Abstract
A laboratory study was carried out to characterize the physical, chemical and mechanical properties of lime fluxed (varying basicity 0–2) hematite iron ore pellets. Lime was used as additive as well as fluxing agent for making iron ore pellets. The effect of additives on different properties of pellets was studied. The findings show that on increasing the addition of lime, more of calcium-alumino-silicate phases were produced as confirmed by SEM-EDAX analysis. These phases have low melting points, which enhances sticking behaviour of pellets, as well as imparts strength to the pellets (resulting increasing compressive strength, tumbler, abrasion and shatter index) but decreases the porosity. The low basicity pellets were found predominantly oxide-bonded, while the high basicity pellets were mostly slag-bonded. This means that the pellet should be fired at sufficiently high enough temperature to generate liquid phases to get the sufficient strength but not so high as to cause the pellets to stick to each other. The obtained properties of these fluxed pellets were compared with the properties of iron ore lump and pellets, which are being used conventionally in the blast furnace for production of iron and steel.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Global blast furnace iron production from January to November 2014 was 1083 million tonnes of which India alone produced 49.16 million tonnes occupying third position in the world. Global direct reduced iron (DRI) production for the same period was 55.43 million tonnes of which India produced 16.66 million tonnes occupying first position [1].
About 1600 million tonnes of iron ore was mined worldwide in 2013, of which almost 98 % was used in iron and steel making [2]. Mined ore can be used directly as lump or converted into sinter or pellets, and reduced either by direct reduction or in a blast furnace. Around 25 % of mined ore is converted into pellets and its demand is increasing [3].
In India, nearly 60 % of mined ore is fines [4]. Agglomeration of ore fines is essential to utilize these fines. The agglomerates quality plays a vital role in decreasing the consumption of coke and increasing the productivity. In most of the integrated steel plants, the burden mix for the blast furnace is decided according to the availability of iron ore agglomerates [5]. In India, typical pellet use in burden is nearly 15–20 % only. These pellets are exclusively acidic pellet [6]. Acid pellets are known for their poor high temperature softening–melting characteristics and reducibility [7, 8].
The industrial use of various additives for acidic pellets has been reviewed by Eisele and Kawatra (2003) [9]. Mostly 0.5–0.7 % bentonite is being used as additive for making acidic pellets which increases the input of acidic oxide in the pellets [10]. Acid oxides have adverse effect on iron–steel making economics. Some forms of sodium bentonite contain more than 65 % SiO2 by weight or 85 % SiO2 and Al2O3. Besides the adverse effect on pellet chemistry, this additional silica blocks porosity inhibiting the flow of reducing gases into the interior of the pellet. This lowers the reducibility of pellets which increases the energy requirements and increases the costs of handling and disposal of the increased slag volume [11]. An increase in silica content in pellets by 1 %, leads to increase the cost of making steel by US$ 4–7/tonne [12]. In the case of directly reduced pellets, every percent of acid gangue addition is associated with an increased energy consumption of 108 MJ/tonne [13]. Hence, the use of pellets having lower gangue concentrations, results in improvement of energy and flux consumptions. Cost savings in energy for each 1.0 % reduction in silica can reach US$ 2.5/tonne of hot metal [14].
Inorganic materials, like hydrated lime, as an additive have a slight advantage over bentonite when silica levels are the primary concern of steel makers. Addition of lime results in poor quality green balls which are fragile and easily broken, lowering production rates in iron making [15].
In India, in recent years, attention has been given to use of fluxed pellets in blast furnaces due to their good strength, reducibility, swelling and softening–melting characteristics [16]. In general, the quality of pellets is influenced by the nature of ore or concentrate, associated gangue, type and amount of flux/additive added and their subsequent treatment to produce pellets. These factors in turn result in the variation of physicochemical properties of the coexisting phases and their distribution during pellet indurations. Hence, properties of these pellets are largely governed by the form and degree of bonding achieved between ore particles and the stability of these bonding phases during reduction [17].
In fluxed pellets, bonding is achieved through silicate melt formation during induration. The amount of gangue in the ore or concentrate, fluxes and binder, influences the amount and chemistry of the silicate melt. CaO fluxes silicates reacts with iron oxide to form calcium ferrites. Panigrahy et al. (1984) found that, with increasing basicity more liquid phase was formed during induration and hence more strength and less porosity were observed [18].
