Abstract
Titanium diboride ultrafine powder was prepared from sucrose, tetrabutyl titanate and boric acid by the sol–gel and microwave carbothermal reduction method. The influence of reaction temperature, ratio of Ti to C and Ti to B on the synthesis of titanium diboride was studied. The results indicated that the carbothermal temperature, the content of carbon and the amount of H3BO3 show obvious effects on the formation of TiB2. 1,300 °C was the optimum synthesis temperature and pure TiB2 could be prepared. The microstructure of prepared TiB2 was investigated by field emission-scanning electron microscopy (FE-SEM), which results showed that the crystalline size of the prepared titanium diboride at 1,300 °C was about 3–5 μm. The quantities of the crystalline phases of the powders prepared at different temperatures were analyzed by Rietveld refinement method.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Titanium boride combines a lot of important characteristics, i.e., high melting point (about 3,526 °C), high elastic modulus (about 541 GPa), high hardness, good chemical inertness, and high wear resistance, that make it a promising candidate for high temperature structural materials [1, 2]. Those properties also make TiB2 attractive in many fields of engineering, such as cutting tools, ballistic armor, dispersoid for metal/ceramic matrix composite, and as a cathode for electrochemical reduction of aluminum (Hall–Heroult cell) with a possibility of reduced cell voltage [3].
TiB2 powders can be usually synthesized by following methods [4–12]; (i). By carbothermic, aluminothermic, silicothermic or magnesiothermic reduction of TiO2–B2O3 mixtures at high temperature usually over 1,600 °C; (ii). By electrolysis of fused salts containing mineral rutile and boric oxide dissolved in mixed electrolytes; (iii). By the reduction of TiO2 with carbon and boron carbide; (iv). By Self-propagating-high-temperature synthesis (SHS) technique. However, it is always necessary to have a high temperature and a long production period in those methods, and the synthesized powders usually have a relatively large crystallite size and poor sinterability.
The microwave sintering method has been developed for fabricating metals, ceramics, and composites [13–15]. Compared with conventional sintering process, the microwave sintering technique allows lower sintering temperature and shorter soaking time.
Recently, the preparation of ultrafine powders has received much attention as it can improve the microstructure of the sintered sample, and enhance its mechanical properties. The sol–gel process is a well-known chemical route to prepare oxide-based materials. Moreover, the use of molecular precursors and the control of the synthesis conditions make it possible to prepare homogeneous and pure multicomponent systems. However, to the knowledge of authors, there is no report on the preparation of TiB2 by a homogeneous sol–gel and microwave heating process. The aim of the present work was to investigate the low temperature preparation of TiB2 ultrafine powder using a novel approach of combining sol–gel and microwave carbothermal reduction.
2 Experimental procedure
Starting materials utilized in present paper were analytical reagent grade tetrabutyl titanate (≥99.8 Wt%, Beijing Chem. Co. Ltd., Beijing, China, C16H36O4Ti), H3BO3 (≥99 Wt%, Beijing Chem. Co. Ltd., Beijing, China), sucrose (≥99 Wt%, Beijing Chem. Co. Ltd., Beijing, China, C12H22O11). TiB2 is obtained by the following reaction,
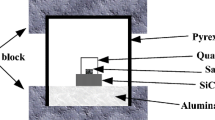
A stoichiometric amount of H3BO3 was dissolved in distilled water, then a stoichiometric amount of sucrose was added, and after complete mixing, a homogenous transparent solution was achieved. The solution was then slowly mixed with tetrabutyl titanate precursor to get the gel; and the gel was heated in a temperature range of 120–140 °C for 24 h to get a dried gel. Finally, the dried gel precursor was fired in microwave furnace (The sketch of microwave heating furnace is shown in Fig. 1. Model: MW-L0316V, 3 kW, 2.45 GHz, by Changsha Longtech CO., Ltd, Hunan province, China) at 900–1,300 °C for 2 h in flowing Ar atmosphere (Ar purity = 99.9999 wt%) to obtain ultrafine TiB2 powder. The heating rate was about 20 °C min−1, the plateau reaction temperature could be held within ±5 °C. The gas outlet valve was normally opened on heating and closed at 600 °C on cooling. Two series of specimens were prepared: (1). in one series (designated as TC series), the molar ratio of TiO2/B2O3 in starting precursor was 1:1(theoretical ratio by Eq. 1), and the amount of carbon was changed. (2). the molar ratio of TiO2/C in the starting precursor was 1:5 (theoretical ratio by Eq. 1), and the amount of B2O3 was changed.
