. Information on the preparation of these compounds by borothermal reduction of oxides and precipitation from the vapor-gas phase is scarce. An interesting feature was revealed: in most of the cited works on the methods of obtaining, the information on the purity of the reaction products are not given.
REFERENCES
Diagrammy sostoyaniya dvoinykh metallicheskikh sistem. Spravochnik (State Diagrams of Binary Metal Systems: Handbook), Lyakishev, N.P., Ed., Moscow: Mashinostroenie, 1996, vol. 1.
Serebryakova, T.I., Neronov, V.A., and Peshev, P.D., Vysokotemperaturnye boridy (High Temperature Borides), Moscow: Metallurgiya, 1991.
Svoistv, poluchenie i primenenie tugoplavkikh soedinenii. Spravochnik (Properties, Production, and Application of Refractory Compounds: Handbook), Kosolapova, T.Ya., Ed., Moscow: Metallurgiya, 1986.
Kosolapova, T.Ya., Chemical properties of refractory compounds, Zh. Vses. Khim. O-va im. D.I. Mendeleeva, 1979, vol. 34, no. 3, pp. 244–249.
Grigor’ev, O.N., Ceramics and cermets based on nonoxide refractory compounds, Powder Metall. Met. Ceram., 2013, vol. 51, nos. 11–12, pp. 697–708.
Zhao, G., Huang, C., Liu, H., Zou, B., Zhu, H., and Wang, J., Microstructure and mechanical properties of TiB2–SiC ceramic composites by reactive hot pressing, Int. J. Refract. Met. Hard Mater., 2014, vol. 42, pp. 36–41. https://doi.org/10.1016/j.ijrmhm.2013.10.007
Mroz, C., Annual minerals review: titanium diboride, Am. Ceram. Soc. Bull., 1995, vol. 74, no. 6, pp. 158–159.
Artem’ev, A.A., Sokolov, G.N., Dubtsov, Yu.N., and Lysak, V.I., Formation of the composite structure of wear-resistant deposited metal with boride hardening, Izv. VUZov, Poroshk. Metall. Funkts. Pokrytiya, 2011, no. 2, pp. 44–48.
Ordan’yan, S.S., Stepanenko, E.K., Dmitriev, A.I., and Shchemeleva, M.V., Interaction in the system B4C–TiB2, Sverkhtverd. Mater., 1986, no. 5, pp. 27–29.
Heydari, M.S. and Baharvandi, H.R., Comparing the effect of different sintering methods for ceramics on the physical and mechanical properties of B4C–TiB2 nanocomposites, Int. J. Refract. Met. Hard Mater., 2015, vol. 61, pp. 224–232. https://doi.org/10.1016/j.ijrmhm.2015.04.003
Dudina, D.V., Hulbert, D.M., Jiang, D., Unuvar, C., Cytron, C.J., and Mukherjee, A.K., In situ boron carbide-titanium diboride composites prepared by mechanical milling and subsequent spark plasma sintering, J. Mater. Sci., 2008, vol. 43, pp. 3569–3576. https://doi.org/10.1007/s10853-008-2563-8
Hulbert, D.M., Jiang, D., Dudina, D.V., and Mukherjree, A.K., The synthesis and consolidation of hard materials by spark plasma sintering, Int. J. Refract. Met. Hard Mater., 2009, vol. 27, no. 2, pp. 367–375. https://doi.org/10.1016/j.ijrmhm.2008.09.011
Nikzad, L., Orru, R., Licheri, R., and Cao, G., Fabrication and formation mechanism of B4C–TiB2 composite by reactive spark plasma sintering using unmilled and mechanically activated reactants, J. Am. Ceram. Soc., 2012, vol. 95, no. 11, pp. 3463–3471. https://doi.org/10.1111/j.1551-2916.2012.05416.x
Nikzad, L., Licheri, R., Ehadzadeh, T., Orru, R., and Cao, G., Effect of ball milling on reactive spark plasma sintering of B4C–TiB2 composites, Ceram. Int., 2012, vol. 38, no. 8, pp. 6469–6480. https://doi.org/10.1016/j.ceramint.2012.05.024
Scherbakov, V.A., Gryadunov, A.N., and Alymov, M.I., Synthesis and characteristics of B4C–TiB2 composites, Adv. Mater. Technol., 2016, no. 4, pp. 16–21. https://doi.org/10.17277/amt.2016.04.pp.016-021