Hence, in the production of acid, basic or fluxed pellets, the characterisation of bonding and crystalline phases is of prime importance for understanding the basis for the production of desired quality pellets. In this direction, investigations have been carried out by several researchers on the bonding behaviour in fluxed hematite pellets of basicity 0.5–1.00 [5, 19, 20] and basicity 0.08–1.15 [21]. Friel and Erickson (1980) have studied on fluxed magnetite pellets using good quality dolomite with MgO contents 1–2 wt% and CaO/SiO2 ratios from 0.6 to 1.3 [20]. Specifically, compositions within these ranges had good physical properties (i.e. tumble >98 %, compressive strength >2670 N) and they were significantly more reducible but softened more at higher temperatures than acid pellets. Based on the literature, it is clear that the mechanical properties (shatter, tumbler, abrasion, crushing strength etc.) and as physical properties (porosity, density etc.) of hardened fluxed hematite pellets with varying basicity have not been extensively studied. The aim of the present study was to optimize the obtained properties (i.e. physical, microstructural, chemical and mechanical) of three types of distinct chemical compositional fluxed fired hematite iron ore pellets and comparing the properties of lumpy hematite iron ore or conventionally used pellets, to tell about the suitability of these fluxed pellets for using burden in the blast furnace.
Experimental
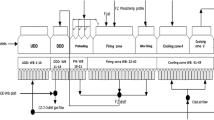
Figure 1 illustrates the experimental program for making and characterizing the pellets.
Materials and Their Characterization
Hematite iron ore was procured from the mines of Barbil, Odisha (India) and limestone from mines of Rajasthan (India). Lime was made in the laboratory by heating the limestone at 1050 °C. The chemical analyses of both samples, done using XRF analysis (model-ARL OPTIM’X X-Ray analyzer), are shown in Table 1. X-ray diffraction analysis was performed by RIGAKU D-MAX IIIB under the following condition: CuKα as emission radiation source (λ = 1.54178 Å), voltage = 40 kV, intensity = 30 mA, scan rate 3°/min in the range of 3°–85°.
The analysis of iron ore indicated that the gangue content in the iron ore were mainly alumina and silica with a negligible amount of MgO, Mn, P, S and alkali. The detailed phase analysis of iron ore and lime fines was presented in earlier work by the authors [15]. The percentage of Fe2O3 in iron ore was around 95 %. Similarly in the lime, CaO was the major phase with small amounts of SiO2 and Al2O3; no major foreign particles were observed.
Burnt lime and iron ore fines samples were ground separately to the required fineness using a 5 kg capacity laboratory ball mill to 80 % passing 0.045 mm to get optimum fineness for pellet making as per industrial practice [22].
Preparation of Iron Ore Pellets
The iron ore fines were thoroughly mixed with different amount of lime fines (i.e. 0, 2, and 4 %) in dry condition. Pellets were made on a disk type pelletizer (diameter 760 mm, rim height 120 mm, disk angle 45°, r.p.m. 20). The determination of the optimum moisture content (OMC) for different mixtures was performed before making pellets as reported in previous work by the authors [15] and the conditions are listed in Table 2. The average diameter of the pellets made was 14–16 mm. The green pellets were oven dried at 110 °C for 1/2 h followed by hardening at temperatures of either 1100, 1200 or 1300 °C for 1 h then cooled to room temperature for further characterization (Fig. 1).
Testing Methodology
The apparent porosity, true porosity, sealed porosity, apparent density and true density of hardened iron ore pellets were determined by the boiling water method as per ASTM C-20-00 (2010) and water pycnometer as per ASTM C135-96 (2009) respectively [23, 24]. The cold crushing strength of the oven dried, hardened iron ore pellets as well as oven dried lump iron ore samples was determined using a low range universal testing machine (SHIMADZU, type: SBL, P/N: 340-43120-01, capacity—5 kN) at a speed of 1.5 cm/min as per ASTM E382-07 (12) [25]. Tumbler, abrasion and shatter indices of hardened pellets and oven dried lump iron ore samples were determined as per ASTM E279-97(2010) as well as ASTM D3038-07 (2012) [26, 27].
The surface characteristics and structural changes in some of the hardened iron ore pellets were examined using a scanning electron microscope (FEI Quanta-200FEG) at 20 kV on scan rate 10 µs with ETD detector, on the fractured surface after crushing strength measurement. EDAX analysis was also performed to determine the phases present in the pellets after hardening.