X-ray diffraction patterns were recorded from 20° to 100° (2θ) with a step width of 0.02°, using a Philips X’Pert PRO diffractometer (Cu Kα radiation, Ni filter and silicon internal standard, 40 kV, 40 mA, time per step 3.80 s). The Rietveld refinement method, an established way for the quantitative determination of crystalline phases, was used to refine the structure of the phases in the samples. The PANalytical X’pert Highscore plus program has been used for the refinement analysis. The microstructure of the samples was directly studied using a field emission scanning electron microscopy (FESEM; JSM-6700F, JEOL, JAPAN) equipped with energy–dispersive spectroscopy (EDS; Oxford, UK) without carbon/gold coating.
3 Results and discussions
Figure 2 shows the XRD patterns of TC series samples with different TiO2:B2O3:C ratio fired at 900–1,300 °C. It indicates that the inceptive formation temperature of TiB2 by sol–gel and microwave carbothermal reduction way is about 900 °C. The extent of TiB2 formation is influenced by amount of C addition and annealing temperature. For specimens with theoretical carbon addition (TC-1), there is an increase in TiB2 formation with temperature rise up to 1,300 °C, and pure TiB2 crystal phase can be prepared at 1,300 °C (Fig. 2a). This temperature is much lower than that of the conventional method (over 1,500 °C) [16]. Excess amount of carbon plays a negative effect on the preparation of pure TiB2, it can be concluded from the XRD patterns showed in Fig. 2b.
Table 1 shows the preparation condition and the content of phase composition of TC series specimens with different carbon annealed at 900–1,300 °C. From the results, it can be seen: (1). The amount of TiC formed increase with carbon content, and decreases with temperature rise. It is interesting to note that TiC may be a transitory phase; the temperature of its formation commences at about 900 °C. For specimens with theoretical carbon addition (TC-1), it is perhaps transformed to formation TiB2 at high temperature (1,300 °C). (2). Over 1,000 °C, TiO2 phase (rutile) can not be observed, this shows that the carbothermal reduction process is complete at this temperature. (3). The lattice parameter of TiB2 decreased with increasing temperature and decreasing carbon content, especially in the parameter of a. The lattice parameter of TiB2 (TC-1 specimen) at 1,300 °C is a = b = 3.027(3)Å and c = 3.230(2)Å, which is little different with that of the pure tetragonal TiB2 phase (a = b = 3.028Å and c = 3.228Å, ICSD reference code 98–001–3159).
Figure 3 shows the XRD patterns of TB series samples fired at 900–1,300 °C. It illustrates that Pure TiB2 is prepared for all the TB series specimens at 1,300 °C (Fig. 3a). When B2O3 addition is 15% (molar ratio) more than that theoretical content and the annealing temperature is 900 °C, the extent of TiB2 formation is more than 50% (Table 2). This indicates that for synthesizing TiB2 by tetrabutyl titanate, H3BO3 and sucrose, the excessive amount of B2O3 is effective in promoting TiB2 preparation at low temperature (Fig. 3b).
SEM images of the synthesized TiB2 powders at 1,300 °C for 2 h are shown in Fig. 4. From the SEM images (Fig. 4a), the granular particle sizes of the synthesized TiB2 powders distribute over 3–5 μm, and agglomerations appear in the samples. Some of the particles are plate-like, clear growth steps (Fig. 4b) are observed in them. And the grain growth of those particles not only occurred along the a–b plane but also along the c-axis of the TiB2 unit cell, which growth of the plate-like crystals along the c-axis is evidenced in Fig. 4c. The growth of these plate-like crystals was driven by the reduction in the substantial particle surface energy at the beginning of the growth process. Subsequently, the crystal growth of a, b and c-axis became less efficient when the effective particle surface area was decreased, and then those plate-like crystals joined together to form big granular particle with growth steps (Fig. 4a). EDS analysis (Fig. 4d) of the granular particles in Fig. 4a shows the only presence of Ti and B, which undoubtedly indicates that the pure TiB2 powders are prepared at 1,300 °C.
SEM photograph and EDS results of TC-1 sample prepared at 1,300°C by sol–gel and microwave carbothermal method (a) granular-shaped TiB2 particles (b) growth steps along the (a–b) plane of TiB2 particles (c) growth steps along the a–b, and c plane of TiB2 particles (d) EDS results (atomic ratio%) of the selected points in Fig.4a
Figure 5 shows the SEM images of the synthesized powders at 1,100 °C for 2 h. Three kinds of particles with different shape were found, the first ones is hexagonal –shaped (as the selected points in Fig. 5a), its crystallite sizes mainly distribute over 2–4 μm, and the EDX analysis (Fig. 5c) shows that those particles are TiB2. This shape is in accordance with the theoretical crystal structure of TiB2. The second ones is octahedral-shaped (as the selected point 1, 2 and 5 in Fig. 5b), its EDX analysis (Fig. 5c) indicates that these particles are TiC. The third ones is granular with size about 1 μm (as selected point 3 and 4 in Fig. 5b), the EDS results (Fig. 5c) demonstrates it is also TiB2.
