Abstract
Currently, the most common fluxing additive to pellets is dolomite CaCO3 · MgCO3, in which the magnesium oxide content can form from 17 to 22%. If the magnesium oxide in pellets increases, then it is necessary to increase the dolomite dosage. Thus, the iron content decreases, which entails a decrease in yield ratio at subsequent processing. One of the fluxes containing magnesium is brucite. Compared with dolomite, magnesium oxide content in pure brucite is more than 3 times higher. The basis of Flumag M flux is brucite. The magnesium oxide content in it is not less than 55%. The paper presents a series of laboratory studies on the Flumag M flux dosage effects on the pelletizing ability of the charge and such properties of iron ore pellets as compressive, impact and abrasion strength. We have made the tests on raw and fired pellets with Flumag M flux. The comparative analysis of strength properties of the pellets obtained with the use of Flumag M and limestone was performed. The binder content, bentonite and magnetite concentrate, for all experiments remained unchanged. The experiment results indicate that Flumag M does not interfere with charge pelletizing ability. The strength of raw pellets for discharge and compression with Flumag M flux has small deviations from the pellets with the addition of limestone. Roasted pellets with the addition of Flumag M flux have higher strength than ones with limestone. The higher difference in strength properties is observed at the flux content of 2%.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The technological development of solid-phase iron’s direct reduction, where strong iron-ore pellets represent the main charge, is a progressive field for contemporary metallurgical production.
Requirements of metal plating processes are mainly caused by the physicochemical changes, which occur in the iron-ore material during these processes. These requirements were comprehensively analyzed by the authors from [1–5].
Raw material has the following general requirements for metal plating shaft furnaces:
—high iron content at low sulfur contents, phosphorus, alkali, and nonferrous metal impurities, which largely affect the steel quality and performance indicators of its casting in electrical furnaces;
—high reducibility;
—high hot strength, which determines the pellet ability to retain integrity upon reduction;
—low susceptibility to sinter formation, swelling, and strain upon direct reduction of iron in solid-phase processes, which determines high gas permeability of charge in furnace;
A narrow particle size distribution of the agglomerated raw material possessing sufficient strength in initial state and upon reduction with high onset fusion temperature and low content of fine fraction (less than 5 mm) is necessary for metal plating furnaces with the aim to develop optimal gas-dynamic conditions, which provide high performance and high degree of metal plating. According to the experimental data, the following data is shown: the 9–16-mm pellets at content less than 5-mm fraction of less than 5% and the compression strength higher than 250 kg/pellet in the cold state.
With the incorporation of metal plating shaft furnaces, additional external and internal friction forces arose upon hot discharge due to large strain of reduced pellets, which determine the form of burden yield in furnace. With an increase in the internal friction coefficient, the motion of charge uniformity in the shaft furnace is distorted, which leads to gas-dynamic model violation of reduction and destabilization of the effective product quality. Friction coefficients are determined by the surface’s state and structure, as well as the degree of pellet strain in charge bottom lifts with a high degree of reduction [8, 9].
Many years fabricating iron-ore pellets showed that the addition of various fluxing or flux-strengthening components is one of the most widespread approaches to control physical and metallurgical characteristics of pellets [10–18].
The addition of dolomite to the pellet charge instead of limestone prevents the formation of fusible eutectics, which allows for a temperature increase to reduce pellets in metal plating furnaces. However, the compression strength of pellets decreases with the retention of temperature–time calcination conditions (from 242 to 160 kg/pellet), which is caused by the lack of liquid-phase binder upon high-temperature strengthening on a roast machine. To obtain high strength of roasted pellets with the addition of magnesium oxide, it is necessary to increase temperature in the roast machine area up to 1300–1320°C [6, 7, 10, 15].
The addition of MgO results in the decrease in the reduction ability of the pellets, as well as improvement of strength characteristics of the pellets upon recovery. The pellets with the basicity of 1.2, where limestone represents a fluxing additive (0.6% MgO), possess the highest reducing ability. In spite of the decrease in the reducing ability of the pellets upon the addition of MgO, it remains higher than at the basicity of 0.5 fluxed in the presence of CaO. Basicity is usually 0.5–0.8 upon the MIDREX process, while it varies from 0.2 to 1.2 in the HyL-III process [12–16].