Xu, C., Cai, Y., Flodstrom, K., Li, Z., Esmaelizadeh, S., and Zhang, G.-J., Spark plasma sintering of B4C ceramics: the effects of milling medium and TiB2 addition, Int. J. Refract. Met. Hard Mater., 2012, vol. 30, no. 1, pp. 139–144. https://doi.org/10.1016/j.ijrmhm.2011.07.016
Skorokhod, V. and Krstic, V.D., High strength-high toughness B4C–TiB2 composite, J. Mater. Sci. Lett., 2000, vol. 19, pp. 237–239. https://doi.org/10.1023/A:1006766910536
Wang, Y.-J., Peng, H.-X., Ye, F., and Zhou, Y., Effect of TiB2 content on microstructure and mechanical properties of in-situ fabricated TiB2/B4C composites, Trans. Nonferrous Met. Soc. China, 2011, vol. 21, no. 2, pp. 369–373. https://doi.org/10.1016/S1003-6326(11)61608-7
Yue, X.Y., Zhao, S.M., Yu, L., and Ru, H.Q., Microstructures and mechanical properties of B4C–TiB2 composite prepared by hot pressure sintering, Key Eng. Mater., 2010, vol. 434, pp. 50–53. https://doi.org/10.4028/www.scientific.net/KEM.434-435.50
Skorokhod, V.V., Processing, microstructure, and mechanical properties of B4C–TiB2 particulate sintered composites. Part I. Pressureless sintering and microstructure evolution, Powder Metall. Met. Ceram., 2000, vol. 39, pp. 414–423. https://doi.org/10.1023/A:1026625909365
Skorokhod, V.V., Processing, microstructure, and mechanical properties of B4C–TiB2 particulate sintered composites. Part II. Fracture and mechanical properties, Powder Metall. Met. Ceram., 2000, vol. 39, pp. 504–513. https://doi.org/10.1023/A:1011378825628
Baharvandi, H.R., Hadian, A.M., and Alizadex, A., Processing and mechanical properties of boron carbide-titanium diboride ceramic matrix composites, Appl. Compos. Mater., 2006, vol. 13, pp. 191–198. https://doi.org/10.1007/s10443-006-9012-0
Mukhopadhyay, A., Venkateswaran, T., and Bikramjit, B., Spark plasma sintering may lead to phase instability and inferior mechanical properties: a case study with TiB2, Scr. Mater., 2013, vol. 69, no. 2, pp. 159–164. https://doi.org/10.1016/j.scriptamat.2013.02.027
Gidikova, N., Vanadium boride coatings on steel, Mater. Sci. Eng., A, 2000, vol. 278, nos. 1–2, pp. 181–186. https://doi.org/10.1016/S0921-5093(99)00596-1
Grigor’ev, O.N., Galanov, B.A., and Kotenko, V.A., Resistance of B4C–VB2 composites to abrasion and friction when paired with steel, Ogneupory Tekh. Keram., 2005, no. 10, pp. 2–8.
Grigor’ev, O.N., Koval’chuk, V.V., Zaporozhets, O.I., Bega, N.D., Galanov, B.A., Prilutskii, E.V., Kotenko, V.A., Kutran’, T.N., and Dordienko, N.A., Synthesis and physicomechanical properties of B4C–VB2 composites, Powder Metall. Met. Ceram., 2006, vol. 45, nos. 1–2, pp. 47–58. https://doi.org/10.1007/s11106-006-0041-x
Trach, Y., Schulze, B., Makota, O., and Bulgakova, L., The liquid-phase oxidation of olefins by molecular oxygen in the presence of metal borides and MoO3, J. Mol. Catal. A: Chem., 2006, vol. 258, nos. 1–2, pp. 292–294. https://doi.org/10.1016/j.molcata.2006.05.069
Trach, Y.B., Bulgakova, L.V., Makota, O.I., Suprun, W.Ya., Schulze, B., and Stark, C.B.W., Vanadium diboride catalyzed oxidation of cyclooctene by molecular oxygen: kinetic study, J. Mol. Catal. A: Chem., 2009, vol. 302, nos. 1–2, pp. 124–128. https://doi.org/10.1016/j.molcata.2008.12.008
Yu, X. and Light, S., A novel high capacity, environmentally benign energy storage system: super-iron boride battery, J. Power Sources, 2008, vol. 179, no. 1, pp. 407–411. https://doi.org/10.1016/j.jpowsour.2007.12.060
Gurin, V.N., Methods of refractory compounds synthesis and prospects for their application to create new materials, Zh. Vses. Khim. O-va im. D.I. Mendeleeva, 1979, vol. 24, no. 3, pp. 212–222.