The growth of lamellar TiB2 crystals along a favored orientation with a lateral growing mechanism is also shown in Fig. 6. From this figure, it can be clearly seen that TiB2 nucleated and grew in a pool of “amorphous phase. “The amorphous phase” was consumed through the grain growth, and those small grains were subsequently joined together to form plate-like crystals with an orientation parallel to the pellet surface. The “amorphous phase” is undetectable by XRD due to its non-crystalline nature. Based on the SEM results of Figs. 4–6, a possible microstructure transformation of the prepared TiB2 crystals can be proposed as shown in Fig. 7.
4 Conclusions
Ultrafine TiB2 was formed by sol–gel and microwave carbothermal reduction process using tetrabutyl titanate, H3BO3 and sucrose as studying materials. The initial crystallization temperature of the TiB2 powder is about 900 °C, whereas that of the fully crystallized TiB2 appeared at about 1,300 °C. The carbothermal temperature, the content of carbon and the amount of H3BO3 show obvious effects on the formation of TiB2.
The principal factors influencing ultrafine TiB2 powder synthesis are: ① Formulation. For promoting ultrafine TiB2 formation at lower temperatures, use of tetrabutyl titanate, H3BO3 and sucrose is recommended at TiO2:B2O3:C = 1:1:5 molar ratios. ② Temperature. The suitable firing temperature is 1,300°C. The TiB2 powders prepared at 1,300 °C are granular with size 3–5 μm.
References
Edirisinghe MJ, McCollum JI (1993) History and recent development in SHS, Ceram International, vol 19. pp 113–20
Millet P, Hwang T (1996) Preparation of TiB2 and ZrB2. Influence of a mechanochemical treatment on the borothermic reduction of titania and zirconia. J Mater Sci 31:351–355
Khanra AK, Godkhindi MM (2005) Comparative studies on sintering behavior of self-propagating high-temperature synthesized ultra-fine titanium diboride powder. J Am Ceram Soc 88(6):1619–1621
Campbell IE, Sherwood EM (1967) High-temperature materials and technology. John Wiley & Sons, New York, p 349
Bates SE, Bur WE, Frey CA, Sastry SL, Kelton KF (1995) Synthesis of titanium boride (TiB)2 nanocrystalline by solution-phase processing. J Mater Res 10(10):2599–2612
Axelbaum RL, Dufaux DP, Crey CA (1996) Gas-phase combustion synthesis of titanium boride (TiB 2) nanocrystallites. J Mater Res 11(4):948–954
Merzhanov AG (1995) History and recent development in SHS. Ceram Int 21:371–379
Quabdesselam M, Munir ZA (1987) The sintering of combustion-synthesized titanium diboride. J Mater Sci 22:1799–1807
Khanra AK, Pathak LC, Mishra SK, Godkhindi MM (2004) Effect of nacl on the synthesis of tib2 powder by a self-propagating-high-temperature synthesis (SHS) technique. Mater Lett 58:733–738
Khanra AK, Godkhindi MM, Pathak LC (2007) Sintering behaviour of ultra-fine titanium diboride powder prepared by self-propagating high-temperature synthesis (SHS) technique. Mater Sci Eng A 454:281–287
Jun L, Bing L (2007) Preparation of the TiB2 coatings by electroplating in molten salts. Mater Lett 61(6):1274–1278
Kang SH, Kim DJ (2007) Synthesis of nano-titanium diboride powders by carbothermal reduction. J Eur Ceram Soc 27(2–3):715–718
Saitou K (2006) Microwave sintering of iron, cobalt, nickel, copper and stainless steel powders. Scripta Mater 54:875–879
Fang Y, Cheng JP, Agrawal DineshK (2004) Effect of powder reactivity on microwave sintering of alumina. Mater Lett 58:498–501
Purushotham Yadoji, Ramesh Peelamedu, Dinesh Agrawal, Rustum Roy (2003) Microwave sintering of Ni-/Zn ferrites: comparison with conventional Sintering. Mater Sci Eng B 98:269–278
Subramanian C, Murthy TSRCh, Suri AK (2007) Synthesis and consolidation of titanium diboride. Int J Refract Mater 25:345–350
Acknowledgements
This work was financially supported by “Science Fund for Distinguished Young Scholars of Henan Province, China” (Contact No. 0512002400)” “The Fund of The Hubei Province Key Laboratory of Refractories and Ceramics Ministry-Province Jointly-Constructed Cultivation Base for State Key Laboratory, China (Contact No. G0603)”.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s10971-008-1771-7
Rights and permissions
About this article
Cite this article
Zhang, H., Li, F. Preparation and microstructure evolution of diboride ultrafine powder by sol–gel and microwave carbothermal reduction method. J Sol-Gel Sci Technol 45, 205–211 (2008). https://doi.org/10.1007/s10971-007-1656-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-007-1656-1