The pellets containing 3–5% MgO, where dolomite is a fluxing additive, possess the highest metallurgical characteristics. Magnesite, olivine, and dunite can act as magnesium-containing additives to the charge of pellets. The studies showed that addition of 1–2% of olivine (Mg,Fe)2SiO4 to super-rich Olenegorskii concentrate (0.4–0.6% SiO2) increases both cold (by the factor of 1.5–1.7) and hot (by the factor of 1.5–3.0) strength of pellets [8].
At present, researchers in the field of iron-ore raw material production are focused on magnesium hydroxide Mg(OH)2, which represents an insoluble base.
An increase in the magnesium oxide content in pellets results in the formation of the pyroxene phase (Mg,Fe,Ca)2Si2O6 possessing high melting point (1540–1550°C) [13].
Natural minerals of magnesium hydroxide, which constitutes the main content of brucite rocks, was named after American mineralogist A. Bruce, and its chemical formula is Mg(OH)2. Its pure mineral composition is 69.12% MgO and 30.88% H2O.
Brucite is a mineral that mainly consists of magnesium hydroxide, as well as possesses lower porosity at heating and lower dissolving ability in the sinter burden materials as compared to magnesite.
Brucite contains less inclusions, which form the basis of slurries. It contains more magnesium oxide and possesses lower losses upon sintering as compared to magnesite. This is one of the advantages, because low-porous materials avoid fast cohesion of magnesium oxide in the metal upon heating.
Brucite generally has less inclusions. The silica content is lower than that in serpentinite, while the calcium oxide content is less than that in dolomite. If a magnesium-containing additive contains a large SiO2 content, a “thick drop structure” in the slaggy phase forms and, as a result, the agglomerate strength decreases [11, 16, 17].
Laboratory experiments using magnesium fluxing material represented by Flumag M based on brucite for the preparation of iron-ore pellets were carried out in the Stary Oskol Technological Institute, National University of Science and Technology “MISiS” [18].
Flumag M based on brucite contains at least 55% of magnesium oxide, at least 6% of silica, less than 1% of Fe2O3, and less than 0.03% of sulfur according to chemical analysis; losses on ignition are less than 35%.
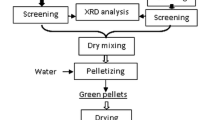
A series of experiments on charge dosage into pellets were performed. At the Institute under laboratory conditions, limestone, bentonite, and Flumag M fluxing additive were dispersed on a disc grinder and the material was sieved through 0.071 mm sieve. Magnetite concentrate chosen for studies contained 97.65% of the particles with the size less than 0.045 mm.
Qualitative characteristics of charge components are given in Table 1.
In order to evaluate the Flumag M fluxing additive as flux-strengthening components in the fabrication of iron-ore pellets, the pellets fabricated using limestone at analogous quantities were used for comparative analysis.
The bentonite dosage was constant in all experiments and corresponded to 0.6%.
A total of 2 kg of magnetite concentrate was taken for the preparation of pellets in order to provide the necessary amount of the test specimens.
Raw pellets were prepared in a laboratory pelletizer (Fig. 1). Then, they were exposed to particle size distribution analysis and compression and discharge strength and moisture content.
The discharge strength n, times, was calculated using the following equation:
where ni is the number of discharges of one pellet until loss of integrity.
The test results were calculated exactly to the integer value. The charge pelletizing ability was identified as the material ability to form pellets with particular strength characteristics.
There is no standard procedure on the determination of pelletizing ability of fine-dispersed material. Thus, the researchers suggest various approaches to the determination of charge pelletizing ability. The staff of the Division of the Ugarov Stary Oskol Technological Institute, National University of Science and Technology “MISiS” suggested the procedure of pelletizing ability tests for the pelletizing study of iron-ore concentrates, which is described in detail in [1]. In accordance with this procedure, the pelletizing ability is performed according to the yield of the grains larger than 5 mm and the duration of pelletizing process corresponds to 20 min.
The moisture content and strength of raw pellets was determined according to the procedures accepted in industrial enterprises [19]. The laboratory study results of raw pellets are given in Table 2.
The weight fraction of moisture (W) was calculated as follows:
where m1 is the container mass with shot before drying, g; m2 is the container mass with shot after drying, g; and m is the mass of the empty container, g.