Merzhanov, A.G. and Borovinskaya, I.P., Self-propagating high-temperature synthesis in chemistry and technology of refractory compounds, Zh. Vses. Khim. O-va im. D.I. Mendeleeva, 1979, vol. 24, no. 3, pp. 223–227.
Samsonov, G.V., Svoistva elementov. Chast’ 1. Fizicheskie svoistva. Spravochnik (Properties of Elements, Part 1: Physical Properties: Handbook), Samsonov, G.V., Ed., Moscow: Metallurgiya, 1976.
Samsonov, G.V. and Perminov, V.P., Magnietermiya (Magnesiothermy), Moscow: Metallurgiya, 1971.
Fiziko-khimicheskie svoistva okislov. Spravochnik (Physicochemical Properties of Oxides: Handbook), Samsonov, G.V., Ed., Moscow: Metallurgiya, 1978.
Kieffer, R. and Benesovsky F., Hartmetalle, Vienna: Springer-Verlag, 1965.
Kislyi, P.S., Kuzenkova, M.A., Bodnaruk, N.I., and Grabchuk, B.L., Karbid bora (Boron Carbide), Kiev: Naukova Dumka, 1988.
Popovich, A.A., Reva, V.P., Vasilenko, V.N., Popovich, T.A., and Belous, O.A., Mechanochemical method of obtaining powders of high-melting compounds (review), Powder Metall. Met. Ceram., 1993, vol. 32, no. 2, pp. 131–136.
Cirakoglu, M., Bhaduri, S., and Bhaduri, S.B., Combustion synthesis processing of functionally graded materials in the Ti–B binary system, J. Alloys Compd., 2002, vol. 347, nos. 1–2, pp. 259–265. https://doi.org/10.1016/S0925-8388(02)00499-1
Hwang, J. and Lee, J.K., Preparation of TiB2 powders by mechanical alloying, Mater. Lett., 2002, vol. 54, no. 1, pp. 1–7. https://doi.org/10.1016/S0167-577X(01)00526-2
Tang, W.-M., Zheng, Z.-X., Wu, Y.-C., Wang, J.-M., Lu, J., and Liu, J.-W., Synthesis of TiB2 nanocrystalline powder by mechanical alloying, Trans. Nonferrous Met. Soc. China, 2006, vol. 16, no. 3, pp. 613–617. https://doi.org/10.1016/S1003-6326(06)60108-8
Calka, A. and Oleszak, D., Synthesis of TiB2 by electric discharge assisted mechanical milling, J. Alloys Compd., 2007, vol. 440, nos. 1–2, pp. 346–348. https://doi.org/10.1016/j.jallcom.2006.09.073
Makarenko, G.N., Krushinskaya, L.A., Timofeeva, I.I., Matsera, V.E., Vasil’kovskaya, M.A., and Uvarova, I.V., Formation of diborides of groups IV–VI transition metals during mechanochemical synthesis, Powder Metall. Met. Ceram., 2015, vol. 53, nos. 9–10, pp. 514–521.
Peters, J.S., Cook, B.A., Harringa, J.L., and Russell, A.M., Microstructure and wear resistance of low temperature hot pressed TiB2, Wear, 2009, vol. 266, nos. 11–12, pp. 1171–1177. https://doi.org/10.1016/j.wear.2009.03.027
Tayeh, T., Douin, J., Jouannigot, S., Zakhour, M., Nakh, M., Silvain, J.-F., and Bobet, J.-L., Hardness and Young’s modulus behavior of Al composites reinforced by nanometric TiB2 elaborated by mechanosynthesis, Mater. Sci. Eng., A, 2014, vol. 591, pp. 1–8. https://doi.org/10.1016/j.msea.2013.10.065
Millet, R. and Hwang, T., Preparation of TiB2 and ZrB2. Influence of a mechano-chemical treatment on the borothermic reduction of titania and zirconia, J. Mater. Sci., 1996, vol. 31, pp. 351–355. https://doi.org/10.1007/BF01139151
Chen, B., Yang, L., Heng, H., Chen, J., Zhang, L., Xu, L., Qian, Y., and Yang, J., Additive-assisted synthesis of boride, carbide and nitride micro/nanocrystals, J. Solid State Chem., 2012, vol. 194, pp. 219–224. https://doi.org/10.1016/j.jssc.2012.05.032
Smirnyagina, N.N., Tsyrenzhapov, B.B., and Milonov, A.S., Phase equilibria in the Me–B–C–O (Me = Ti, Zr, and V) systems, Russ. J. Phys. Chem. A, 2006, vol. 80, no. 11, pp. 1855–1859.