Results
Physical Properties
Fusion Behavior and Colour Changes After Hardening
After hardening, a colour change of the pellets was observed. It was seen that with increasing lime content, the colour of the pellets faded from brown to whitish at temperatures below 1300 °C. At 1300 °C, the colour of all pellets was black and few pellets were agglomerated. The sticking tendency among the pellets, increased with temperature; therefore temperature was restricted to 1300 °C.
Porosity and Density
Increasing induration temperature from 1100 to 1200 °C, the apparent porosity of pellets (i.e. acid to neutral) were observed increasing trends, but for basic pellets showed decreasing trends even at 1300 °C. The minimum apparent porosity of basic pellets was obtained as 10 %, which is well above the value of lump iron ore (i.e. 8.1 %). Similar trend was also observed for true porosity. With increasing lime content, apparent and true porosity were increased from temperature 1100 to 1200 °C. But, the reverse effect was observed in case of 1300 °C (Fig. 2). The decreasing trend in density of pellet was observed by increasing lime content as shown in Fig. 3.
Mechanical Properties
Crushing Strength
The crushing strength of dried and hardened pellets increased with both basicity and temperature as shown in Fig. 4. The crushing strength of all types of pellets fired at 1300 °C are well above the acceptable limit for pellets being used in the blast furnace (200 kg/pellet). The highest strength 586 kg/pellet was observed for basic pellets which are much above the accepted value for industrial practice.
Shatter, Tumbler and Abrasion Indices
Strength properties (tumbler, abrasion and shatter indices) are commonly used to assess the resistance to degradation during handling before reduction in the blast furnace. The variation in properties of hardened iron ore fluxed pellets and lumpy iron ores are shown in Fig. 5. It is observed that tumbler and abrasion indices of hardened iron ore pellets at 1100–1200 °C decreased compare to lump iron ore but increased with increasing hardening temperature. At 1300 °C induration temperature, the tumbler and abrasion indices of the pellets were superior for lump iron ore and also fulfilled the industrial requirement of minimum 94 % tumbling index and a maximum 5 % abrasion index [6]. The results indicate a lower value of shatter index (for −5 mm size fraction) for all types of pellets at 1100–1200 °C whereas higher values were observed for temperature of 1300 °C as already observed in the case of tumbler and abrasion indices.
Microstructure
SEM images of 1100 °C indured pellets are shown in Fig. 6a. At this temperature, presence of more porosity and free particles indicates that there is no formation of slag. More free particles were observed in case of basic pellets than acid pellets. With increasing temperature free particles decreased (Fig. 6b). At 1300 °C, particles got fused and agglomerated resulting in less porosity and more compacted structure (Fig. 6c). At 1300 °C in case of high basicity pellets, a distinct network of slag (slag bridge) was observed in the pellets.
a SEM micrographs of 1100 °C indured pellets having different basicity. b SEM micrographs of 1200 °C indured pellets having different basicity. c SEM micrographs and EDAX analysis of 1300 °C indured pellets having different basicity. (Spot 1 Aluminium silicate slag phases at lower basicity; Spot 2 porosity; Spot 3 calcium–aluminium silicate slag phases at higher basicity and Spot 4 metallic phases)
From the EDAX analysis of acid pellets at 1300 °C, it was observed that aluminium silicate phases exist in lower amount. With increasing basicity, aluminium silicate slag phase transformed to calcium aluminium silicate phase (6c).
Discussion
The addition of lime resulted in the formation of more calcium silicate melt phase which fills up the pores between solid particles and exerts capillary pressure to pull them together due to interfacial forces, thereby reducing the porosity [5]. Therefore, apparent and true porosity values decreased on increasing temperature above 1200 °C, and increasing lime content. But internal porosity was closed by this slag, resulting in increased sealed porosity. The apparent and true density of pellets decreased with increasing lime content due to the lower specific gravity of lime. On the other hand, higher temperature (1300 °C) resulted in higher values of both due to volume change owing to slag formation in the pellets causing a decrease in volume. The slag formed a strong bond inside the pellets [28, 29].This bond offered high resistance to abrasion and tumbling, most likely due to their hard fine-grained structure, resulting in increasing crushing strength, shatter and tumbling index. Based on the tumbler, abrasion, and shatter indices results, pellets hardened at 1300 °C were hard and strong. On the other hand, all the other pellets were more porous, soft and friable and hence, are liable to produce deleterious fines (−5 mm fraction) during handling or in blast furnaces, rotary kilns, etc. Pellets, hardened at lower temperature (1100–1200 °C) mainly oxide bonded, cannot bear a sudden load. The shatter test values are much lower than for the lump ore. As seen from Figs. 2, 4 and 5 there is a definite correlation between porosity and strength (tumbler, abrasion, and shatter indices). The trends clearly show increasing strength with decreasing porosity. At higher induration temperature, slag formed bridge between particles after cooling the pellets. These bridge is mainly responsible for high strength. With increasing lime content the silicate slag phase transformed to calcium silicate slag, which created high strength in the pellets at high temperature as evidenced from the SEM-EDAX analysis. This behaviour has also been reported by other researchers [30].