Calculations were performed exactly up to the second decimal.
Divergence between the results of parallel measurements was less than 0.3%. Divergence between the results of three measurements was less than 0.4%.
The test results were calculated exactly up to the whole integer.
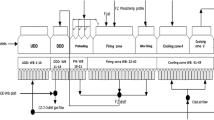
Heat treatment of the pellets was carried out in a laboratory furnace according to the developed temperature–time mode, which was maintained constant in all experiments. The maximum temperature of annealing corresponded to 1280°C (Fig. 2).
The compression strength of the pellets was determined according to the procedure in accordance with GOST [20] using a hydraulic press, which applies a maximum load on the pellet.
The compression strength of pellet Pav was calculated as follows
where Pi is the compression strength of one pellet, kg (N), and n is the number of pellets for the determination of strength, units.
The results were rounded up to one decimal value, lg/pellet. The dispersion and abrasive strength [21] were evaluated in a laboratory drum and determined as follows:
where m1 is the mass of >5 mm fraction after testing in drum, kg; m2 is the mass of <5 mm and >0.5 mm fraction after testing in drum, kg; and m3 is the mass of the <0.5 mm fraction after testing in drum, kg.
The abrasion wear resistance of the pellets was calculated using the following equation:
The impact and abrasion wear strength were evaluated on two shots. The mean value of two parallel measurements was taken as the final result.
Analysis of the test results showed that the Flumag M fluxing additive can be used in the production of iron-ore pellets. According to the concentrate features of various deposits under different process conditions, the fluxing additive dosage should be optimized at each particular process condition and can differ from the results above.
CONCLUSIONS
The Flumag M fluxing additive does not prevent the pelletizing process with the addition to the iron-ore concentrate as a charge component.
The Flumag M (brucite) is a technologically pure supplier of magnesium oxide into iron-ore charge and provides raw pellets upon palletization without worsening their qualitative characteristics.
Analysis of the roasted pellet characteristics shows that the compression strength of the roasted pellets slightly decreases with the addition of the Flumag M fluxing additive, which can be rationalized by the decrease in the amount of the liquid-phase binder when using magnesium oxide as a fluxing additive. This factor confirms the necessity for the increase in temperature of pellet annealing when using magnesial fluxing materials up to the temperatures of 1300–1330°C.
The impact and abrasion wear strength of the pellets is slightly higher with the addition of the Flumag M fluxing agent than those using limestone. The largest difference in abrasion wear strength characteristics was recorded at the content of 2% of Flumag M.
With an increase in the dosage of the Flumag M fluxing agent, the compression strength of pellets tends to decrease; however, this can be controlled by the change of the temperature–time mode of heat treatment.
REFERENCES
Gimmel’farb, A.I., Nemenov, A.M., and Tarasov, B.E., Metallizatsiya i elektroplavka zhelezorudnogo syr’ya (Metallization and Electric Smelting of Iron Ore Raw Materials), Moscow: Metallurgiya, 1981.
Tulin, N.A., Kudryavtsev, V.S., Pchelkin, S.A., et al., Razvitie beskoksovoi metallurgii (Development of Non-Coke Metallurgy), Moscow: Metallurgiya, 1987.
Carvalho, R., Supply availability of DR grade pellet, Proc. 3rd World DRI & Pellet Congr., Abu Dhabi, 2015. https://www.metalbulletin.com/events/download.ashx/ document/speaker/7663/a0ID000000X0kBmMAJ/Presentation.
Vasil’ev, S.S. and Vasil’ev, E.N., Changes in strength of roasted non-fluxed pellets from rich Lebedinskii concentrate during recovery process, in Pryamoe poluchenie zheleza i poroshkovaya metallurgiya (Direct Production of Iron and Powder Metallurgy), Moscow: Metallurgiya, 1976, no. 2, p. 5.
Nobuhiko, T., Development of Iron-Making Technology: Nippon Steel Technical Report No. 101, Tokyo: Nippon Steel, 2012, pp. 79–88.
Alekseev, L.F., Gorbachev, V.A., Kudinov, D.Z., and Shavrin, S.V., Struktura i razrushenie okatyshei pri vosstanovlenii (Structure and Destruction of Pellets during Recovery), Moscow: Nauka, 1983.