Krishnarao, R.V. and Subrahmanyam, J., Studies on the formation of TiB2 through carbothermal reduction of TiO2 and B2O3, Mater. Sci. Eng., A, 2003, vol. 362, nos. 1–2, pp. 145–151. https://doi.org/10.1016/S0921-5093(03)00523-9
Huang, B., Chen, S., Yao, Z., Zhang, M., Jing, Y., Li, B., and Xiong, W., Study of carbothermal synthesis of TiB2 assisted by extended high-energy milling, Powder Technol., 2015, vol. 275, pp. 69–76. https://doi.org/10.1016/j.powtec.2014.12.025
Yu, J., Ma, L., Zhang, Y., Gong, H., and Zhou, L., Synthesis of TiB2 powders via carbothermal reduction of TiO2, HBO2 and carbon black, Ceram. Int., 2016, vol. 42, no. 4, pp. 5512–5516. https://doi.org/10.1016/j.ceramint.2015.12.108
Kang, S.H. and Kim, D.J., Synthesis of nano-titanium diboride powders by carbothermal reduction, J. Eur. Ceram. Soc., 2007, vol. 27, nos. 2–3, pp. 715–718. https://doi.org/10.1016/j.jeurceramsoc.2006.04.053
Zhang, H. and Li, F., Preparation and microstructure evolution of diboride ultrafine powder by sol-gel and microwave thermal reduction method, J. Sol-Gel Sci. Technol., 2008, vol. 45, no. 2, pp. 205–211. https://doi.org/10.1007/s10971-007-1656-1
Kudaka, K., Iizumi, K., Izumi, H., and Sasaki, T., Synthesis of titanium carbide and titanium diboride by mechanochemical displacement reaction, J. Mater. Sci. Lett., 2001, vol. 20, pp. 1619–1622. https://doi.org/10.1023/A:1017906012176
Nekahi, A. and Firoozi, S., Effect of KCl, NaCl and CaCl2 mixture on volume combustion synthesis of TiB2 nanoparticles, Mater. Res. Bull., 2011, vol. 46, no. 9, pp. 1377–1383. https://doi.org/10.1016/j.materresbull.2011.05.013
Nasiri-Tabrizi, B., Adhami, T., and Ebrahimi-Kahrizsangi, R., Effect of processing parameters on the formation of TiB2 nanopowder by mechanically induced self-sustaining reaction, Ceram. Int., 2014, vol. 40, no. 5, pp. 7345–7354. https://doi.org/10.1016/j.ceramint.2013.12.078
Zarrinpoor, H., Firoozi, S., and Milani, V., Ignition and chemical mechanisms of volume combustion synthesis of titanium diboride, Ceram. Int., 2016, vol. 42, no. 9, pp. 11217–11223. https://doi.org/10.1016/j.ceramint.2016.04.032
İpekçi, M., Acar, S., Elmadağlı, M., Hennicke, J., Balcı, Ö., and Somer, M., Production of TiB2 by SHS and HCl leaching at different temperatures: characterization and investigation of sintering behavior by SPS, Ceram. Int., 2017, vol. 43, no. 2, pp. 2039–2045. https://doi.org/10.1016/j.ceramint.2016.10.174
Javadi, A., Pan, S., Cao, C., Yao, G., and Li, X., Facile synthesis of 10 nm surface clean TiB2 nanoparticles, Mater. Lett., 2018, vol. 229, pp. 107–110. https://doi.org/10.1016/j.matlet.2018.06.054
Nozari, A., Ataie, A., and Neshmati-Manesh, S., Synthesis and characterization of nano-structured TiB2 processed by milling assisted SHS route, Mater. Charact., 2012, vol. 73, pp. 96–103. https://doi.org/10.1016/j.matchar.2012.08.003
Zhou, L., Yang, L., Shao, L., Chen, B., Meng, F., Qian, Y., and Xu, L., General fabrication of boride, carbide and nitride nanocrystals via a metal-hydrolysis-assisted process, Inorg. Chem., 2017, vol. 56, no. 5, pp. 2440–2447. https://doi.org/10.1021/acs.inorgchem.6b02501
Rao, L., Gillan, E.G., and Kaner, R.B., Rapid synthesis of transition-metal borides by solid-state metathesis, J. Mater. Res., 1995, vol. 10, no. 2, pp. 353–361. https://doi.org/10.1557/JMR.1995.0353