Conclusions
Following conclusions were drawn from the present study:-
It is possible to use lime-fluxed iron ore pellets as a raw material to minimise flux input in blast furnaces and avoid bentonite addition as an additive as used in conventionally prepared pellets.
The preparation of fluxed iron ore pellets with high basicity (1.17) is possible without sticking at induration temperature up to 1300 °C with the help of lime as an effective binder.
The hardened pellets show higher crushing strength (586.76 kg/pellet) and lower apparent density (2.69 g/cc) than lump iron ore (400 kg/cm2 and 3.1 g/cc respectively). This is due to formation of calcium silicate in basic pellets.
The values of shatter, tumbler and abrasion resistance of fluxed hardened (1300 °C) pellets were superior to acid pellets and lumpy iron ore.
The apparent and true porosity of hardened (1300 °C) fluxed pellets were higher than lumpy ore. For example, apparent porosity 9.96 % for basic pellets is comparable with value for lump ore (i.e. 8.1 %).
With increasing hardening temperature, apparent porosity increased by 42.8 % at 1200 °C. But at 1300 °C, slag formation within the pellets resulted in less porosity (as low as 9.96 %) which enhanced the crushing strength (586.76 kg/pellet) compared to 400 kg/cm2 for lump ore.
References
World Iron Production (2014), http://www.worldsteel.org/statistic/BFI-production.html)
United States Geological Survey, Iron Ore Statistics and Information (Reston, VA, 2014), (available at http://www.minerals.usgs.gov/minerals/pubs/commodity/iron_ore/2013)
United Nations Conference on Trade and Development, The Iron Ore Market 2009–2011 (Geneva, Switzerland, 2010), (available at http://www.unctad.org/infocomm/Iron/Flyer_2010_English.PDF)
Indian Mineral Yearbook, Part-III, edn 51, (2012), (available at http://www.ibm.nic.in/writereaddata/files/07092014125520IMYB_2012_iron%20ore.pdf)
S. Dwarapudi, T.K. Ghosh, A. Shankar, V. Tathavadkar, D. Bhattacharjee, R. Venugopal, Effect of pellet basicity and MgO content on the quality and microstructure of hematite pellets. Int. J. Miner. Process. 99, 43 (2011)
IBM Iron and Steel Vision 2020. Chapter 4; Agglomeration (2012), (available at http://www.ibm.gov.in/ch4.pdf)
M. Onoda, O. Tsuchiya, T. Sugiyama, I. Fujita, Quality improvements of lime fluxed pellets, in Proceedings of ISS-AIME 40th Iron Making Conference, (Toronto, Ontario, 1980), p. 286
S. Dwarapudi, T.K. Ghosh, A. Shankar, V. Tathavadkar, D. Bhattacharjee, R. Venugopal, Effect of pyroxenite flux on the quality and microstructure of hematite pellets. Int. J. Miner. Process. 96, 45 (2010)
T.C. Eisele, S.K. Kawatra, A review of binders in iron ore pelletization. Miner. Process. Extr. Metall. Rev (2003). doi:10.1080/08827500306896
S.P.E. Forsmo, A.J. Apelqvist, B.M.T. Björkman, P.O. Samskog, Binding mechanisms in wet iron ore green pellets with a bentonite binder. Powder Technol. (2006). doi:10.1016/j.powtec.2006.08.008
R.P. de Souza, C.F. de Mendonca, T. Kater, Production of acid iron ore pellet for direct reduction using an organic binder. Mining Eng. Magn. 36, 1437 (1984)
V.M. Chizhikov, R.M. Vainshtein, S.N. Zorin, T.I. Zainetdinov, G.A. Zinyagin, A.A. Shevchenko, Production of iron ore-pellets with an organic binder. Metallurgist 47, 3 (2003)
R.H. Heerema, H. Kortmann, T. Kater, V.C. ven den Boogaard Improvements of acid, olivine and dolomite fluxed iron ore pellets using an organic binder, in 5th International Symposium on Agglomeration, (Brighton, 1989), p. 227