Halt, J.A. and Kawatra, S.K., Review of organic binders for iron ore concentrate agglomeration, Miner. Metall. Process., 2014, vol. 31, no. 2, pp. 73–94.
Kovalev, D.A., Vanyukova, N.D., Ivashchenko, V.P., et al., Teoreticheskie osnovy proizvodstva okuskovannogo syr’ya (Theoretical Basics for Production of Agglomerates), Dnepropetrovsk: IMA-Press, 2011.
Chen, M., Zhang, W., Zhao, Z., et al., High temperature softening behaviors of iron blast furnace feeds and their correlations to the microstructures, Proc. 6th Int. Symp. on High-Temperature Metallurgical Processing, Chichester: Wiley, 2015, pp. 67–74.
Okrkr, S.I. and Onukwuli, O.D., Effect of basicity on metallurgical properties of pellets produced from Itakpe iron ore concentrates, Discovery Innovation, 1999, vol. 11, nos. 3–4, pp. 170–176.
Abzalov, V.M., Gorbachev, V.A., Evstyugin, S.N., et al., Fiziko-khimicheskie i teplotekhnicheskie osnovy proizvodstva zhelezorudnykh okatyshei (Physicochemical and Thermal Basics of Iron Ore Pellets Production), Leont’ev, L.I., Ed., Yekaterinburg: Molodezhn. Inf. Tsentr, 2015.
Kalenga, M.K. and Garbers-Craig, A.M., Investigation into how the magnesia, silica and alumina contents of iron ore sinter influence its mineralogy and properties, J. South. Afr. Inst. Min. Metall., 2010, vol. 10, pp. 447–456.
Poveromo, J.J., Grade pellet quality and supply, Proc. AISTech’2014 Annual Meeting, Indianapolis, Warrendale, PA: Assoc. Iron Steel Technol., 2015, pp. 751–762.
Forsmo, S.P.E., Samskog, P.O., and Bjorkman, M.T., A study on plasticity and compression strength in wet iron ore green pellets related to real process variations in raw material fineness, Powder Technol., 2008, vol. 181, no. 3, pp. 321–330.
Wang, Z., Chu, M., Chen, S., et al., Effects of B–Mg additive on metallurgical properties of oxidized pellets, Adv. Mater. Res., 2011, vols. 284–286, pp. 1232–1236.
Umadevi, T., Roy, A.K., and Prabhu, P.C., Influence of magnesia on iron ore sinter properties and productivity—use of dolomite and dunite, Steel Res. Int. J., 2009, vol. 80, no. 11, pp. 800–807.
Zborshchik, A.M., Teoreticheskie osnovy metallurgicheskogo proizvodstva (Theory of Metallurgical Production), Donetsk: Donsk. Nats. Tekh. Univ., 2008.
Timofeeva, A.S., Nikitchenko, T.V., and Kozhukhov, A.A., Role of magnesium oxide in formation of physical, chemical and metallurgical properties of iron ore pellets, Chern. Metall., Byull. Nauchno-Tekh. Ekon. Inf., 2018, no. 5 (1421), pp. 23–27.
GOST (State Standard) 12764–73: Iron Ores, Concentrates, Agglomerates and Pellets, Method for Determination of Moisture, Moscow: Izd. Standartov, 1974. http://docs.cntd.ru/document/1200024455. Accessed February 9, 2019.
GOST (State Standard) 24765–81: Method for the Determination of Compression Strength, Moscow: Izd. Standartov, 1981. http://docs.cntd.ru/document/gost-24765-81. Accessed February 9, 2019.
GOST (State Standard) 15137–77: Iron and Manganese Ores, Agglomerates and Pellets, Determination of Tumbler Strength, Moscow: Izd. Standartov, 1978. http://docs.cntd.ru/document/gost-15137-77. Accessed February 9, 2019.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by A. Muravev
About this article
Cite this article
Kozhukhov, A.A., Timofeeva, A.S. & Nikitchenko, T.V. Effect of Flumag M Flux on Strength Properties of Iron Ore Pellets. Steel Transl. 50, 375–380 (2020). https://doi.org/10.3103/S0967091220060030
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0967091220060030