Rabiezadeh, A., Hadian, A.M., and Ataie, A., Synthesis and sintering of TiB2 nanoparticles, Ceram. Int., 2014, vol. 40, no. 10, pp. 15775–15782. https://doi.org/10.1016/j.ceramint.2014.07.102
Kim, J.W., Shim, J.-H., Ahn, J.-P., Cho, Y.W., Kim, J.-H., and Oh, K.H., Mechanochemical synthesis and characterization of TiB2 and VB2 nanopowders, Mater. Lett., 2008, vol. 62, no. 16, pp. 2461–2464. https://doi.org/10.1016/j.matlet.2007.12.022
Karasev, A.I., Manufacture of powders of technical titanium, zirconium, chromium, and tungsten borides by the boron carbide method, Powder Metall. Met. Ceram., 1973, vol. 12, no. 10, pp. 777–780.
GOST (State Standard) 5744–85: Abrasive Grains from Boron Carbide. Specifications, Moscow: Izd. Standartov, 1998.
GOST (State Standard) 7885–86: Carbon Black for Rubber Industry. Specifications, Moscow: Izd. Standartov, 1987.
Levinskii, Yu.V. and Petrov, A.P., Making alloyed titanium diboride powders, Powder Metall. Met. Ceram., 1993, vol. 32, no. 6, pp. 480–483.
Subramanian, C., Murthy, T.S.R.Ch., and Suri, A.K., Synthesis and consolidation of titanium diboride, Int. J. Refract. Met. Hard Mater., 2007, vol. 25, no. 4, pp. 345–350. https://doi.org/10.1016/j.ijrmhm.2006.09.003
Fard, H.S.P. and Baharvandi, H., Preparation of titanium diboride powders from titanium alkoxide and boron carbide powder, Bull. Mater. Sci., 2011, vol. 34, art. ID 883. https://doi.org/10.1007/s12034-011-0209-y
Yu, J., Ma, L., Abbas, A., Zhang, Y., Gong, H., Wang, X., Zhou, L., and Liu, H., Carbothermal reduction synthesis of TiB2 ultrafine powders, Ceram. Int., 2016, vol. 42, no. 3, pp. 3916–3920. https://doi.org/10.1016/j.ceramint.2015.11.059
Krutskii, Yu.L., Bannov, A.G., Antonova, E.V., Sokolov, V.V., Pichugin, A.Yu., Maksimovskii, E.A., Krutskaya, T.M., Netskina, O.V., and Bataev, I.A., Synthesis of fine dispersed titanium diboride from nanofibrous carbon, Ceram. Int., 2017, vol. 43, no. 3, pp. 3212–3217. https://doi.org/10.1016/j.ceramint.2016.11.146
Kuvshinov, G.G., Mogilnykh, Yu.L., Kuvshinov, D.G., Yermakov, D.Yu., Yermakova, M.A., Salanov, A.N., and Rudina, N.A., Mechanism of porous filamentous carbon granule formation on catalytic hydrocarbon decomposition, Carbon, 1999, vol. 37, no. 8, pp. 1239–1246. https://doi.org/10.1016/S0008-6223(98)00320-0
Krutskii, Yu.L., Nepochatov, Yu.K., Pel’, A.N., Skovo-rodin, I.N., Dyukova, K.D., Krutskaya, T.M., Kuchumova, I.D., Matts, O.E., Tyurin, A.G., Emurlaeva, Yu.Yu., and Podryabinkin, S.I., Synthesis of polydisperse boron carbide and synthesis of a ceramic on its basis, Russ. J. Appl. Chem., 2019, vol. 92, no. 6, pp. 750–758. https://doi.org/10.1134/S0044461819060045
Blott, S.J. and Pye, K., Gradistat: A grain size distribution and statistics package for the analysis of unconsolidated sediments, Earth Surf. Process. Landforms, 2001, vol. 26, no. 11, pp. 1237–1248. https://doi.org/10.1002/esp.261
Saburov, V.P., Cherepanov, A.N., Zhukov, M.F., Galevskii, G.V., Krushenko, G.G., and Borisov, V.T., Plazmokhimicheskii sintez ul’tradispersnykh poroshkov i ikh primenenie dlya modifitsirovaniya metallov i splavov (Plasma-Chemical Synthesis of Ultrafine Powders and Their Application for the Modification of Metals and Alloys), Novosibirsk: Nauka, 1995.