Schmitt J A method for improving the process and quality of iron ore pellets made with organic binders, in 66th Annual University of Minnesota Mining Symposium, Duluth, (2005), p. 19
A. Sarkar, A.K. Mandal, O.P. Sinha, Pelletisation behavior of fluxed iron ore pellets of varying basicities made with waste fines. Int. J. Sci. Eng. 5, 9 (2013)
A.R. Firth, J.F. Garden, J.D. Douglas, Phase equilibria and slag formation in the magnetite core of fluxed iron ore pellets. ISIJ Int. 48, 1485 (2008)
S.C. Panigrahy, B.C. Jena, M. Rigaud, Characterization of bonding and crystalline phases in fluxed pellets using peat moss and bentonite as binders. Metall. Trans. B 21, 463 (1990)
S.C. Panigrahy, P. Verstraeten, J. Dilewijns, Influence of MgO addition on the mineralogy of iron ore sinter. Metall. Trans. B 15, 23 (1984)
F.W. Frazer, H. Westenberger, K.H. Boss, W. Thumm, The relationship between basicity and swelling on reduction of iron-ore pellets. Int. J. Miner. Process. 2, 353–365 (1975)
J.J. Friel, E.S. Erickson, Chemistry, microstructure, and reduction characteristics of dolomite-fluxed magnetite pellets. Metall. Trans. B 11, 233 (1980)
T. Umadevi, P. Kumar, N.F. Lobo, M. Prabhu, P.C. Mahapatra, M. Ranjan, Influence of pellet basicity (CaO/SiO2) on iron ore pellet properties and microstructure. ISIJ Int. 51, 14 (2011)
K.M.K Sinha, T. Sharma, Reduction of iron ore pellets by statistical design of experiments. Int. J. Eng. Res. Sci. Technol. 3, 1 (2014), (available at http://www.ijerst.com/currentissue.php)
ASTM Standard C20-00 Standard test methods for apparent porosity, water absorption, apparent specific gravity, and bulk density of burned refractory brick and shapes by boiling water, ASTM Int., West Conshohocken, (2010). doi:10.1520/C0020-00R10
ASTM Standard C135-96 Standard test method for true specific gravity of refractory materials by water immersion, ASTM Int., West Conshohocken, (2009). doi:10.1520/C0135-96R09E01
ASTM E382-12 Standard test method for determination of crushing strength of iron ore pellets, ASTM Int., West Conshohocken (2012). doi:10.1520/E0382-12
ASTM E279-97 Standard test method for determination of abrasion resistance of iron ore pellets and sinter by the tumbler test, ASTM Int., West Conshohocken (2010). doi:10.1520/E0279-97R10
ASTM D3038-93 Standard test method for drop shatter test for coke, ASTM Int., West Conshohocken (2010). doi:10.1520/D3038-93R10
M. Specht, C. Seaton, A. Morales, Self reduced iron ore pellets using Plexicoke as reductant, in Symposium on Chemistry, Structure and Reactivity of Coals, Tar Sands and Oil Shale, vol. 37 (San Francisco, 1992), p. 608
A. Takashi, F. Kiyoshi, F. Hidekazu, Development of carbon iron composite process, JFE Technical Report 13, 20 (2009) (available at http://www.jfe-steel.co.jp/en/research/report/013/pdf/013-02.pdf)
J. Pal, S. Ghorai, M.C. Goswami, S. Ghosh, D. Ghosh, D. Bandyopadhyay, Development of fluxed iron oxide pellets strengthened by CO2 treatment for use in Basic Oxygen Steel Making. ISIJ Int. 49, 210 (2009)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mandal, A.K., Sarkar, A. & Sinha, O.P. Utilization of Lime Fines as an Effective Binder as well as Fluxing Agent for Making Fluxed Iron Ore Pellets. J. Inst. Eng. India Ser. D 97, 69–75 (2016). https://doi.org/10.1007/s40033-015-0079-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40033-015-0079-3