Cheng, Y., Shigeta, M., Choi, S., and Watanabe, T., Formation mechanism of titanium diboride nanoparticles by RF induction thermal plasma, Chem. Eng. J., 2012, vol. 183, pp. 483–491. https://doi.org/10.1016/j.cej.2011.12.040
Yeh, C.L. and Wang, H.J., Combustion synthesis of vanadium borides, J. Alloys Compd., 2011, vol. 509, no. 7, pp. 3257–3261. https://doi.org/10.1016/j.jallcom.2010.12.004
Rhodes, C., Stuart, J., Lopez, R., Li, X., Waje, M., Mullings, M., Lau, J., and Licht, S., Evaluation of properties and performance of nanoscopic materials in vanadium diboride/air batteries, J. Power Sources, 2013, vol. 293, pp. 244–252. https://doi.org/10.1016/j.jpowsour.2013.03.071
Solov’ev, N.E., Makarov, V.S., Meshchaninova, L.N., and Ugai, Ya.A., Interaction of oxides of 3d transition metals with boron, J. Alloys Compd., 1992, vol. 178, nos. 1–2, pp. 131–138. https://doi.org/10.1016/0925-8388(92)90254-7
Smirnyagina, N.N., Sizov, I.G., Semenov, A.P., and Vandanov, A.G., Thermodynamic analysis of vanadium borides synthesis on surface of carbon steels in vacuum, Fiz. Khim. Obrab. Mater., 2001, no. 2, pp. 63–67.
Smirnyagina, N.N., Sizov, I.G., and Semenov, A.P., Thermodynamic modeling of the vacuum synthesis of transition-metal borides, Inorg. Mater., 2002, vol. 38, no. 1, pp. 39–44. https://doi.org/10.1023/A:1013699326953
Markovskii, L.Ya., Vekshina, N.V., Bezruk, E.T., Sukhareva, G.E., and Voevodskaya, T.K., A magnesium-thermic method for the preparation of metal borides, Sov. Powder Metall. Met. Ceram., 1969, vol. 8, no. 5, pp. 350–354.
Shi, L., Gu, Y., Chen, L., Yang, Z., Ma, J., and Qian, Y., Low-temperature synthesis of nanocrystalline vanadium diboride, Mater. Lett., 2004, vol. 58, nos. 22–23, pp. 2890–2892. https://doi.org/10.1016/j.matlet.2004.05.013
Meerson, G.A. and Samsonov, G.V., Vacuum-thermal production of refractory metals borides, Zh. Prikl. Khim., 1954, vol. 27, no. 10, pp. 1115–1120.
Krutskii, Yu.L., Maksimovskii, E.A., Krutskaya, T.M., Popov, M.V., Netskina, O.V., Nikulina, A.A., Cherkasova, N.Yu., and Kvashina, T.S., Synthesis of highly dispersed zirconium diboride for fabrication of special-purpose ceramic, Russ. J. Appl. Chem., 2017, vol. 90, no. 10, pp. 1579–1585. https://doi.org/10.1134/S1070427217100044
Funding
This work was carried out in accordance with the state task of the Ministry of Science and Higher Education of the Russian Federation (code FSUN-2020-0008).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated by V. Selikhanovich
About this article
Cite this article
Krutskii, Y.L., Cherkasova, N.Y., Gudyma, T.S. et al. Diborides of Some Transition Metals: Properties, Application and Production. Review. Part 1. Titanium and Vanadium Diborides. Steel Transl. 51, 93–106 (2021). https://doi.org/10.3103/S0967091221020029
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0967091221020029